Abstract
Background
Laparoscopic adjustable silicone gastric banding (LASGB) and laparoscopic vertical banded gastroplasty (LVBG) are the most frequently performed restrictive operations for morbid obesity. The question of whether bariatric restrictive procedures increase or reduce gastroesophageal reflux disease (GERD) remains open. This study aimed to compare the long-term results of LASGB with those of LVBG in terms of postoperative GERD and esophageal motility function.
Methods
From February 1999 to December 2000, 175 patients underwent bariatric surgery. After 75 of these patients were excluded from the study, the remaining 100 patients were randomly assigned to one of two treatment groups: LASGB or LVBG. The end points of the study were evaluation of clinical and instrumental GERD and esophageal function. The follow-up protocol included clinical assessment using the Gastroesophageal Reflux Health-Related Quality-of-Life (GERD-HRQOL) scale at 3, 12, and 96 months. Esophageal manometry, 24-h pH monitoring, and endoscopy were performed at 12 and 96 months.
Results
At 12 months, GERD had developed in 13 (26%) LASGB and 11 (21.6%) LVBG patients. In the majority of cases, GERD resulted from pouch dilation or poor compliance and required either reoperation (ten after LASGB and three after LVBG) or endoscopic dilation of the neopylorus (four after LVBG). In all, 71 patients completed the 96-month follow-up protocol. The findings showed that three (11.5%) of 26 LASGB patients and four (9%) of 45 LVBG patients were receiving proton pump inhibitor (PPI) therapy for GERD. Postoperative lower esophageal sphincter (LES) pressure and esophageal motility did not differ from preoperative data except for the presence of aperistaltic waves in one LASGB and two LVBG symptomatic GERD patients.
Conclusions
No significant association between gastric restrictive procedures and GERD or esophageal function was found during long-term follow-up assessment. The increased occurrence of GERD in the early follow-up period often is due to a technical defect or poor patient compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity has become the most common chronic health problem in Western countries, and its prevalence is rising. In the United States alone, obesity currently is the second leading cause of preventable death, with more than 50% of adults estimated as being overweight or obese and 5% as being morbidly obese [1, 2].
Obesity increases the incidence of other medical problems including coronary artery disease, hypertension, peripheral vascular disease, pulmonary insufficiency, sleep apnea, diabetes, osteoarthritis, and gastroesophageal reflux disease (GERD), together with psychosocial disabilities. Obese individuals also are more likely to experience the development of cancer. Furthermore, the risk of premature death is greater for the severely obese than for the nonobese [3].
Bariatric surgery is the only effective treatment for morbid obesity. Numerous studies have described the effect that these procedures can have on GERD symptoms, the objective measures of GERD, and the mixed pattern of outcomes [4, 5]. However, the relationship between restrictive bariatric surgery and GERD is difficult to analyze; and the question about the extent to which weight loss and surgical treatment can resolve GERD remains open.
Mason [6] introduced the principles of vertical banded gastroplasty (VBG). Later, Deitel et al. [7] showed that the laparoscopic vertical banded gastroplasty (LVBG) procedure works on principles used in the surgical treatment of GERD, such as repositioning and retaining the gastroesophageal junction within the abdomen and constructing an elongated intraabdominal esophagus by converting part of the lesser curvature into a compressible tube. Some studies also have reported relief of heartburn and regurgitation in VBG-treated patients, with regression of esophagitis among those undergoing surgery with conversion of adjustable gastric silicone banding to VBG [8, 9].
In recent years, laparoscopic adjustable gastric silicone banding (LASGB) has gained wider acceptance because of its relative simplicity, minimal invasiveness, safety, and efficacy [10, 11]. In Europe, it currently is the most common operation for morbid obesity. Although LASGB has proved to be effective for weight reduction, its effect on esophageal function and GERD remains unclear [12, 13].
This study aimed to evaluate the long-term results of bariatric restrictive surgery in terms of postoperative GERD and esophageal motility function in a group of morbidly obese patients randomly assigned to undergo LVBG or LASGB.
