Abstract
Background
Laparoscopy is slowly becoming an established technique for liver resection. This procedure still is limited to centers with experience in both hepatic and laparoscopic surgery. Preliminary reports include mainly minor resections for benign liver conditions and show some advantage in terms of postoperative recovery. The authors report their experience with laparoscopic liver resection, the evolution of the technique, and the results.
Methods
From 1999 to 2006, 70 laparoscopic liver resections were performed using a procedure similar to resection by laparotomy.
Results
There were 38 malignant tumors (54%) and 32 benign lesions (46%). The malignant tumors were mainly hepatocellular carcinomas (19 of 24 patients had cirrhosis). The tumor mean size was 3.8 ± 1.9 cm (range, 2.2–8 cm). There were 19 major hepatectomies, 34 uni- or bisegmentomies, and 17 atypical resections. The operative time was 227 ± 109 min. Conversion to laparotomy was required for seven patients (10%), mainly for continuous bleeding during transection. Nine patients (13%) required blood transfusion. One patient had both brisk bleeding and gas embolism from a tear in the section line of the right hepatic vein requiring laparoscopic suture. Blood loss and transfusion requirements were significantly lower in recent than in early cases and in resections with prior vascular control than in those without such control. Postoperative complications were experienced by 11 patients (16%), including one bleed from the hepatic stump requiring hemostasis and two subphrenic collections requiring percutaneous drainage. One cirrhotic patient died of liver failure after resection of a partially ruptured tumor. No ascites was observed in other cirrhotic patients. The mean hospital stay was 5.9 days.
Conclusion
The study results confirm that laparoscopic liver resection, including major hepatectomies, can be safely performed by laparoscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For technical and carcinologic reasons, the laparoscopic approach was late to develop in solid organ surgery such as that performed on the spleen [12, 15], the pancreas [13], the adrenals [14, 33], and especially the liver. Mobilization and exposure of the liver still are difficult in the absence of specific nontraumatic instruments for safe handling of such a fragile and heavy organ. The “hidden” vascular anatomy of the liver exposes it to the risk of massive bleeding, which can be difficult to control by laparoscopy. Uncontrolled air embolism has long been feared because of the high-pressure pneumoperitoneum [18, 30]. Dissemination of malignant tumors during the procedure also is expected [17, 32], as in the early experience of colon and gallbladder laparoscopic surgery.
In addition, most experienced liver surgeons have little experience in laparoscopy and therefore are not keen to advocate laparoscopic liver resections. Improvement in surgeons’ laparoscopic skills and their growing experience in laparoscopic handling of the liver with laparoscopic instruments have allowed better exposure and mobilization of the liver. At the same time, the development of new coagulation/section technologies have facilitated parenchymal transection. Both experimental studies and clinical reports have shown that carbon dioxide (CO2) embolism is a rare and well-tolerated event during laparoscopic liver resections [9, 28].
Although most reported laparoscopic liver resections still are minor hepatectomies for benign liver tumors [29], an increasing number of large series involving laparoscopic liver resections for benign and malignant tumors are being published [5, 22, 34]. Case-control studies have suggested that laparoscopy is even slightly better than laparotomy for left lateral lobectomies [24] and minor liver resections [26]. Finally, the results from a few series of laparoscopic resections of both hepatocellular carcinoma [3, 5, 23] and colorectal liver metastases [25] have suggested that tumor dissemination has not been enhanced by laparoscopy.
Currently, laparoscopic access is becoming a recognized technique for liver resection. However this advanced procedure still is limited to centers with experience in both hepatic and laparoscopic surgery. We report our experience with laparoscopic liver resection and compare earlier experiences with recent results and anatomic resections using prior vascular control with other resections.
