Abstract
Introduction
Interventional therapy for weight regain after gastric bypass surgery has been tempered by higher complications associated with revisional surgery. Endolumenal reduction of post-bypass stomal and pouch dilatation offers the promise of a safer approach. Questions still remain regarding safety and efficacy with these procedures. We report intra- and postoperative results to date utilizing an endolumenal suturing platform for this patient subset.
Methods
Patients who had regained significant weight 2+ years after Roux-en-Y gastric bypass (RYGB) after losing ≥50% of excess body weight (EBW) post RYGB underwent endolumenal stomal and pouch reduction if they endoscopically displayed post-bypass stomal and/or pouch dilatation. The platform was utilized to endolumenally reduce stoma size by creating circumferential folds with a tissue anchoring system. Anchors were also utilized to approximate gastric pouch tissue. Information regarding patient baseline status and data on procedural safety, intraoperative performance, postoperative weight loss, and anchor durability were recorded to date with use of this system.
Results
In 20/21 subjects we were able to successfully place anchors (one patient had occult G-G fistula which impaired visualization). Weight regain post RYGB averaged 59 lbs (N = 20). Stomal diameter was reduced on average by 53%, with pouch reduction averaging 41%. The number of anchors placed on average per case was 5.3. Operating room (OR) time averaged 91 min. There were no significant complications. Three- and 12-month esophagogastroduodenoscopy (EGD) results revealed preservation of most of the intraoperative stoma and pouch reduction, and presence of fibrotic tissue folds with continued presence of anchors at their original locations. Mean percentage excess weight loss (%EWL) at 6 months was 18% to date (N = 18). Mean weight loss at 6 months was 17.3 ± 15 lbs.
Conclusion
Clinical study of this endolumenal tissue approximation system has shown intraoperative safety and efficacy in reducing stoma and pouch dilatation post RYGB. Follow-up anchor durability to date is encouraging. Continuing weight loss is being tracked through ongoing endoscopic and clinical follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Weight regain after Roux-en-Y gastric bypass (RYGB) surgery is an unfortunate reality for a significant percentage of post-RYGB patients. Studies have shown that as many as 35% of post-Roux-en-Y patients will regain weight at the 5–10 year mark [1, 2]. Billions of US dollars are spent annually on nonsurgical approaches to address weight gain (pharmaceuticals, diets, lifestyle changes, etc.) [3]. These interventions have typically fallen short of both patients’ and clinicians’ expectations, with excess weight loss (EWL) results of less than 5% at the 12 month mark [4].
Restriction of the gastrum and outlet are long-held tenets of surgical intervention for the obese patient population. Campos and others have validated that principle with data revealing that pouch size is independently correlated with weight loss after primary RYGB [5, 6]. Most patients who have regained weight post RYGB have evidence of a dilated stoma and/or gastric pouch [7]. Weight regain is the most common indication for revisional surgery [8]. It is estimated that over 250,000 patients will be potential candidates for revisional intervention by 2014 [9].
Traditional surgical intervention to revise the bypass, although effective, is underutilized. The revisional gastric bypass rate (R-RYGB) (open or laparoscopic) is only 3–13%, despite a much higher incidence of weight regain [10]. Intuitively, this can be at least partially attributed to both patient and clinical concern over the higher complication rates associated with revision. Studies have shown complication rates as high as 30–50% for revisional bypass, with mortality rates twice those for the primary procedure [11, 12]. The expense and lack of insurance coverage for revisional bypass is also a hurdle, with the cost in the tens of thousands of US dollars [13]. These realities have helped push the initiative for a safer, incisionless procedural approach for this growing patient population. We report on our prospective single-site series, part of a large multicenter registry, to address stomal and/or pouch dilatation through an endolumenal surgical approach. An incisionless approach to intervention for these patients brings the promise of better intraoperative safety, fewer postoperative complications, and equivalent efficacy.
Materials and methods
Materials
The Incisionless Operating Platform™ (IOP) with the tissue anchoring system (USGI Medical, San Clemente, CA, USA) was utilized for all cases. The IOP consists of the Transport®, a flexible, steerable, multilumen access device for passage of instrumentation (Fig. 1), an endoscopic grasper and tissue approximation device, the g-Prox®, and the g-cath™ tissue anchors (USGI Medical, San Clemente, CA, USA) (Figs. 2, 3). The Transport®, which combines many features of a standard endoscope and a laparoscopic trocar, provides four operating lumens (6, 6, 4, and 4 mm). Lumens were used for insertion of the g-Prox®/g-cath™ for tissue grasping and approximation, an endoscope (Olympus GIF N-180; Olympus America, Inc., Center Valley, PA, USA) for visualization, and various endoscopic graspers and instrumentation as necessary for the procedure. Insufflation was delivered from a standard, high-flow CO2 insufflator connected to a luer lock on the TransPort® handle. Tissue folds were placed circumferentially around the stoma and additionally, as necessary, in the gastric pouch, to reduce the size of the pouch and stoma to approximate the original post-RYGB anatomy.
