Abstract
Background
Patients undergoing general anesthesia for laparoscopic cholecystectomy are at high risk for postoperative nausea and vomiting (PONV). This study compared ramosetron and ondansetron in terms of efficacy for PONV prevention after laparoscopic cholecystectomy.
Methods
For this study, 120 patients scheduled to undergo laparoscopic cholecystectomy were randomized (in double-blind fashion) to receive 4 mg of ondansetron (group O4, n = 40), 8 mg of ondansetron (group O8, n = 40), or 0.3 mg of ramosetron (group R, n = 40) intravenously after surgery. Postoperative nausea, retching, vomiting, pain, and side effects were assessed at 2 h, 24 h, and 48 h after surgery.
Results
No statistical differences were observed among the three groups with regard to patient characteristics and information on surgery and anesthesia. The ratio of complete response (no PONV for 2 h) was higher for groups O8 and R than for group O4 as follows: 80% (n = 32) for groups O8 and R versus 58% (n = 23) for group O4 during the first postoperative 2 h (p = 0.04), 90% (n = 36) for groups O8 and R versus 76% (n = 30) for group O4 over 24 h (2–24 h) (p = 0.09), and 98% (n = 38) for groups O4 and O8 versus 100% (n = 40) for group R over the next 24 h (24–48 h) after surgery (p = 0.36). During the first 2 h after surgery, rescue antiemetics were used for significantly fewer patients in groups O8 and R (20%) than in group O4 (42.5%) (p = 0.04). Postoperative pain and the use of rescue analgesics were comparable among the groups. There was no clinically serious adverse event due to the study drugs.
Conclusion
Ramosetron 0.3 mg and ondansetron 8 mg are more effective than ondansetron 4 mg for the prevention of PONV (2 h). Ramosetron 0.3 mg is as effective as ondansetron 8 mg for the prophylaxis of PONV after laparoscopic cholecystectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postoperative nausea and vomiting (PONV), one of the most common and distressing adverse events experienced by patients after anesthesia and surgery [1, 2], may prolong recovery, delay patient discharge, and increase hospital costs [1, 2]. Prevention and treatment of PONV help to accelerate postoperative recovery and increase patient satisfaction [3, 4].
Laparoscopic cholecystectomy (LC) is routinely performed for cholelithiasis. When no prophylactic antiemetic is given, PONV occurs frequently after LC with a relatively high incidence (40–75%) [5–7]. Because this undesirable side effect can be a barrier to early recovery and discharge, the prophylactic use of antiemetics may be justified.
Numerous studies have investigated the prevention and treatment of PONV for patients scheduled to undergo LC using a variety of antiemetics including anticholinergics [8, 9], antihistamines [10], phenothiazines [11], butyrophenones [12], and benzamide [6, 13]. However, these agents may cause undesirable adverse effects such as excessive sedation, hypotension, dry mouth, dysphoria, hallucinations, and extrapyramidal signs [14]. Serotonin subtype 3 (5-HT3) antagonists prevent serotonin from binding to 5-HT3 receptors on the ends of the vagus nerve’s afferent branches, which send signals directly to the vomiting center in the medulla oblongata and in the chemoreceptor trigger zone of the brain [15]. By preventing activation of these receptors, 5-HT3 antagonists interrupt one of the pathways leading to vomiting [15]. Findings have demonstrated that several 5-HT3 antagonists (ondansetron, granisetron, tropisetron, dolasetron, and ramosetron) currently available are highly efficacious for PONV. Ondansetron, the most commonly used prophylactic 5-HT3 antagonist, was found to be more effective than traditional antiemetics, including droperidol and metoclopramide, in reducing the incidence of PONV [16–18].
Ramosetron, a new 5-HT3 receptor antagonist, has higher potency and prolonged activity than previously developed 5-HT3 antagonists as an antiemetic after chemotherapy [19, 20] or surgery [21–23]. To the best of our knowledge, no reports have compared the efficacy of ramosetron with that of ondansetron for preventing PONV after LC. We therefore conducted a prospective, randomized, double-blind study to compare the efficacy and tolerability of ramosetron and ondansetron for the prophylaxis of PONV in patients undergoing LC.