Patients and methods
Patients with a diagnosis of morbid obesity based on National Institutes of Health (NIH) Conference Consensus Development guidelines [14] were included in a prospective randomized controlled clinical study. The details on the reoperation rate, the early and late complications rate, and the percentage of excess body-weight loss (EWL) have been published elsewhere [15].
In the current study, we evaluated the long-term effect of LASGB and LVBG on postoperative GERD and esophageal function. The inclusion criteria required a history of obesity exceeding 5 years, documented previous weight loss attempts, a body mass index (BMI: weight in kilograms divided by the square of the height in meters) of 40–50 kg/m2, and an age of 18–60 years. The exclusion criteria ruled out contraindications to creation of a pneumoperitoneum (e.g., glaucoma), large esophageal hiatal hernia (>3 cm), symptomatic GERD, pregnancy, drug or alcohol abuse, psychological disorders (e.g., bulimia, depression), hormonal or genetic obesity-related disease, and previous gastric surgery.
The patients were evaluated by a dietitian to exclude “sweet” eaters and “binge” eaters because restrictive bariatric procedures are contraindicated for these two patient categories. Eligibility was based on clinical history, thorough physical examination, blood chemistry, hormonal status, and instrumental evaluation. Patients then were randomly assigned to one of two treatment groups: LAGB or LVBG. Preoperative GERD symptoms were assessed using the Gastroesophageal Reflux Health-Related Quality-of-Life (GERD-HRQOL) scale [16].
All the patients underwent preoperative upper gastrointestinal (GI) endoscopy. Findings of possible hiatal hernia or esophagitis were recorded in detail, and esophagitis was graded according to the Savary–Miller classification [17].
Stationary manometry [18–20] of the esophagus was performed before and after the operation using a low-compliance pneumohydraulic system (Dyno 2000 Menfis Biomedica, Bologna, Italy). Medications exerting a possible effect on esophageal motility were discontinued 5 days before examination. Analyzed parameters included upper and lower esophageal sphincter (LES) pressure, sphincter relaxation, and amplitude of peristaltic contractions.
Lower esophageal sphincter pressure was calculated as both the mid-expiratory pressure at the respiratory inversion point and the average of all pressures recorded in the high-pressure zone (as analyzed by computer). Esophageal body motility and LES relaxation were assessed by recording the changes in pressure elicited by 10 wet swallows, with the side holes of the catheter positioned inside the LES and 5, 10, 15, and 20 cm higher up. Residual pressure of LES was defined as the minimal pressure (nadir) recorded in the LES during swallowing.
Pre- and postoperative 24-h esophageal pH monitoring was performed as described elsewhere [21]. The 24-h pH-monitoring data were downloaded from the digital data logger into a personal computer and analyzed using pH monitoring dedicated software (pH day software; Menfis Biomedica, Bologna, Italy). Reflux was evaluated using the DeMeester scoring system (DMS) [22] and the area under the H+ parameter (AUH+) [23].
Surgical technique
The surgical technique has been described previously [15, 24]. For LASGB, the LapBand (Bioenterics, Carpinteria, CA, USA) was used for all patients. A calibration balloon tube (Bioenterics) was passed transorally by the anesthetist into the stomach and filled with 25 ml of saline solution. Dissection of the retrogastric tunnel started at a point on the lesser curve level with the equator of the balloon. The LASGB was correctly positioned, and the tubing was connected to the access port positioned subcutaneously in the left upper abdomen.
For LVBG, laparoscopic dissection started on the lesser curvature of the stomach 6 cm from the gastroesophageal junction. A transgastric window was created using an endoluminal 12-mm-diameter calibrating tube. The transgastric window was created with a 21-mm-diameter circular stapler, and the gastric pouch was constructed with a linear stapler inserted through the gastric window and directed toward the angle of His. Finally, a polypropylene mesh band was wrapped flat around the gastric pouch outlet and sutured to itself to create a 5-cm circumference to calibrate the gastric pouch outlet.
