Abstract
Background
Tension, ischemia, and technical error are factors leading to anastomotic complications such as leak, stricture, and ulceration with bleeding. Currently, surgeons evaluate tissue ischemia without any simple routine measurement technique. A new tissue surface probe, T-Stat 303, provides continuous measurement of tissue hemoglobin oxygen saturation (StO2) and may have clinical utility for intraoperative assessment of blood flow in areas of surgical anastomosis. This pilot study aimed to determine local StO2 during gut stapling using various staple sizes for the purpose of assessing the tool’s ability to measure changes and the reproducibility of those changes with stapling.
Methods
Measurements were made in nine anesthetized adult swine during laparotomy. Various staple heights were used to transect small bowel and colon. Serosal and mucosal surface measurements were obtained at baseline and on each side of the transection using the T-Stat device adjacent to the staple line and 2 cm away from it.
Results
Both small bowel and colon mucosal StO2 adjacent to the staple line showed significant ischemia compared with baseline (p < 0.001) and 2 cm away from the staple line (p < 0.001) using all staple heights. The serosa of both small bowel and colon adjacent to the staple line was not significantly different from baseline serosa (p > 0.11) except when the grey stapler was used (baseline, 58 ± 6.6 vs. staple line, 51 ± 15.1; p = 0.022). The baseline mucosa of the small bowel and colon did not differ from mucosa 2 cm away from the staple line (p > 0.08). The small bowel serosa 2 cm away from the staple line did not differ from baseline mucosa, whereas the colon serosa 2 cm away significantly increased after stapling compared with baseline mucosa (p < 0.012). No statistically significant StO2 difference was found between the various staple load sizes.
Conclusion
Mucosal ischemia occurs after gastrointestinal stapling and is not affected by various staple heights. The T-Stat probe provides a real-time method for assessment of gut ischemia by surgeons during surgical procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Avoidance of significant perioperative morbidity and mortality associated with intestinal surgery is dependent on well-formed, functioning, enteric anastomoses. Tension, tissue ischemia, and technical error are known factors that can lead to anastomotic complications such as leak, stricture, and ulceration with bleeding [1, 2]. Tension and technique are factors that can be visibly detected and corrected and that can improve with surgeon experience.
Currently, surgeons evaluate tissue ischemia by subjective observation without any simple routine measurement technique. A new tissue surface probe, T-Stat 303 Microvascular Tissue Oximeter (Spectros Corporation, Portola Valley, CA, USA) provides continuous measurement of tissue hemoglobin oxygen saturation (StO2) and may have clinical utility for intraoperative assessment of blood flow in areas of surgical anastomosis.
This pilot study aimed to determine local tissue StO2 during gut stapling using various staple sizes for the purpose of assessing the tool’s ability to measure changes and the reproducibility of those changes with stapling. We sought to evaluate mucosal versus serosal measurements and to determine whether proximity to the staple line correlated with possible tissue ischemia.
Materials and methods
The T-Stat 303 Microvascular Tissue Oximeter was used to detect mucosal and serosal StO2 in swine under institutional animal care committee approval.
Before use, the T-Stat device is calibrated by exposing the probe to requested colors for the purpose of standardizing its readings. Functionally, the oximeter emits low-power white light from a handheld flexible or endoscopic probe placed on or near tissue. The light penetrates approximately 2 mm and diffuses into the surrounding tissues. It reemerges colored according to the oxygenation level of the hemoglobin in capillaries. Measurements are continuous, typically requiring 5 to 50 ms depending on the intensity of the reflected light. The device shows the StO2 as a percentage [3].
After induction of anesthesia using a combination of intramuscular ketamine, acepromazine, and atropine, nine 25- to 35-kg adult swine were intubated and ventilated using isoflurane and oxygen. An intravenous line was placed in an ear vein for medication and fluid administration. End-tidal carbon dioxide (EtCO2), arterial oxygen saturation (SpO2), heart rate, and body temperature were intermittently monitored and maintained throughout the procedures.
After iodine prep, an abdominal incision from the sternum to the lower abdomen was made using a scalpel and electrocautery. After brief exploration, small bowel was defined as approximately 10 cm from the ligament of Trietz, from which a baseline serosal StO2 was obtained. A 1-cm enterotomy was performed approximately 10 cm proximal to the intended staple site using electrocautery on the antimesenteric border, allowing for passage of the T-Stat probe and measurement of a baseline mucosal StO2 to be recorded.