Methods
This study is an Institutional Review Board (IRB)-approved prospective evaluation of 21 post-Roux-en-Y gastric bypass patients who underwent a restorative, endolumenal procedure at Monmouth Medical Center between February and July 2008. Informed consent was obtained. Demographic information obtained at screening included patient age, gender, pre-bypass weight, post-bypass nadir weight, and amount of weight regained post nadir. All patients underwent a thorough presurgical history and physical examination with documentation of comorbid conditions as well as a nutritional evaluation. Additionally, upper endoscopy was performed to document pouch and stoma dilatation. Pouch length was measured using the markings on a standard gastroscope, with the measurement being made from the most proximal wall of the stoma back to the top of the gastric pouch. Pouch was characterized as bulbous, spherical, or tubular. The longest diameter of the stoma was measured using an endoscopic grasper with a premeasured jaw width of 8 mm.
Inclusion criteria for the study included patient age greater than 18 and less than 65 years old. Patients must have initially achieved ≥50% loss of excess body weight (EBW) after their primary surgery, and their primary surgery must have been completed at least 2 years prior to undergoing the restorative procedure. They had to be at reasonable risk with regard to general anesthesia and be able to provide written informed consent. Patients were excluded from this study if they presented with esophageal stricture or other anatomical abnormality that would preclude the passage and/or use of the endolumenal instruments, if they had significant impairment of mobility that would limit compliance with postoperative exercise regimen, or if the investigator determined another causal factor for weight gain other than pouch or stomal dilatation. Intraoperative data recorded included OR time, pre- and postprocedural stoma and pouch size, number of anchors placed, and any intra- or immediate postoperative complications. The patients were followed post procedure with weight checks, endoscopy, and nutritional evaluations at time points noted in Table 1.
Statistics
All analysis was done by using SAS version 8.2 under independent statistical oversight. Ideal body weight (IBW) was calculated using a body mass index (BMI) of 25 kg/m2 to determine excess body weight (EBW) and %EWL [14] Statistical significance was set at the 95% confidence interval (p < 0.05).
Results
We enrolled a total of 21 patients, of whom 20 successfully had anchors placed during the procedure. Mean patient age was 46 (31–57) years, and 70% were women. Weight regain post RYGB averaged 59 lbs (N = 21). Mean post-RYGB nadir weight was 198 lbs, and mean weight at time of the procedure was 257 lbs. One patient (1/21) had an occult gastro-gastric fistula (missed on screening EGD) which impaired insufflation and visualization and contributed to a small mucosal tear in the distal esophagus. The case was discontinued with no anchors placed, and there were no patient adverse sequelae. Patients tolerated the procedure well and there were no serious intra- or postoperative complications.
Out of an abundance of caution, we opted to have our first four study patients admitted and observed overnight, but quickly transitioned to discharging the remaining 16 patients the same day. No patients required narcotics for pain control and only one complained of a sore throat. Stoma and pouch size were measured intraoperatively before and at the conclusion of the procedure. Procedural results are noted in Table 2. Representative endoscopic photographs of the gastrojejunostomy before and immediately after the anchors were placed are shown in Fig. 4.
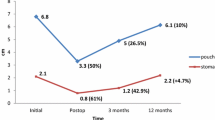
The mean duration of the procedure including pre- and postprocedural endoscopy was 91 min (range 50–130 min), with an average of 5.3 total tissue anchors placed per patient. The mean time of the first ten versus the last ten consecutive procedures decreased from 101 to 82 min, which we felt was due to both the operating teams’ increased proficiency with placement of tissue anchors as well as availability of improved versions of the devices during the second half of the study. Eighteen of 20 patients completed 6-month follow-up. Two patients opted to withdraw from follow-up because of time and travel constraints. Mean 6-month weight loss was 17.3 ± 15 lbs (N = 18), which translated into a mean of 18% EWL. The patients lost a mean of 29% of their weight gain post nadir at 6 months. The majority of subjects (54%) continue to report increased satiety as compared with pre-revision levels at 6 months post procedure. The maximum weight loss for an individual patient in the study was 48 lbs, with a %EWL of 42%, and 75% loss of their regained weight. A subset of patients in whom we achieved a pouch length post procedure of ≤5 cm was significantly correlated with superior weight loss at 6 months, with mean %EWL of 30% (N = 7, p < 0.03). Mean weight loss for this subset was 27.1 ± 15.3 lbs at 6 months, with a loss of 52% of their regained weight (Table 3).