Materials and methods
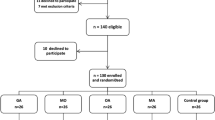
After institutional review board approval and informed consent from each patient had been obtained, this study enrolled 120 American Society of Anesthesiology (ASA 1 or 2) patients (ages 25 to 65 years) scheduled for elective LC under general anesthesia. The patients who had gastrointestinal disease, those pregnant or menstruating, those with a history of motion sickness or postoperative emesis, and those who had taken antiemetics within 24 h before surgery were excluded from the study.
In a randomized double-blind manner, the patients received a single dose of ondansetron 4 mg (group O4), ondansetron 8 mg (group O8), or ramosetron 0.3 mg (group R) intravenously at the end of surgery. Randomization was conducted using the sealed envelope method. Study medications were prepared by a blinded nurse in identical 5-ml syringes and administered according to the list.
No patient received preanesthestic medication. At arrival in the operating room, all patients were given standard anesthesia. Patients were monitored during anesthesia by continuous electrocardiogram, noninvasive blood pressure, pulse oximetry, and capnography.
Anesthesia was induced with 2 mg/kg of propofol and 4 ng/ml of remifentanil in a target-controlled infusion. Before endotracheal intubation, 0.6 mg/kg of rocuronium was administered. Anesthesia was maintained with desflurane 3% to 6% (inspired concentration), medical air in oxygen (fraction of inspired oxygen [FiO2] = 0.5), and intravenous (IV) remifentanil 2 to 3 ng/ml. Ventilation was mechanically controlled and adjusted to maintain an end-tidal concentration of carbon dioxide at 35 to 40 mmHg throughout the surgery using an anesthetic/respiratory analyzer (Ultima; Datex, Helsinki, Finland). Muscle relaxation was achieved with rocuronium, as required.
The intravenous fluid used during surgery was Ringer’s lactate infused at 15 to 20 ml/kg/h. The nasopharyngeal temperature was monitored and maintained at 37 ± 1°C throughout surgery using a warming pad. A nasogastric tube was placed to promote emptying of gastric contents, which were suctioned and removed before endotracheal extubation.
At the end of the procedure, desflurane and remifentanil administration were stopped. Antagonism of muscle relaxation was achieved with 0.02 mg/kg of atropine and 0.04 mg/kg of neostigmine administered intravenously, and then the trachea was extubated when spontaneous ventilation of the patient was adequate.
After the operation, the patients were transferred to the postanesthesia recovery room. All the patients were observed for 48 h postoperatively. The incidence of nausea and vomiting was recorded during three assessment periods (0–2 h, 2–24 h, and 24–48 h) by a nursing staff blinded to the treatment drug. Nausea was defined as a subjectively unpleasant sensation associated with awareness of the urge to vomit, whereas retching was defined as the labored spasmodic, rhythmic contraction of the respiratory muscles without expulsion of gastric contents, and vomiting was defined as the forceful expulsion of gastric contents from the mouth [1]. A complete response was defined as the absence of PONV.
The rescue antiemetic used was metoclopramide 10 mg IV for patients who experienced two or more episodes of vomiting. The severity of nausea was recorded using a verbal rating scale (VRS) with choice options ranging from 0 (none) to 100 (most severe).
In the same study period, patients were asked to evaluate their level of pain during the procedure using the VRS. An IV bolus dose of analgesics (ketorolac 30 mg) was administered if the VRS exceeded 30. Other postoperative adverse effects, such as headache and dizziness, also were recorded.
Calculations based on previous studies [23, 24] showed that 40 patients per group would be required by power analysis (for a power of 80% and a type 1 error of 5%) using sample-size software (PASS 2005; NCSS, Kaysville, UT, USA) to demonstrate a 20% difference in values for a complete response 24 h after surgery. Data were analyzed using analysis of variance (ANOVA) (patient demographics) or chi-square test (incidence variables), and post hoc comparisons were made with Bonferroni’s correction. Values are expressed as counts or as means ± standard deviation. A p value less than 0.05 was considered statistically significant.