Outcome assessment
All the patients underwent upper GI evaluation with hydrosoluble contrast medium on the postoperative day 1 (LASGB) or 2 (LVBG). The follow-up protocol included clinical assessment according to the GERD-HRQOL scale [16] at 3, 12, and 96 months. Esophageal manometry and 24-h pH monitoring were performed at 12 and 96 months. Esophagogastroduodenoscopy was performed at 12 and 96 months. Postsurgical antireflux medication was prescribed according to severity of symptoms, 24-h pH monitoring, and endoscopic findings.
Statistical analysis
The primary end point of the study was the incidence of GERD in the LASGB and LVBG groups at 12- and 96-month follow-up evaluations of patients randomized to receive LASGB or LVBG and described in a previously published study [15]. The GERD rates after the LASGB procedure are reportedly 10–57% [6, 9], whereas LVBG is not thought to affect GERD rates [11, 25].
The secondary end points were the effect that the interventions had on esophageal function, particularly the differences between pre- and postoperative LES pressure (LESp) and esophageal motility. Categorical variables were compared using a χ2 test, with Yates correction and Fisher’s exact test (two-tailed) used when necessary. Continuous variables were compared using Student’s t-test or the Mann–Whitney U test, depending on distribution. All P values were two-sided. A P value <0.05 indicated a statistically significant difference. Data were analyzed on an intention-to-treat basis. All calculations were done with SPSS, version 10.0 (SPSS Inc., Chicago, IL, USA).
Results
From February 1999 to December 2000, 175 patients underwent bariatric surgery at our institution. Of these patients, 75 were excluded from the study because of a BMI exceeding 50 kg/m2 (35 patients), a BMI less than 40 kg/m2, comorbidities (5 patients), a specific contraindication to pneumoperitoneum (4 patients), previous gastric surgery (6 patients), GERD (14 patients), and refusal to enter the protocol (11 patients) (Fig. 1).
The remaining 100 patients were randomized into two treatment groups: LASGB (n = 49) or LVBG (n = 51). The two groups were comparable in terms of sex, age, mean body weight, BMI, percentage of EWL, and laboratory test results. The short-term follow-up results from this randomized clinical trial in terms of weight loss, operating time, hospital stay, mortality, and morbidity have been published elsewhere [15].
Preoperative GERD symptoms were assessed by means of a standard form based on the GERD-HRQOL scale [16]. Preoperative upper GI endoscopy excluded malignancy in all patients and showed a small hiatal hernia (type 1) in six LASGB patients (12.2%) and five LVBG patients (9.8%). Preoperative manometry and 24-h pH monitoring were performed for all the patients. At 12 months, all the patients from both groups were available for the follow-up assessment. At 96 months, 67 patients (26 in the LASGB group and 41 in the LVBG group) were available for long-term analysis.
LASGB-treated group
At the 12-month follow-up assessment, 13 LASGB-treated patients (26%) presented with GERD symptoms, confirmed by pH-metry, positive DMS score, and AUH+ for only 6 patients. For the remaining 7 patients, the DMS score was positive but AUH+ was normal. All patients were treated with proton pump inhibitor (PPI) therapy, although a band desufflation was required in four cases. Because clinical and instrumental GERD did not improve with medical therapy and band desufflation for three patients (6.1%), two of these patients underwent reoperation with band removal, and the remaining patient with grade 2 esophagitis underwent band removal followed by a Roux-en-Y gastric bypass. All the patients with clinical GERD who had a discrepancy between the DMS score and AUH+ underwent band removal for pouch dilation without band slippage in the course of the following 36 months.