Patients and methods
From February 1999 to January 2006, we performed 70 liver resections by laparoscopy. Patients referred for benign or malignant liver lesions were routinely considered for laparoscopic hepatectomy. The indication for resection was not modified by the use of the laparoscopic approach, especially for benign lesions, which were resected only in the presence of symptoms or uncertain diagnosis on biopsy. The indications for laparoscopic segmental and atypical resections were tumors smaller than 4 cm located in the inferior or anterior segments of the liver (segments III, IV, V, and VI) or in left segment I. The indications for major hepatectomies (more than 3 segments) were either larger tumors or localized Caroli’s or polycystic diseases. Tumors invading or adjacent to the portal pedicle or hepatic veins were not considered suitable for laparoscopy. Patients were informed concerning the innovative nature of the procedure, and prior consent was obtained.
Liver resections were defined according to Couinaud’s classification. Prior vascular control (of the portal vessels and hepatic vein) and ligation of the portal pedicle were carried out before the parenchymal transection whenever possible. This procedure was typically sought for right hepatectomy (segments V, VI, VII, and VIII), left hepatectomy (segments II, III, and IV), and left lateral lobectomy (segments II and III). These resections were defined as anatomic with prior vascular control (APVC). Other resections including segmental (one or two segments) and atypical liver resections were performed without prior vascular control.
Surgical procedure
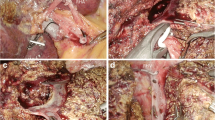
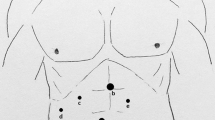
Pneumoperitoneum was performed using the open laparoscopy technique, and the abdominal pressure was maintained at less than 11 mmHg. The patients were in supine position. Trocar placement was based on the patient’s morphology and the type of resection. Four or five trocars were generally used. A central trocar was placed on the midline above the umbilicus for the laparoscope. In all cases, 0° laparoscopes were used. Two 10-mm working ports were placed on either side of the median trocar, and two 5-mm trocars were placed laterally for exposure. Intraoperative ultrasound was routinely performed. The part of liver to be removed then was mobilized.
For APVC resections (right hepatectomy, left hepatectomy, and left lateral lobectomy), the portal pedicles were first dissected. The portal branch, the arterial branch, and the biliary canal were separated extraparenchymally and secured with clips or a linear stapler. The concerned hepatic vein then was dissected and taped before the transection. For the last seven patients, the hepatic vein was sectioned using a linear roticulating stapler (Endo GIA roticulator; Tyco Healthcare, Elancourt, France) before transection was started.
For segmental and atypical resections, there was no prior vascular control. The transection line was marked on the capsule using diathermy. The parenchymal transection was performed using an ultrasonic dissector (Dissectron; Satelec Medical, Merignac, France) for the first 10 patients (14%), harmonic shears (Ultracision; Ethicon, Issy les Moulineaux, France) for the following 31 patients (44%), and laparoscopic Ultrashears (Ligasure; Tyco Healthcare) for the last 29 patients (42%). A Pringle maneuver was never used.
The resected liver was placed in a plastic bag and extracted without any fragmentation through a suprapubic horizontal incision. The hepatic stump was scrutinized for any bleeding while the level of pneumoperitoneum pressure was lowered. None of the patients had any abdominal drainage. Intraoperative complications, postoperative course, and outpatient follow-up evaluation were studied. The collected data included duration of surgery, blood loss, perioperative transfusions, conversion to laparotomy, postoperative complications, and hospital stay.
The results of our early experience (40 resections from 1999 to 2003) were compared with recent results (30 resections from 2004 to 2006). In addition, APVC resections were compared with resections that had no prior vascular control (segmental and atypical resections) in terms of blood loss, transfusions, conversion to laparotomy, operative time, and postoperative complications. Statistical analysis was performed with JMP 6.0 software (SAS Institute Inc., Cary, NC, USA) using Student’s t-test and Fisher’s exact test. All p values less than 0.05 were considered statistically significant.