Results
One of the 121 patients enrolled the study was withdrawn because the patient underwent conversion to open cholecystectomy. Consequently, 120 patients (59 men and 61 women) were included in the study. The three groups were comparable in terms of patient characteristics, operation time, anesthesia time, and administered anesthetics (Table 1).
The ratio of complete response, defined as no PONV 2 h after surgery, was higher in groups O8 and R than in group O4 (Table 2), 80% (n = 32) in group O8 and R versus 58% (n = 23) in group O4 during the first 2 h after surgery (p = 0.04), 90% (n = 36) in groups O8 and R versus 76% (n = 30) in group O4 during 24 h after surgery (2–24 h) (p = 0.09), and 98% (n = 38) in groups O4 and O8 versus 100% (n = 40) in group R during the next 24 h (24–48 h) after the operation (p = 0.36).
No statistical difference was observed between postoperative pain evaluated using the VRS and the extent of rescue analgesics use (Table 3). The incidences of the most common adverse events, such as headache and dizziness, were similar among the three groups (Table 3), and no clinically important treatment-emergent adverse events were found.
Discussion
Our study showed that ondansetron 8 mg and ramosetron 0.3 mg were more effective than ondansetron 4 mg in preventing PONV during 2 h after LC and that there was no difference in the incidence of complete response between the ramosetron 0.3 mg and ondansetron 8 mg groups. The incidences of common adverse events, such as headache and dizziness, also were similar among the three groups. These results suggest that ramosetron 0.3 mg is comparable with ondansetron 8 mg in terms of the antiemetic effect and side effects.
This study differs from previous investigations in that an ondansetron 8 mg group was added and compared with the ramosetron 0.3 mg group. In this study, the ratio for a complete response of ondansetron 4 mg during 2 h after LC was 58%, and it was increased by up to 80% with ondansetron 8 mg in this study.
In previous studies, some investigators chose ondansetron 8 mg rather than ondansetron 4 mg for the prophylaxis of PONV [24–26]. In addition, Paventi et al. [27] compared the efficacy of ondansetron 4 mg with that of ondansetron 8 mg for the prevention PONV after laparoscopic cholecystectomy, concluding that single-dose ondansetron 8 mg was more effective than ondansetron 4 mg in the prevention of PONV. On the other hand, ramosetron 0.6 mg did not provide further benefit compared with ramosetron 0.3 mg with regard to the incidence of complete response after different types of surgeries including thyroidectomy [22], laparoscopic cholecystectomy [23], and gynecologic surgery [28, 29].
The etiology of PONV is not entirely known. It probably is multifactorial, with risk factors including age, sex, obesity, history of motion sickness, previous PONV, operative procedure, anesthetic technique, and postoperative pain [1]. Published evidences suggest that appropriate antiemetic treatment is recommended for patients with more than two risk factors [2]. In this study, these factors were well balanced between the treatment groups. Besides suspected risk factors, patient-controlled anesthesia (PCA) with opioids is associated with a high risk of PONV, potentially exceeding 30% [30].
There is a possibility that combined antiemetics with different sites of activity would be more effective than one drug alone for prophylaxis against PONV in high-risk groups. Combination antiemetic therapy with serotonin antagonists (granisetron, ondansetron, tropisetron) plus dexamethasone, metoclopramide, or droperidol is known to be highly effective in preventing PONV [2, 13, 31–34]. We did not use this combination therapy in the current study because there was no need to apply PCA, including opioids. The result of this investigation showed that postoperative pain was not severe and that consumption of rescue analgesic was reduced 2 h after surgery. Therefore, simple prophylaxis with a single drug was sufficient to prevent PONV after LC. Therefore, the use of PCA and combination antiemetic therapy seemed not to be cost effective for this patient population.