Durability of plications was confirmed at 3 months, with anchors visible in 13/14 (93%) patients who completed their 3-month EGD. The one patient in whom we were unable to visualize the anchor had only one very large stomal plication placed. Of the 12-month EGDs done to date, 100% (4/4) had visible anchors, with average %EWL of 22%. Figure 5 illustrates images from two of the first four 12-month follow-up endoscopies.
Discussion
One of the challenges in offering patients a less invasive alternative to traditional revisional surgery has been our ability to reliably recreate pouch and stoma restriction in a consistent and durable fashion with an endolumenal approach. The gastrointestinal (GI) tract can be unforgiving when foreign material is introduced. Thorough understanding of the biological and mechanical factors involved in the healing of GI tissue is critical to successful wound healing. Dubay et al. in discussing the biology of acute wound failure, noted that successful wound healing occurs when a dynamic balance is met between the loads placed across a provisional matrix, and the feedback and feedforward responses of repair cells [15].
Surgeons are cognizant of the risk of putting too much tension on GI tissue when performing anastomoses. Hypoperfusion at the site ensues, with the resultant ischemia impairing collagen proliferation and eventually precipitating wound dehiscence [16]. Conversely, unlike with cutaneous wound healing, full inclusion of the submucosal layer, along with muscularis and serosal incorporation, is critical to successful healing of gastrointestinal tissue. The bulk of collagen, blood supply, and lymphatics are in the submucosa, providing early crucial tensile strength to the new wound. Without inclusion of tissue deep to the submucosal layer in any tissue fold brought together, the wound is apt to fail because of lack of mechanical tension and of an adequate inflammatory response to trigger early collagen proliferation at the site [17]. The upper GI wound is at greatest risk 2–3 days after suture is placed. This is when shear stress is high, and before collagen proliferation has begun to take place [18].
The expandable tissue anchors are placed under direct visualization and the delivery system provides a tactile, “surgical” feel when plicating tissue. The anchors spread the force load across the wound and can accommodate but not break when under tension. This theoretically should provide an optimal environment for wound healing, collagen deposition, and good scar formation. Preclinical work by Seaman et al. has demonstrated the potential of the anchor “basket” design for reliable and durable plication of gastric tissue [19]. The platform allows for adjustable tensioning and even distribution of forces across a greater surface area, theoretically resulting in better tissue fibrosis and remodeling of the newly placed tissue folds, which we feel should contribute to greater long-term wound durability.
Previous to our case series work as part of this endolumenal registry, Herron et al. [20] reported on encouraging feasibility work with the platform for this incisionless revision, and Bessler et al. presented early clinical work with the platform for revision of pouch and stoma dilatation [21].
Weight regain after bypass surgery has a significant impact on quality of life for these patients [2] in addition to the impact on their overall physical and mental health. Along with reporting on standard weight loss metrics used for primary bariatric procedures (i.e., %EWL), we additionally report a metric that measured success of losing the weight that was regained post bypass (% regained weight loss, or %RWL). We feel that this may be a more appropriate metric for defining success in this revisional bariatric population, since the goal of this surgical intervention is to revise the pouch and stoma to approximate immediate post-bypass anatomy and thereby provide a tool to help patients lose their regained weight.
This study allowed for broad inclusion criteria. Patients were not excluded due to psychosocial issues, amount of excess body weight, or the amount of time passed since their original bypass. Considering the broad spectrum of included patients, we are quite encouraged by both the anchor durability observed at 1 year and the 6-month weight loss results to date. The four patients to date at 12 months who have had follow-up endoscopy have averaged 24% EWL at the 1 year mark.
There were some technical device issues that impacted the level of procedural success early on in the study. Among them, the steering and torque of the TransPort® was suboptimal and the lumens were often too tight to allow instruments to easily pass and maneuver. These issues were addressed by the manufacturer, which resulted in improved device performance at the end of the study and in our subsequent cases. Finally, one of the most significant gratifying endpoints was the safety profile of this revisional approach. There were no significant complications or rehospitalizations in our study patients. After feeling comfortable with the postoperative course of these patients, the last 16 patients were discharged the same day. All patients returned to their activities of daily living upon discharge.