An Endo-GIA stapler (Covidien, Norwalk, CT, USA) with either a grey (2 mm) or green (4.8 mm) staple load was used to transect the small bowel. Immediately after transection, serosal and mucosal surface measurements were obtained proximal to the transection using the T-Stat device adjacent to the staple line and 2 cm away from it. After a second enterotomy, the same measurements were obtained approximately 10 cm distal to the transection site in the same manner described previously.
This protocol was repeated distally in both the ileum in the colon using a different staple height (Fig. 1). White (2.5 mm) and green (4.8 mm) staple loads were used in the colon. These staple loads were used to compare small and large staple heights.
Statistical analysis
Continuous variables were compared using the Student’s t-test. A p value less than 0.05 was considered statistically significant. All data analyses were performed using Intercooled STATA Version 9.0 (StataCorp, College Station, TX, USA).
Results
Baselines
Baseline StO2 measurements (n = 36) taken on the small bowel and colon mucosa and serosa were planned for gastrointestinal transection. The baseline mucosal StO2 in the jejunum and ileum were not significantly different (48.5 ± 7.0 vs. 47.5 ± 8.0; p > 0.05). The baseline serosal StO2 in the jejunum and ileum also were not significantly different (59 ± 6.5 vs. 58.5 ± 7.5; p > 0.05). These findings suggest that blood flows to the mucosa and serosa throughout the small bowel are similar. Therefore, all small bowel measurements can be combined.
The same was true for the colon (n = 36). The baseline mucosal StO2 values in the right and left colon were not significantly different (47 ± 12.0 vs. 41.5 ± 13.5; p > 0.05). The baseline serosal StO2 values in the right and left colon also were not significantly different (63 ± 5.0 vs. 61.5 ± 6.5; p > 0.05). These findings suggest that blood flows to the mucosa and serosa throughout the colon are similar. Therefore, all colon measurements can be combined.
The baseline mucosal StO2 throughout the small bowel and colon was significantly less than the baseline serosal StO2 (n = 72). The small bowel mucosal baseline StO2 was significantly less than the small bowel serosal baseline (48.0 ± 7.6 vs. 58.5 ± 6.5; p < 0.001). The colon mucosal baseline StO2 was significantly less than the colon serosal baseline (44.0 ± 12.7 vs. 62.5 ± 6.0; p < 0.001). The results suggest greater StO2 in the serosa than in mucosa for both the small and large bowel.
Effects of stapling
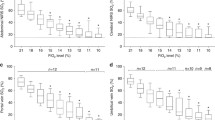
The mean StO2 and standard deviations for the small bowel and colon mucosa and serosa using different staple sizes are presented in Table 1. A graphic representation of the numeric data is seen in Figs. 2, 3, 4, 5.
Serosa
Stapling caused a decrease in StO2 adjacent to the staple line in small bowel serosa despite staple height (Fig. 2). After stapling, StO2 adjacent to the staple line significantly decreased compared with StO2 2 cm away from it. These findings were similar for both staple heights. The grey staple load caused a significant decrease in StO2 adjacent to the staple line compared with baseline StO2 and StO2 2 cm away from the staple line. In spite of this finding, the changes in StO2 that occurred due to the different staple heights were not significantly different.
The colon serosa responded in a similar fashion (Fig. 3). The colon serosal StO2 2 cm away from the staple line increased significantly compared with baseline StO2 in response to stapling. In addition, the StO2 adjacent to the staple line decreased significantly compared with StO2 2 cm away from it. Comparison of different staple heights showed that these findings and the changes in StO2 as a result of stapling were not different.
Mucosa
The mucosal StO2 adjacent to the staple line significantly decreased compared with baseline StO2 and StO2 2 cm away from it for both the small bowel and the colon using various staple heights. After stapling, the small bowel mucosal StO2 2 cm away from the staple line did not differ from baseline StO2, whereas the StO2 adjacent to the staple line significantly decreased compared with both baseline StO2 and StO2 2 cm away from the staple line (Fig. 4). These results and the changes in StO2 were not significantly different with the use of different staple heights.
The colon responded to stapling in the same manner as the small bowel (Fig. 5). The colon mucosal StO2 2 cm away from the staple line did not differ from baseline StO2 after stapling. The StO2 adjacent to the staple line significantly decreased compared with both baseline StO2 and StO2 2 cm away from the staple line. Again, these findings and the changes in StO2 were not significantly different with the use of different staple heights.