Postoperative manometry showed abnormal aperistaltic waves of the esophageal body in patients with symptomatic GERD, especially in those with pouch dilation (30% of aperistaltic waves in 10 patients [20%]). At the 96-month follow-up assessment, 23 patients were unavailable because three refused to complete the follow-up evaluation; nine (18.4%) underwent band removal because of pouch dilation with or without band slippage; three (6.1%) underwent band removal due to GERD; seven (14.3%) were noncompliant or referred for severe food intolerance, poor weight loss, or weight regain and subsequently underwent reoperation with band removal and conversion to another bariatric procedure (VBG for 3 and gastric bypass for 4 patients); and 1 (2%) with band erosion required reoperation with band removal. Three patients (11.5%, 3/26) with clinical and instrumental GERD were still receiving PPI therapy without an endoscopic finding of esophagitis. Postoperative manometry showed normal peristalsis and LES pressure in two patients (7.6%) and 20% of aperistaltic waves with normal LES pressure in one patient (3.8%).
LVBG-treated group
At the 12-month follow-up assessment, 11 patients (21.6%) presented with GERD symptoms, confirmed by pH-metry in only five cases. The remaining 6 patients had a positive DMS score but a normal AUH+. Three patients (5.9%) (two with grade 2 esophagitis at endoscopic control) whose clinical and instrumental GERD did not improve with medical therapy underwent reoperation with conversion to Roux-en-Y gastric bypass. Among the patients with clinical GERD who had a discrepancy between their DMS score and AUH+, four patients exhibited stenosis at the gastric pouch outlet, which was treated successfully by endoscopic dilation. Pouch dilation was noted in two patients.
Postoperative manometry showed abnormal aperistaltic waves of the esophageal body in the patients with symptomatic GERD, especially in those with stenosis at the gastric pouch outlet (25% of aperistaltic waves in 6 patients [11.8%]). At the 96-month follow-up assessment, 10 patients were unavailable because six refused to complete the follow-up evaluation and 4 underwent reoperation (one had gastric bypass for a staple line leak, and three had late conversion to gastric bypass for severe GERD).
Four patients (9.7%; 4/41) with clinical and instrumental GERD were still receiving PPI therapy without an endoscopic finding of esophagitis. Postoperative manometry showed normal peristalsis and LES pressure in two patients and 18% of aperistaltic waves with normal LES pressure in two other patients. Tables 1, 2, 3, 4 and 5 present the effect of LASGB and LVBG on GERD symptoms, manometry, pH-monitoring, and endoscopy.
Discussion
In recent years, the minimally invasive approach has become the preferred technique for bariatric surgery [25]. For morbid obesity, LASGB and LVBG are the two restrictive procedures most frequently performed [26]. However, there are no definitive data on the relationship between the type of bariatric restrictive procedure and GERD or on the outcomes after these two types of interventions for obese patients [27, 28]. To date, conflicting results have come mostly from small retrospective series with short-term follow-up evaluation, with some claiming significant improvement, others showing no difference, and still others reporting worsening of GERD symptoms and findings after a bariatric restrictive procedure [9, 29, 30]. The effect on esophageal motility also is unclear [31].
The literature contains divergent reports on the incidence of GERD symptoms after LASGB. Gutschow et al. [32] noted a reduction in heartburn during the early follow-up period (≤12 months) compared with the preoperative situation when the band is only slightly or not inflated. They found worsening of symptoms during the mid- and long-term follow-up evaluation after band inflation to narrow the esophageal outlet. As a result, esophageal clearance is progressively reduced, leading to stasis of ingested food and refluxed acidic material, with increasing rates of heartburn, regurgitation, and dysphagia, especially if the proximal pouch is dilated.
Dixon and O’Brien [10] and Merrouche et al. [4] described a rapid improvement in reflux symptoms after surgery during the first postoperative weeks and suggested LASGB as an antireflux procedure, but their studies reported only short-term results. Øvrebø et al. [31] observed an initial worsening of GERD symptoms after LASGB and an increase in reflux score. Reflecting these changes, 24-h pH monitoring data showed a marked increase in esophageal acid exposure.