Results
Between February 1999 and January 2006, 70 (15%) of 467 liver resections were performed by laparoscopy. This percentage increased continuously over the years, reaching 25% in 2005. The 70 resections involved 37 women and 33 men with a mean age of 56 ± 13 years (range, 29–78 years). According to the American Society of Anesthesiologists (ASA) physical status score, 11 patients (16%) were classified as ASA 3, 36 (51%) as ASA 2, and 23 (33%) as ASA 1. The liver parenchyma was normal in 33 patients and pathologic in 37 patients (18 fibrosis or chemotherapy modification cases and 19 cirrhosis cases).
Of the 70 patients, 38 had malignant tumors (54%) and 32 had benign lesions (Table 1). The malignant tumors were mainly hepatocellular carcinomas (19 of 24 patients with cirrhosis). The mean size of the resected tumor was 3.8 ± 1.9 cm (range, 2.2–8 cm). The mean surgical margin for the malignant tumors was 11 mm (range, 1–32 mm). Preoperative portal vein embolization was performed for two patients before a right hepatectomy in a cirrhotic liver.
The types of liver resection are detailed in Table 2. In 33 cases, the resection was anatomic with vascular control before liver division. Extraparenchymal portal control and section were possible in all 33 patients. The concerned hepatic vein was controlled in all left hepatectomies, in all but one right hepatectomy because of difficulties mobilizing the right liver (92%), and in all except two left lobectomies (87.5%) due to the volume of the tumor in the one case and minor bleeding from an injured diaphragmatic vein in the other. The right hepatic vein also was sectioned before liver transection in seven recent cases. No complications arose during vascular control.
The mean operative time was 227 ± 109 min. The mean operative blood loss was 397 ± 356 ml (range, 100–2,300 ml). Bleeding occurred because of parenchymal transection in all cases except one, in which abundant bleeding came from a tear in the section line of the right hepatic vein at the end of a right hepatectomy. This was treated laparoscopically using sutures. Nine patients (13%) required blood transfusion (2.8 ± 1.3 packed red cell units). The patient who experienced the tear of the right hepatic vein had gas embolism, as detected by transesophageal ultrasonography, which resulted in a transient fall in arterial blood pressure. There were no other cases of detectable gas embolism.
Conversion to laparotomy was required for seven patients (10%). Only two cases of conversion occurred in the last 3 years of the study. Conversion was caused by continuous diffused bleeding during the parenchymal transection (3 patients), exposure difficulties (2 patients: 1 segmentectomy V and 1 bisegmentectomy V–VI), unsatisfactory progression during parenchymal section (1 patient: trisegmentectomy V–VI–VII), and an anatomic variant of portal branches (1 patient: right hepatectomy). In none of these cases was the conversion decided in an emergency or because of life-threatening bleeding or major vessel injury.
Laparoscopic radiofrequency also was used for four patients. In three cases, radiofrequency was performed to treat a second tumor, in one case for an insufficient free margin. In this patient, the tumor was seen close to the hepatic stump, on which radiofrequency was subsequently performed.
Postoperative complications were experienced by 11 patients (16%) (Table 3). Bleeding from the liver stump required reoperation by laparoscopy for one patient on the first postoperative day of a segmentectomy IV. Hemostasis was achieved using bipolar coagulation and secured by a suture. One blood and one biliary collection were treated by percutaneous drainage. One cirrhotic patient (child C) who underwent surgery for a partially ruptured tumor of the left lobe died of liver failure. No ascites and no transient liver failure occurred in the remaining 18 cirrhotic patients.
The mean hospital stay was 5.9 ± 5.6 days. During the period of the survey, none of the patients treated for a malignant tumor had any peritoneal carcinomatosis or port-site recurrence.
The results for the first 40 laparoscopic resections performed during the learning period (from 1999 to 2003) were compared with those for 30 resections performed from 2004 onward. Although the patients’ characteristics were similar, blood loss, operative time, and hospital stay were significantly less in the second group (Table 4). The conversion rate also was lower in the second period (7% vs 12.5%), although the difference was not statistically significant.