Discussion
T-Stat 303
The T-Stat 303 Microvascular Tissue Oximeter provided reproducible, continuous, real-time tissue hemoglobin oxygen saturation measurements throughout the small bowel and colon at baseline and after bowel transection using various staple loads. This noninvasive device evaluates StO2 levels, which have been found to correspond to arterial blood oxygen saturation (SaO2), and more strongly to mixed venous oxygen saturation (SvO2). It is suggested that a decrease in blood flow or capillary perfusion, as in shock or hypovolemia, causes a decrease in SvO2 and StO2 [4, 5]. Because of these findings, we used the T-Stat device to measure StO2 changes in response to gut stapling.
A few studies have already documented the T-Stat’s success in measuring StO2 in the gastrointestinal tract. Using the probe endoscopically, small bowel and colon mucosa can be assessed for chronic mesenteric ischemia. One study evaluated three patients with stomach and small bowel mesenteric ischemia endoscopically before and after vascular stenting. The results suggested that the area of ischemia in the small bowel had significantly lower StO2 (16–45% StO2) than found in patients without ischemia (66% ± 5% StO2). The stomach measurements were less (55–57% StO2) but not significantly different than those in the normal group (67–75% StO2). Additionally, after celiac or superior mesenteric artery stenting, a significant StO2 increase in the small bowel occurred with resolution of symptoms [6].
Another study evaluated the T-Stat’s ability to take rectal mucosal and serosal StO2 measurements and detect changes in the rectal serosa after colorectal anastomoses. This study evaluated 11 rectal anastomoses. Standard deviations for mucosal measurements were high (±4–26), whereas those for serosal measurements were much lower (±2–13). A 9% decrease in StO2 was observed in the rectal stump serosa after ligation of the mesenteric arteries [7].
These studies along with the current study suggest that the T-Stat 303 Microvascular Tissue Oximeter can provide reproducible tissue hemoglobin oxygen saturation measurements in the normal gastrointestinal tract and also can exhibit the changes that occur in ischemic or stapled mucosa and serosa.
Although T-Stat is easy to use and fast, some concerns did arise with our experience that may question the reproducibility of the results. The presence of stool, bile, blood, or food would interfere with the passage of light. The angle of the probe relative to the tissue surface should be 90º. Suboptimal angles lead to inaccurate measurements. If the T-Stat 303 is used endoscopically, the scope light may interfere with the light passed from the probe. One particular concern was probe opposition to tissue, resulting in misleading decreased perfusion and StO2 [8]. Anything causing tissue compression, such as pressure from an instrument or distention from a leak test, may lead to inaccurate results as well.
The T-Stat device and its nonreusable probes are expensive: $29,600 for the T-Stat 303 Microvascular Tissue Oximeter, $350 for the T-Stat 5-mm probe (used in our experiment), and $120 for the T-Stat endoscopic probes (Spectros Corporation, April 2008).
Baselines
Despite the different vascular arcades that perfuse the bowel with blood and oxygen, the results suggest no differences among StO2 values for mucosa and serosa between different locations within the small bowel (jejunum versus ileum) or colon (right versus left). Additionally, the serosa StO2 throughout both the small bowel and colon was significantly greater than the mucosa StO2. This is in opposition to the classic teaching that the mucosa demonstrates higher oxygen delivery because of its close proximity to the highly vascularized submucosa but consistent with past studies comparing mucosal and serosal StO2 using spectrophotometry [9, 10].
Effects of stapling
The goal of enteral stapling requires appropriate apposition of tissue to create an anastomosis without compromising the blood supply leading to ischemia. As a result, vendors have manufactured various staple configurations to accommodate different tissue thicknesses. In our study, we used extreme staple sizes (small versus large) to transect both small bowel and colon to evaluate mucosal and serosal changes in StO2.
Both serosa and mucosa adjacent to staple line perfusion decreased in response to stapling as expected. The mucosal changes were more significant. Stapling also caused an increase in perfusion to the colon serosa 2 cm away from the staple line. Mechanical tissue compression by the staple led to these changes. Although significant changes in perfusion occurred, these changes were similar among the various staple heights. The belief that a significant decrease in perfusion occurs due to an inadequate staple height leading to ischemia and leak is questioned.