Also unclear is the effect of LASGB on esophageal motility. Band placement may increase resting LESp and lead to impairment of LES relaxation [9, 29]. Yet some studies claim that the band makes no difference in terms of esophageal motility disorders, whereas others show that postoperative esophageal dysmotility and gastroesophageal reflux commonly occur after LASGB [30].
In the current study, we found a worsening of GERD symptoms in 26% of patients, as measured by the GERD-HRQOL scale after LASGB at the 12-month follow-up assessment. The 24-h pH monitoring data indicated a significant increase in acid exposure for six patients (12%), whereas for seven patients (14%), the DMS scores were higher and AUH+ was normal. This could be explained by stagnation of food in the LASGB pouch and the distal esophagus, which leads to acid fermentation, resulting in a pH decreased to slightly below 4, a positive DMS score, and a positive percentage of total time pH <4 [33], but without changing the normal AUH+ value.
The patients with a discrepancy between DMS score and AUH+ were noted to have pouch dilation with band dislocation and had undergone reoperation with band removal in the first 48 months of the follow-up period. These patients also were found to have altered esophageal motility with 30% of aperistaltic waves and increased synchronous waves, which may have been caused by band outlet obstruction, band dislocation, or pouch dilation (Table 3).
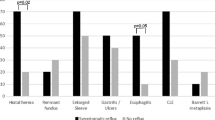
Over the course of the long-term follow-up period, symptoms improved, as measured by the GERD-HRQOL scale (Table 1), and no statistically significant differences emerged for alterations in esophageal motility or LESp. At the 96-month follow-up assessment, three patients (11.5%) presented with clinical and instrumental GERD, one of which (3.8%) had normal LESp but 20% of aperistaltic waves. No significant change in postoperative upper GI tract endoscopy was found, and no GERD complications were recorded at the 96-month follow-up assessment (Table 5).
The LVBG approach was introduced as a possible antireflux procedure by Deitel et al. [7]. These authors observed a reduction in heartburn from 77 to 22% and in regurgitation from 55 to 3% after LVBG. Hiatus hernias identified before surgery were absent after the procedure.
Nevertheless, GERD symptoms continue to be reported in patients after LVBG. These symptoms can result from postprandial esophageal loading or true reflux from the distal stomach [7, 8]. Staple-line disruption may allow passage of acid into the pouch and the esophagus. Additionally, large pouches may include acid-secreting mucosa.
Among the patients with an intact LVBG undergoing conversion to Roux-en-Y gastric bypass for severe GERD symptoms, 96% are reported to experience a complete or near complete resolution of heartburn symptoms, 88% discontinue antireflux medications, and all patients with preoperative endoscopically documented esophagitis have complete resolution without progression to Barrett’s esophagus [7, 8]. Øvrebø et al. [31] found that in LVBG-treated patients, heartburn and acid regurgitation did not increase and that the reflux score was unchanged after surgery. The LVBG procedure was not followed by significant changes in esophageal acid exposure. These authors reported data from a short-term follow-up assessment and did not perform manometry. Similarly, Näslund et al. [34] failed to observe any influence of VBG on gastroesophageal reflux. Other studies claiming the antireflux properties of VBG operations have rather short follow-up periods and lack manometry and pH-metry data.
In our study, the GERD-HRQOL questionnaire showed GERD symptoms in 11 LVBG patients (21.6%) at the 12-month follow-up assessment. At pH-metry, a discrepancy between DMS score and AUH+ value was found in six patients (11.7%) (Table 4). For four of these patients, stenosis of the gastric pouch outlet (2 patients with pouch dilation) was successfully treated by endoscopic dilation. In these patients, pouch outlet obstruction followed by pouch dilation caused food stagnation, leading to acid fermentation, with worsening of symptoms and 25% of aperistaltic waves shown by manometry. At 96 months (Table1), four patients (9.7%) were still receiving PPI therapy. No statistically significant differences in esophageal motility or LESp alterations (Table 3) and no esophagitis at endoscopy were found at 96 months (Table 5).