The reported incidence of PONV is 40% to 75% within the first 24 h after LC when no prophylactic antiemetic is given [5–7]. In addition to the general risk factors of PONV, the central action of carbon dioxide, stretching of the peritoneum, and increased blood pressure in the peritoneal cavity after gas insufflation during laparoscopic surgery all have been considered to provoke nausea and vomiting [13] by reducing intestinal blood flow [35] and releasing emetogenic substances, including serotonin [36].
A placebo group was not included in this study for ethical reasons (i.e., so that all patients were relieved of the distressing PONV experience). In addition, the prophylactic effect of ondansetron and ramosetron on PONV already had been established in a number of previous studies [10, 13, 17, 18, 23, 24, 37, 38].
A variety of pharmacologic approaches (antihistamines, butyrophenones, dopamine receptor antagonists) have been investigated for the prevention and treatment of PONV. However, use of the traditional antiemetics such as droperidol and metoclopramide has been limited due to undesirable adverse effects including excessive sedation, hypotension, dry mouth, dysphoria, hallucinations, and extrapyramidal symptoms [1]. Considering the etiopathogenetic mechanism of PONV after LC, 5- HT3 antagonists may be more effective than other antiemetics in preventing and treating PONV without these adverse effects. The most common adverse events caused by 5-HT3 antagonists are headache and dizziness. Ramosetron was associated with less prevalence of side effects than ondansetron when used as an antiemetic for fentanyl-based intravenous PCA after spine surgery [39]. The current study found no difference in the incidence of these side effects among the groups, and there were no clinically important adverse events.
During the maintenance of anesthesia, desflurane, remifentanil, and medical air in oxygen were administered continuously. In the current study, all the patients received general anesthesia without nitrous oxide because of concerns regarding its ability to diffuse into the bowel lumen, causing distention to impair surgical access. Moreover, the use of nitrous oxide also has been suspected to increase the incidence PONV, although this issue is still debated [40, 41]. Instead of nitrous oxide, remifentanil was used during the surgery in the current study. Remifentanil is a synthetic μ-opioid agonist introduced recently into clinical practice [42]. Remifentanil achieves the desired analgesic effect rapidly and reduces respiratory depression postoperatively because of its short context-sensitive half-time regardless of infusion duration [43, 44]. In a previous study, the adjunct use of remifentanil infusion during desflurane anesthesia facilitated early recovery without increasing PONV, pain, or the need for rescue medication after laparoscopic surgery [45].
In conclusion, ramosetron 0.3 mg was more effective than ondansetron 4 mg and as effective as ondansetron 8 mg for the prophylaxis of PONV in patients undergoing laparoscopic cholecystectomy.
References
Watcha MF, White PF (1992) Postoperative nausea and vomiting: its etiology, treatment, and prevention. Anesthesiology 77:161–184
Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, Kovac A, Philip BK, Sessler DI, Temo J, Tramèr MR, Watcha M (2003) Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 97:62–71
Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG (2003) Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 98:46–52
Tramer MR (2004) Strategies for postoperative nausea and vomiting. Best Pract Res Clin Anaesthesiol 18:693–701
Fujii Y (2005) The utility of antiemetics in the prevention and treatment of postoperative nausea and vomiting in patients scheduled for laparoscopic cholecystectomy. Curr Pharmaceut Des 11:3173–3183