Physiologically, tissues respond to stimuli such as trauma or surgery over time. The immediate response may be different from that which is delayed or occurs over days to months. The StO2 measurements in this study were obtained immediately after stapling, thus reflecting the immediate response of tissues to staple compression. Additional studies are needed to evaluate StO2 changes over time. Using the T-Stat device to measure these changes will help to define the complete physiologic response to stapling and teach clinicians to recognize those that deviate from the norm.
In the future, the T-Stat device may have clinical utility in determining the risk of ischemia leading to anastomotic breakdown. To date, a specific level of StO2 that leads to tissue ischemia has not been defined. When this StO2 level is defined, the T-Stat 303 may be used via open, laparoscopic, and endoscopic approaches to stratify the risk of ischemia to an anastomosis or surrounding tissues during surgical procedures. If a significant StO2 change is determined, the surgeon will be able to reassess and act appropriately to avoid complications. Because of the complex physiologic response to stapling, additional studies will be necessary to determine this level of StO2 that corresponds to ischemia before the routine clinical use of T-Stat 303.
Conclusions
Mucosal and serosal baseline StO2 is similar throughout the small bowel and colon. Gastrointestinal stapling results in significant mucosal ischemia and is not affected by various staple heights. The T-Stat probe may provide a fast, continuous, real-time method for surgeons to use in assessing gut ischemia during surgical procedures, but additional studies are needed before its routine clinical use.
References
Almahmeed T, Gonzalez R, Nelson L, Haines K, Gallagher S, Murr M (2007) Morbidity of anastomotic leaks in patients undergoing Roux-en-Y gastric bypass. Arch Surg 142:954–957
Fernandez A, DeMaria E, Tichansky D, Kellum J, Wolfe L, Meador J, Sugerman H (2004) Experience with over 3,000 open and laparoscopic bariatric procedures. Surg Endosc 18:193–197
Benaron D, Parachikov I, Friedland S, Soetikno R, Brock-Utne J, van der Starre P, Nezhat C, Terris M, Maxim P, Carson J, Razavi M, Gladstone H, Fincher E, Hsu C, Clark L, Cheong W, Duckworth J, Stevenson D (2004) Continuous, noninvasive, and localized microvascular tissue oximetry using visible light spectroscopy. Anesthesiology 100:1469–1475
Nagashima Y, Yada Y, Hattori M, Sakai A (2000) Development of a new instrument to measure saturation and total hemoglobin volume in local skin by near-infrared spectroscopy and its clinical application. Int J Biometeorol 44:11–19
Podbregar M, Mozina H (2007) Skeletal muscle oxygen saturation does not estimate mixed venous saturation in patients with severe left heart failure and additional severe sepsis or septic shock. Crit Care 11:R6
Friedland S, Benaron D, Coogan S, Sze D, Soetikno R (2007) Diagnosis of chronic mesenteric ischemia by visible light spectroscopy during endoscopy. Gastrointest Endosc 65:294–300
Karliczek A, Benaron D, Baas P, van der Stoel A, Wiggers T, van Dam G (2007) Reflectance spectrometry as intraoperative assessment of perfusion in rectal anastomosis, a feasibility study (abstract). Eur Surg Res 39(Suppl 1):O.025
Friedland S, Soetikno R, Benaron D (2004) Reflectance spectrophotometry for the assessment of mucosal perfusion in the gastrointestinal tract. Gastrointest Endosc Clin North Am 14:539–553
Haisjackl M, Luz G, Sparr H, Germann R, Salak N, Friesenecker B, Deusch E, Meusburger S, Hasibeder W (1997) The effects of progressive anemia on jejunal mucosal and serosal tissue oxygenation in pigs. Anesth Analg 84:538–544
Germann R, Haisjackl M, Hasibeder W, Sparr H, Luz G, Plattner R, Pernthaler H, Friesenecker B, Falk M (1994) Dopamine and mucosal oxygenation in the porcine jejunum. J Appl Physiol 77:2845–2852
Acknowledgments
The authors offer special thanks to Mr. Mike Lowe, Ms. Ianthia Parker, and Mr. George Quick, Laboratory Technicians at the Duke University Medical Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myers, C., Mutafyan, G., Petersen, R. et al. Real-time probe measurement of tissue oxygenation during gastrointestinal stapling: mucosal ischemia occurs and is not influenced by staple height. Surg Endosc 23, 2345–2350 (2009). https://doi.org/10.1007/s00464-009-0342-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-009-0342-5