In conclusion, we found an increase in GERD symptoms during the early follow-up period among the LASGB-treated patients mainly because of incorrect device positioning, which resulted in slow emptying of the pouch and pouch dilation with food stagnation. In these cases, worsening of GERD symptoms was not due to acid reflux but rather to acidification because of delayed pouch emptying. When this complication occurred, the device was removed, with or without conversion to other bariatric operations, to improve the patient’s quality of life.
Concerning LVBG, we think that incorrect eating habits of poorly compliant patients during the early follow-up period led to gastric pouch dilation with acidification rather than to a real reflux. At long-term follow-up assessment, patients who had undergone a correctly performed operation and followed a proper diet were at less risk for the development of clinical and instrumental GERD. When GERD is present, medical therapy can reduce the risk of severe complications such as esophagitis or Barrett’s esophagus.
In brief, our study found no significant association between restrictive bariatric surgery and postoperative esophageal dysfunction or gastroesophageal reflux. Correct surgical technique and appropriate follow-up nutrition are both crucial to improving the patients’ quality of life. Due to the consistent rate of redo surgery after both LASGB and LVBG, a higher number of cases and possibly a multicentric trial will be useful to confirm the current findings.
References
Flegal KM, Carroll MD, Ogden CL, Johnson CL (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727
Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH (2002) Trends and correlates of class 3 obesity in United States from 1990 through 2000. JAMA 288:1758–1761
Martin LF, Hunter SM, Lauve RM, O’Leary JP (1995) Severe obesity: expensive to society, frustrating to treat, but important to confront. South Med J 88:895–902
Merrouche M, Sabaté JM, Jouet P, Harnois F, Scaringi S, Coffin B, Msika S (2007) Gastroesophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg 17:894–900
Korenkov M, Köhler L, Yücel N, Grass G, Sauerland S, Lempa M, Troidl H (2002) Esophageal motility and reflux symptoms before and after bariatric surgery. Obes Surg 12:72–76
Mason EE (1987) Morbid obesity: use of vertical banded gastroplasty. Surg Clin North Am 67:521–537
Deitel M, Khanna RK, Hagen J (1988) Vertical banded gastroplasty as an antireflux procedure. Am J Surg 155:512–516
Naslund E, Granstrom L, Stockeld D, Backman L (1993) Vertical banded gastroplasty: one treatment for esophagitis and/or weight gain after gastric banding. Obes Surg 3:365–368
Nilsell K, Thörne A, Sjöstedt S, Apelman J, Pettersson N (2001) Prospective randomized comparison of adjustable gastric banding and vertical banded gastroplasty for morbid obesity. Eur J Surg 167:504–509
Dixon JB, O’Brien PE (1999) Gastroesophageal reflux in obesity: the effect of lap-band placement. Obes Surg 9:527–531
Belachew M, Belva P, Desaive C (2002) Long-term results of laparoscopic adjustable gastric banding for the treatment of morbid obesity. Obes Surg 12:564–568
Victorzon M, Tolonen P (2002) Intermediate results following laparoscopic adjustable gastric banding for morbid obesity. Dig Surg 19:354–358
Tolonen P, Victorzon M, Niemi R, Mäkelä J (2006) Does gastric banding for morbid obesity reduce or increase gastroesophageal reflux? Obes Surg 16:1469–1474
NIH Conference (1991) Gastrointestinal surgery for severe obesity. Consensus development conference panel. Ann Intern Med 115:956–961
Morino M, Toppino M, Bonnet G, del Genio G (2003) Laparoscopic adjustable silicone gastric banding versus vertical banded gastroplasty in morbidly obese patients: a prospective randomized controlled clinical trial. Ann Surg 238:835–842