Although based on a small case series, our early data analysis suggests that certain demographic and procedural factors can optimize weight loss success at 6 months. We plan to apply this knowledge to our selection and management of patients to whom we now offer this endolumenal tissue anchoring procedure as a treatment option to approximate tissue to address weight regain post RYGB.
Conclusions
In this case series, this endolumenal procedure was shown to be intraoperatively safe and efficacious for approximating gastric pouch and stoma tissue to reduce stoma and pouch dilatation post RYGB. Patients achieved moderate weight loss at 6 months post procedure. Presence of the tissue anchors was confirmed in early 12-month endoscopic follow-up. Further and longer-term follow-up will be necessary to document durability of the anchors as well as of weight loss.
References
Magro DO, Gelonese B, Delfini R, Pareja BC, Callejas F, Pareja JC (2008) Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg 18(6):648–651 Epub 2008 Apr 8
Christou NV, Look D, MacLean LD (2006) Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg 244(5):734–740
Kruger J, Galuska DA, Serdula MK, Jones DA (2004) Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med 26(5):402–406
Klein S (2003) Medical management of obesity: present and future therapy. Surg Alimentary Tract 7(4):464–467
Campos GM, Rabl C, Mulligan K, Posselt A, Rogers SJ, Westphalen AC, Lin F, Vittinghoff E (2008) Factors associated with weight loss after gastric bypass. Arch Surg 143(9):877–884
Roberts K, Duffy A, Kaufman J, Burrell M, Dziura J, Bell R (2007) Size matters; gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y surgery. Surg Endosc 21:1397–1402
Parikh M, Bessler M (2008) Revision Procedures for Failed Gastric Bypass Bariatric Times e-article September 2007 Surgical Perspective. http://bariatrictimes.com/2007/09/10/revision-procedures-for-failed-gastric-bypass/. Accessed Apr 08
Behrns KE, Smith CD, Kelly KA, Sarr MG (1993) Reoperative bariatric surgery—Lessons learned to improve patient selection and results. Ann Surg 218:646–653
Statistics Related to Overweight and Obesity: The Economic Costs. http://www.win.niddk.nih.gov/statistics/index.htm. Accessed: May 2008
Cohen R, Pinheiro JS, Correa JL, Schiavon C (2005) Laparoscopic revisional bariatric surgery: myths and facts. Surg Endosc 19:822–825
Schwartz RW et al (1988) Gastric bypass revision: lessons learned from 920 cases. Surgery 104:806–812
Nesset EM, Kendrick ML, Houghton SG, Mai JL, Thompson GB, Que FG, Thomsen KM, Larson DR, Sarr MG (2007) A two-decade spectrum of revisional bariatric surgery at a tertiary referral center. Surg Obes Relat Dis 3:25–30
Livingston EH (2005) Hospital costs associated with bariatric procedures in the United States. Am J Surg 190(5):816–820
Dixon JB, McPhail T, O’Brien PE (2005) Minimal reporting requirements for weight loss: current methods not ideal. Obes Surg 15:1034–1039
Dubay DA, Franz MG (2003) Acute wound healing: the biology of acute wound failure. Surg Clin North Am 83(3):463–481
Schroder W, Stippel D, Gutschow C, Leers J, Holscher AH (2004) Post-operative recovery of microcirculation after gastric tube formation. Langenbecks Arch Surg 389:267–271
Thompson SK, Chang EY, Jobe BA (2006) Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery 26:131–136
Thorton FJ, Barbul A (1997) Healing in the gastrointestinal tract. Surg Clin North Am 77:549–573
Seaman DL, Gostout CJ, de la Mora Levy JG, Knipschield MA (2006) Tissue anchors for transmural gut-wall apposition. Gastrointest Endosc 64:577–581
Herron DM, Birkett DH, Thompson CC, Bessler M, Swanstrom LL (2008) Gastric bypass pouch and stoma reduction using a transoral endoscopic anchor placement system: a feasibility study. Surg Endosc 22(4):1093–1099 Epub 2007 Nov
Bessler M, Stevens PD, Milone L, Swanstrom LL (2008) An endosurgical operating system: human experience in endolumenal and NOTES procedures. SAGES 2008 Podium Presentation
Acknowledgement
Disclosures
Frank J. Borao has no conflicts of interest to disclose; the cost of the study was funded by USGI Medical. Steve Gorcey has no conflicts of interest; the cost of the study was funded by USGI Medical. Aaron Capuano has no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borao, F., Gorcey, S. & Capuano, A. Prospective single-site case series utilizing an endolumenal tissue anchoring system for revision of post-RYGB stomal and pouch dilatation. Surg Endosc 24, 2308–2313 (2010). https://doi.org/10.1007/s00464-010-0919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-0919-z