The results for 33 APVC laparoscopic liver resections were compared with those for 37 resections performed without prior vascular control (Table 5). Although major resections were more frequent in the first group, blood loss and transfusion requirements were significantly less for the patients with prior vascular control. The conversion rate also was lower (3% vs 16%) in the APVC group, although this difference did not reach statistical significance. The operative time was similar in the two groups.
Discussion
Our results confirm that liver resection can be performed safely by laparoscopy in selected cases. Although the percentage of liver resections performed by laparoscopy remained low in the current study, it has greatly increased since the beginning of our experience in 1999. Indeed, for the last 2 years, 80% of left lateral lobectomies were performed by laparoscopy. A great number of left and right hepatectomies are currently being performed this way. In the current series, as in others [2, 4, 29], the rate of resected benign lesions still is high. This is not because of a change in the therapeutic policy for benign liver tumors, but because most benign lesions were resected by laparoscopy due to the fact that they generally arise in normal livers [8]. The indications for laparoscopy in the case of malignant tumors were more restrictive at the beginning of our experience because they often occur in livers injured with chemotherapy, cirrhosis, or fibrosis. Until recently, our team considered the following to be a contraindication to laparoscopy: large and subcapsular tumors, tumors arising in the immediate vicinity of major vessels, tumors invading adjacent organs, and tumors located in the posterior segments of the right liver.
The conversion rate in this study was relatively low [4, 5, 16, 34], and became very low during the last 2 years (7%), particularly in major liver resections. This is mainly because of greater confidence in the technique as well as better management of every step of the procedure. The fact that the procedure performed by laparoscopy is strictly similar to that performed by laparotomy in our department is of particular significance [11]. Conversion to laparotomy was never carried out in an emergency for acute massive bleeding.
The only major intraoperative complication (bleeding from the section line of the right hepatic vein) was handled by laparoscopy. Interestingly, this complication was related to inadequate use of a stapler. No other complications occurred during dissection of major hepatic veins. Magnification associated with laparoscopy may render dissection of major hepatic veins safer by laparoscopy than by laparotomy. We found the laparoscopic approach for the left border of the right hepatic vein and for the inferior angle between the left hepatic vein and the inferior vena cava to be quite convenient and safe. This accurate dissection is facilitated by the stability of the image because of a voice-controlled robotic camera holder we systematically use for laparoscopic liver surgery [27]. Extraparenchymal dissection and division of arterial and portal branches and bile ducts was always possible in right and left hepatectomies and in left lateral lobectomies. The use of large resorbable clips was of great interest for the division of portal pedicles because the use of a stapler often is prevented by the lack of space.
Bleeding was generally limited except in nine patients (13%), for whom a transfusion was required. The incidence of this occurrence was slightly higher than in recent series of liver resections [31, 34, 35]. Bleeding and transfusion requirements were greater in patients without previous control of portal and hepatic pedicles. It occurred mainly in patients with resections of segment V or V–VI. In these cases, the two transection planes cross at right angles. Bleeding usually started at the junction between these two planes and at the plane extending from the gallbladder fossa to the right border of the liver. More trocars should be used to have the operating instrument always in the line of the liver transection plane.
It has been shown that the clamping of the hepatic pedicle is well tolerated in hepatic resections by laparoscopy [4, 6, 7, 20]. This was not used in our experience. Segmental resections in the right liver may be a good indication for portal triad clamping. However in our experience, the duration of parenchymal transection in laparoscopic segmental resections in the right liver is considerable and may exceed the time allowed for a safe clamping.
It is generally suggested that small liver resections are more amenable to laparoscopy than larger ones. This may be true for minor resections on the anterior border of the liver or on its surface. However, our results suggest that true anatomic segmental resections are more difficult than major resections. In the current series, bleeding was more profuse, and both the transfusion requirement and conversion were more frequent in segmental resections. It should be underscored that there was no resection of segments 7 or 8 in the current series, although this has been described by others [34]. We consider that such segmental resections should not be undertaken unless major resections are perfectly handled.