Velanovich V, Vallance SR, Gusz JR, Tapia FV, Harkabus MA (1996) Quality-of-life scale for gastroesophageal reflux disease. J Am Coll Surg 183:217–224
Savary M, Miller G (1978) The esophagus: handbook and atlas of endoscopy. Gassinany, Solothurn
Zaninotto G, DeMeester TR, Schwizer W, Johansson KE, Cheng SC (1988) The lower esophageal sphincter in health and disease. Am J Surg 155:104–111
Ergun GA, Kahrilas PJ (1996) Clinical application of esophageal manometry and monitoring. Am J Gastroenterol 91:1077–1089
Frantzides CT, Carlson MA, Madan AK, Stewart ET, Smith C (2003) Selective use of esophageal manometry and 24-hour pH monitoring before laparoscopic fundoplication. J Am Coll Surg 197:358–363
Jamieson JR, Stein HJ, DeMeester TR, Bonavina L, Schwizer W, Hinder RA, Albertucci M (1992) Ambulatory 24-h oesophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 87:1102–1110
Johnson L, DeMeester T (1986) Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol 8:52–58
Rebecchi F, Francia I, Giaccone C, Morino M (2002) Improving the analysis of esophageal acid exposure by a new parameter: area under H+. Am J Gastroententerol 97:568–574
MacLean LD, Rhode BM, Forse RA (1993) A gastroplasty that avoids stapling in continuity. Surgery 113:380–388
Toppino M, Morino M, Capuzzi P, Mistrangelo M, Carrera M, Morino F (1999) Outcome of vertical banded gastroplasty. Obes Surg 9:51–54
Schauer PR, Ikramuddin S (2001) Laparoscopic surgery for morbid obesity. Surg Clin North Am 81:1145–1179
Murray L, Johnson B, Lane A, Harvey I, Donovan J, Nair P, Harvey R (2003) Relationship between body mass and gastrooesophageal reflux symptoms: the Bristol Helicobacter Project. Int J Epidemiol 32:645–650
Delgado-Aros S, Locke GR III, Camilleri M, Talley NJ, Fett S, Zinsmeister AR, Melton LJ III (2004) Obesity is associated with increased risk for gastroesophageal reflux symptoms: a population-based study. Am J Gastroentrol 99:1801–1806
Di Francesco V, Baggio E, Mastromauro M, Zoico E, Stefanelli N, Zamboni M, Panourgia MP, Frulloni L, Bovo P, Bosello O, Cavallini G (2004) Obesity and gastroesophageal acid reflux: physiological mechanisms and role of gastric bariatric surgery. Obes Surg 14:1095–1102
Suter M, Dorta G, Giusti V, Calmes JM (2005) Gastric banding interferes with esophageal motility and gastroesophageal reflux. Arch Surg 140:639–643
Øvrebø KK, Hatlebakk JG, Viste A, Bassøe HH, Svanes K (1998) Gastroesophageal reflux in morbidly obese patients treated with gastric banding or vertical banded gastroplasty. Ann Surg 228:51–58
Gutschow CA, Collet P, Prenzel K, Hölscher AH, Schneider PM (2005) Long-term results and gastroesophgeal reflux in a series of laparoscopic adjustable silicon gastric banding. J Gastroint Surg 9:941–948
Crookes PF, Corkill S, DeMeester TR (1997) Gastroesophageal reflux in achalasia: when is reflux really reflux? Dig Dis Sci 42:1354–1361
Näslund E, Granström L, Melcher A, Stockeld D, Backman L (1996) Gastroesophageal reflux before and after vertical banded gastroplasty in the treatment of obesity. Eur J Surg 162:303–306
Disclosures
Fabrizio Rebecchi, Stefano Rocchietto, Claudio Giaccone, Ahmed Talha, and Mario Morino have no conflicts of interests or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rebecchi, F., Rocchietto, S., Giaccone, C. et al. Gastroesophageal reflux disease and esophageal motility in morbidly obese patients submitted to laparoscopic adjustable silicone gastric banding or laparoscopic vertical banded gastroplasty. Surg Endosc 25, 795–803 (2011). https://doi.org/10.1007/s00464-010-1257-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1257-x