Nesek-Adam V, Grizelj-Stojcic E, Rasic Z, Cala Z, Mrsic V, Smiljanic A (2007) Comparison of dexamethasone, metoclopramide, and their combination in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc 21:607–612
Santon JM (1991) Anaesthesia for laparoscopic cholecystectomy. Anaesthesia 46:317
Thune A, Appelgren L, Haglind E (1995) Prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy: a prospective randomized study of metoclopramide and transdermal hyoscine. Eur J Surg 161:265–268
Sohi HS, Heipel J, Inman KJ, Chinnick B, Cunningham DG, Holliday RL, Girotti MJ (1994) Preoperative transdermal scopolamine does not reduce the level of nausea and frequency of vomiting after laparoscopic cholecystectomy. Can J Surg 37:307–312
Kothari SN, Boyd WC, Bottcher ML, Lambert PJ (2000) Antiemetic efficacy of prophylactic dimenhydrinate (Dramamine) vs ondansetron (Zofran): a randomized, prospective trial of inpatients undergoing laparoscopic cholecystectomy. Surg Endosc 14:926–929
Parlow JL, Meikle AT, van Vlymen J, Avery N (1999) Postdischarge nausea and vomiting after ambulatory laparoscopy is not reduced by promethazine prophylaxis. Can J Anaesth 46:719–724
Fujii Y, Tanaka H, Toyooka H (1999) Prophylactic antiemetic therapy with droperidol in patients undergoing laparoscopic cholecystectomy. J Anesth 13:140–143
Leksowski K, Peryga P, Szyca R (2006) Ondansetron, metoclopramid, dexamethasone, and their combinations compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a prospective randomized study. Surg Endosc 20:878–882
Morgan GE, Mikhail MS, Murray MJ (2002) Adjuncts to anesthesia. In: Morgan GE, Mikhail MS, Murray MJ (eds) Clinical anesthesiology. McGraw-Hill, New York, pp 242–252
Hesketh PJ, Gandara DR (1991) Serotonin antagonists: a new class of antiemetic agents. J Natl Cancer Inst 83:613–620
Tramer MR, Moore RA, Reynolds DJ, McQuay HJ (1997) A quantitative systematic review of ondansetron in the treatment established postoperative nausea and vomiting. Anesthesiology 314:1088–1089
Helmy SA (1999) Prophylactic antiemetic efficacy of ondansetron in laparoscopic cholecystectomy under total intravenous anaesthesia: a randomised, double-blind comparison with droperidol, metoclopramide and placebo. Anaesthesia 54:266–270
Naguib M, el Bakry AK, Khoshim MH, Channa AB, el Gammal M, el Gammal K, Elhattab YS, Attia M, Saddique A (1996) Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron, and metoclopramide in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind comparison with placebo. Can J Anaesth 43:226–231
Shi Y, He X, Yang S, Ai B, Zhang C, Huang D, Dong M, Liu P, Zhou S, Han X (2007) Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: a prospective randomized controlled study. Chemotherapy 53:44–50
Kang YK, Park YH, Ryoo BY, Bang YJ, Cho KS, Shin DB, Kim HC, Lee KH, Park YS, Lee KS, Heo DS, Kim SY, Cho EK, Lim HY, Kim WK, Lee JA, Kim TY, Lee JC, Yoon HJ, Kim NK (2002) Ramosetron for the prevention of cisplatin-induced acute emesis: a prospective randomized comparison with granisetron. J Int Med Res 30:220–229
Fujii Y, Tanaka H (2002) Comparison of granisetron and ramosetron for the prevention of nausea and vomiting after thyroidectomy. Clin Ther 24:766–772
Fujii Y, Tanaka H (2002) Double-blind, placebo-controlled, dose-ranging study of ramosetron for the prevention of nausea and vomiting after thyroidectomy. Clin Ther 24:1148–1153
Fujii Y, Uemura A, Tanaka H (2002) Prophylaxis of nausea and vomiting after laparoscopic cholecystectomy with ramosetron: randomised controlled trial. Eur J Surg 168:583–586
Quaynor H, Raeder JC (2002) Incidence and severity of postoperative nausea and vomiting are similar after metoclopramide 20 mg and ondansetron 8 mg given by the end of laparoscopic cholecystectomies. Acta Anaesthesiol Scand 46:109–113
Grond S, Lynch J, Diefenbach C, Altrock K, Lehmann KA (1995) Comparison of ondansetron and droperidol in the prevention of nausea and vomiting after inpatient minor gynecologic surgery. Anesth Analg 81:603–607