Exposure and mobilization of the right liver remain problematic. The correct positioning of the patient on the operating table with thick cushions behind the right shoulder and buttock is essential. This allows the right liver to fall toward the left hypochodrium, exposing the right triangular ligament. Also, the 5-mm trocar placed in the right subcostal space should be positioned very laterally. Mobilization and exposure remain difficult due to the lack of smooth and safe liver retractors. Great care should be taken to avoid injury of the liver or tumor throughout the whole procedure. Some surgeons advocate the hand-assisted technique to facilitate liver exposure and parenchymal transection [1, 10], especially in the case of cirrhotic liver [21] or tumor located in the posterior segments of the right liver [19]. In addition, it provides the surgeon the tactile sensation lacking during laparoscopy. However, we find that it decreases the vision of the operative field and thus do not use this technique.
Liver resection by laparoscopy is not an easy procedure. It requires knowledge and technical skill in both liver surgery and laparoscopy. In the current series, we greatly benefited from extensive experience in both.
References
Antonetti MC, Killelea B, Orlando R III (2002) Hand-assisted laparoscopic liver surgery. Arch Surg 137: 407–411, discussion 412
Borzellino G, Ruzzenente A, Minicozzi AM, Giovinazzo F, Pedrazzani C, Guglielmi A (2006) Laparoscopic hepatic resection. Surg Endosc 20: 787–790
Champault A, Dagher I, Vons C, Franco D (2005) Laparoscopic hepatic resection for hepatocellular carcinoma: retrospective study of 12 patients. Gastroenterol Clin Biol 29: 969–973
Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, Rotman N, Fagniez PL (2000) Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 232: 753–762
Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL (2006) Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg 243: 499–506
Decailliot F, Cherqui D, Leroux B, Lanteri-Minet M, Ben Said S, Husson E, Duvaldestin P, Stephan F (2001) Effects of portal triad clamping on haemodynamic conditions during laparoscopic liver resection. Br J Anaesth 87: 493–496
Decailliot F, Streich B, Heurtematte Y, Duvaldestin P, Cherqui D, Stephan F (2005) Hemodynamic effects of portal triad clamping with and without pneumoperitoneum: an echocardiographic study. Anesth Analg 100: 617–622
Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, Morino M, Bismuth H, Castaing D, Savier E, Honore P, Detry O, Legrand M, Azagra JS, Goergen M, Ceuterick M, Marescaux J, Mutter D, de Hemptinne B, Troisi R, Weerts J, Dallemagne B, Jehaes C, Gelin M, Donckier V, Aerts R, Topal B, Bertrand C, Mansvelt B, Van Krunckelsven L, Herman D, Kint M, Totte E, Schockmel R, Gigot JF (2003) Laparoscopic liver resection of benign liver tumors. Surg Endosc 17: 23–30
Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J (2002) Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg 9: 242–248
Fong Y, Jarnagin W, Conlon KC, DeMatteo R, Dougherty E, Blumgart LH (2000) Hand-assisted laparoscopic liver resection: lessons from an initial experience. Arch Surg 135: 854–859
Franco D (2001) Right hepatectomy, operative technique. Websurg Gen Dig Surg. http://www.websurg.com/ref/doiot02en155.htm (Retrieved December 28, 2006)
Friedman RL, Fallas MJ, Carroll BJ, Hiatt JR, Phillips EH (1996) Laparoscopic splenectomy for ITP: the gold standard. Surg Endosc 10: 991–995
Gagner M, Pomp A (1997) Laparoscopic pancreatic resection: is it worthwhile? J Gastrointest Surg 1: 20–26
Gagner M, Pomp A, Heniford BT, Pharand D, Lacroix A (1997) Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg 226: 238–246, discussion 246–237
Gigot JF, Ville de Goyet J, Van Beers BE, Reding R, Etienne J, Jadoul P, Michaux JL, Ferrant A, Cornu G, Otte JB, Pringot J, Kestens PJ (1996) Laparoscopic splenectomy in adults and children: experience with 31 patients. Surgery 119: 384–389
Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L, Descottes B, Valleix D, Lachachi F, Bertrand C, Mansvelt B, Hubens G, Saey JP, Schockmel R (2002) Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg 236: 90–97
Gutt CN, Riemer V, Kim ZG, Erceg J, Lorenz M (2001) Impact of laparoscopic surgery on experimental hepatic metastases. Br J Surg 88: 371–375
Hashizume M, Takenaka K, Yanaga K, Ohta M, Kajiyama K, Shirabe K, Itasaka H, Nishizaki T, Sugimachi K (1995) Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc 9: 1289–1291
Huang MT, Lee WJ, Wang W, Wei PL, Chen RJ (2003) Hand-assisted laparoscopic hepatectomy for solid tumor in the posterior portion of the right lobe: initial experience. Ann Surg 238: 674–679
Huscher CG, Lirici MM, Chiodini S (1998) Laparoscopic liver resections. Semin Laparosc Surg 5: 204–210
Inagaki H, Kurokawa T, Nonami T, Sakamoto J (2003) Hand-assisted laparoscopic left lateral segmentectomy of the liver for hepatocellular carcinoma with cirrhosis. J Hepatobiliary Pancreat Surg 10: 295–298
Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T (2005) Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg 189: 190–194
Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL (2003) Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 138: 763–769, discussion 769
Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL (2003) Laparoscopic versus open left lateral hepatic lobectomy: a case–control study. J Am Coll Surg 196: 236–242
Mala T, Edwin B, Gladhaug I, Fosse E, Soreide O, Bergan A, Mathisen O (2002) A comparative study of the short-term outcome following open and laparoscopic liver resection of colorectal metastases. Surg Endosc 16: 1059–1063
Morino M, Morra I, Rosso E, Miglietta C, Garrone C (2003) Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc 17: 1914–1918
Proske JM, Dagher I, Franco D (2004) Comparative study of human and robotic camera control in laparoscopic biliary and colon surgery. J Laparoendosc Adv Surg Tech A 14: 345–348
Ricciardi R, Anwaruddin S, Schaffer BK, Quarfordt SH, Donohue SE, Wheeler SM, Gallagher KA, Callery MP, Litwin DE, Meyers WC (2001) Elevated intrahepatic pressures and decreased hepatic tissue blood flow prevent gas embolus during limited laparoscopic liver resections. Surg Endosc 15: 729–733
Rogula T, Gagner M (2004) Current status of the laparoscopic approach to liver resection. J Long Term Eff Med Implants 14: 23–31
Takagi S (1998) Hepatic and portal vein blood flow during carbon dioxide pneumoperitoneum for laparoscopic hepatectomy. Surg Endosc 12: 427–431
Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, Ijichi M, Hasegawa K (2001) Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg 136: 922–928
Targarona EM, Martinez J, Nadal A, Balague C, Cardesa A, Pascual S, Trias M (1998) Cancer dissemination during laparoscopic surgery: tubes, gas, and cells. World J Surg 22: 55–60, discussion 60–51
Thompson GB, Grant CS, van Heerden JA, Schlinkert RT, Young WF Jr, Farley DR, Ilstrup DM (1997) Laparoscopic versus open posterior adrenalectomy: a case–control study of 100 patients. Surgery 122: 1132–1136
Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B (2006) Laparoscopic liver resection. Br J Surg 93: 67–72
Wu CC, Ho WM, Cheng SB, Yeh DC, Wen MC, Liu TJ, P’Eng F K (2006) Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a “blood transfusion”–free hepatectomy. Ann Surg 243: 173–180
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 2006 Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Meeting, Dallas, Texas, 26–29 April 2006
Rights and permissions
About this article
Cite this article
Dagher, I., Proske, J.M., Carloni, A. et al. Laparoscopic liver resection: results for 70 patients. Surg Endosc 21, 619–624 (2007). https://doi.org/10.1007/s00464-006-9137-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-006-9137-0