Honkavaara P (1996) Effect of ondansetron on nausea and vomiting after middle ear surgery during general anaesthesia. Br J Anaesth 76:316–318
Paventi S, Santevecchi A, Ranieri R (2001) Efficacy of a single-dose ondansetron for preventing postoperative nausea and vomiting after laparoscopic cholecystectomy with sevoflurane and remifentanil infusion anaesthesia. Eur Rev Med Pharmacol Sci 5:59–63
Fujii Y, Tanaka H, Somekawa Y (2004) A randomized, double-blind, placebo-controlled trial of ramosetron for preventing nausea and vomiting during termination of pregnancy. Int J Obstet Anesth 13:15–18
Fujii Y, Saitoh Y, Tanaka H, Toyooka H (2000) Ramosetron for preventing postoperative nausea and vomiting in women undergoing gynecological surgery. Anesth Analg 90:472–475
Gepstein R, Arinzon Z, Folman Y, Shuval I, Shabat S (2007) Efficacy and complications of patient-controlled analgesia treatment after spinal surgery. Surg Neurol 67:360–366
Papadimitriou L, Livanios S, Katsaros G, Hassiakos D, Koussi T, Demesticha T (2001) Prevention of postoperative nausea and vomiting after laparoscopic gynaecological surgery: combined antiemetic treatment with tropisetron and metoclopramide vs metoclopramide alone. Eur J Anaesthesiol 18:615–619
Sanchez-Ledesma MJ, Lopez-Olaondo L, Pueyo FJ, Carrascosa F, Ortega A (2002) A comparison of three antiemetic combinations for the prevention of postoperative nausea and vomiting. Anesth Analg 95:1590–1595
Biswas BN, Rudra A (2003) Comparison of granisetron and granisetron plus dexamethasone for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Acta Anaesthesiol Scand 47:79–83
Coloma M, White PF, Markowitz SD, Whitten CW, Macaluso AR, Berrisford SB, Thornton KC (2002) Dexamethasone in combination with dolasetron for prophylaxis in the ambulatory setting: effect on outcome after laparoscopic cholecystectomy. Anesthesiology 96:1346–5130
Caldwell CB, Ricotta JJ (1987) Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res 43:14–20
Diebel LN, Dulchavsky SA, Wilson RF (1992) Effect of increased intraabdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma 33:45–48
Goll V, Akça O, Greif R, Freitag H, Arkiliç CF, Scheck T, Zoeggeler A, Kurz A, Krieger G, Lenhardt R, Sessler DI (2001) Ondansetron is no more effective than supplemental intraoperative oxygen for prevention of postoperative nausea and vomiting. Anesth Analg 92:112–117
Fujii Y, Saitoh Y, Tanaka H, Toyooka H (1999) Ramosetron vs granisetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Can J Anaesth 46:991–993
Choi YS, Shim JK, Yoon do H, Jeon DH, Lee JY, Kwak YL (2008) Effect of ramosetron on patient-controlled analgesia-related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine 33:E602–E606
Hartung J (1996) Twenty-four of twenty-seven studies show a greater incidence of emesis with nitrous oxide than with alternative anesthetics. Anesth Analg 83:114–116
Tramer M, Moore A, McQuay H (1996) Omitting nitrous oxide in general anesthesia: meta-analysis of intraoperative, awareness, and postoperative emesis in randomized controlled trials. Br J Anaesth 76:186–193
Rosow CE (1999) An overview of remifentanil. Anesth Analg 89:S1–S3
Glass PS, Gan TJ, Howell S (1999) A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 89:S7–S14
Kapila A, Glass PS, Jacobs JR, Muir KT, Hermann DJ, Shiraishi M, Howell S, Smith RL (1995) Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology 83:968–975
Song D, White PF (1999) Remifentanil as an adjuvant during desflurane anesthesia facilitates early recovery after ambulatory surgery. J Clin Anesth 11:364–367
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, J., So, YM., Hwang, J. et al. Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc 24, 812–817 (2010). https://doi.org/10.1007/s00464-009-0670-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-009-0670-5