Abstract
Background
Laparoscopic total extraperitoneal (TEP) inguinal hernia repair is as efficacious as the open Lichtenstein procedure, can be learned with proper training, and causes less postoperative pain, better cosmesis, and earlier return to work. The one major factor preventing the widespread acceptance of TEP is the requirement for general anesthesia (GA). In contrast, open hernia is performed using local or regional anesthesia, thereby having the advantage of quicker recovery, decreased postoperative nausea and vomiting (PONV), fewer hemodyanamic changes, reduced metabolic responses to surgical stress, and better muscle relaxation. This study attempted to evaluate whether laparoscopic TEP can be performed under less invasive anesthesia, such as regional anesthesia, and to determine its feasibility and limitations
Methods
All total of 22 male patients were studied between January 2002 and March 2003 in a tertiary care referral hospital. Epidural anesthesia with 2% lignocaine with adrenaline (Adr) was given via a lumbar epidural catheter, achieving a sensory level of T6. The standard technique for TEP was followed, using three midline infraumbilical ports.
Results
Twenty-two patients (20 unilateral, 2 bilateral) underwent operation. The mean operating time was 67.8 ± 18 (range, 40–110) min. All 22 cases were started with epidural anesthesia, 7 of which (31.9%) were converted to GA; the other 15 (68.1%) were completed under epidural anesthesia. All cases were successfully completed laparoscopically, and there were no conversions. There were no intraoperative complications. There was no significant difference between the cases conducted under epidural anesthesia (67.6 ± 23 min) and those converted to GA (69.3 ± 7.3 min). There was no statistically significant difference between the conversion rates of smaller versus larger hernias in this study (p value 0.22). A significant association of success of the procedure was seen with a sensory level of T6 and above (2/15 conversions to GA; i.e., 13.3%) and cases with a sensory level below T6 (5/7 converted; i.e., 71.4%) and adequate epidural catheter length (p = 0.015). Prevention and management of pneumoperitoneum and subsequent shoulder-tip pain was the key to preventing conversions (6 of 9 converted to GA; i.e., 67%; p = 0.006). There were no significant postoperative complications, and no recurrences were noted during a mean follow-up period of 29 months (range, 20–36 months).
Conclusions
From the present study it is clear that TEP is possible under epidural anesthesia provided a minimal sensory level of T6 is achieved. To achieve that level, an appropriate higher site for catheter insertion and/or adequate intraepidural catheter length needs specific attention. Pneumoperitoneum, shoulder-tip pain, intraoperative straining, and inadequate preperitoneal space are factors whose interplay leads to conversion to GA. The size of the hernia is not related to pneumoperitoneum or conversion to GA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic total extraperitoneal repair of inguinal hernia has now been proved to be a safe and effective method for the repair of inguinal hernia [9]. Laparoscopic total extraperitoneal inguinal hernia repair (TEP) has been shown in various studies to have lower pain scores, shorter hospital stay, and earlier return to work, with recurrence rates comparable to open surgery (Lichtenstein’s tension-free repair and the Bassini repair) [3, 5, 6, 10, 12]. Early fears of a high incidence of complications have been proved wrong, and laparoscopic hernia repair can be accomplished with this procedure with minimum complications [12]. It has been recommended as the method of choice for recurrent and bilateral hernias [14]. Its role, however, in managing primary and unilateral inguinal hernias remains a matter of debate. The reasons quoted for this are that TEP is more costly, has a prolonged and difficult learning curve, incurs more complications, and requires general anesthesia (GA) [1]. The learning curve can be effectively overcome as reported elsewhere [8]. Various studies have shown that TEP is as efficacious as open Lichtenstein repair, and that it can be learned with proper training. In addition, it is relatively cost effective in view of the early return to work is common with TEP [11]. Today, probably one major factor preventing the widespread acceptance of TEP is the requirement for GA, and its attendant complications as compared to the open Lichtenstein repair, which can be performed under local anesthesia.
Although TEP under regional anesthesia has been reported, its feasibility, fallouts, and guidelines have not been described [10, 13]. Regional anesthesia offers many advantages over GA, such as quicker recovery, decreased postoperative nausea and vomiting (PONV), and fewer hemodyanamic changes. Metabolic responses to surgical stress are also reduced. Because the patient retains consciousness, it is likely that there would be an early awareness of complications. Regional anesthesia produces better muscle relaxation, which is an advantage in this procedure [4, 7]. The disadvantage of conducting TEP under epidural anesthesia is that if pneumoperitoneum occurs, irritation of the phrenic nerve causes shoulder-tip pain, which is mediated by C3–4 nerve roots and cannot be blocked with regional anesthesia alone.
The present study was designed to determine the issues peculiar to lap TEP under epidural anesthesia, such as the site of epidural puncture, length of catheter insertion, amount of anaesthetic agent used, use of supplemental intravenous sedation and analgesia, and management of pneumoperitoneum, and to find out whether there is a subset of patients in whom this procedure is preferable.
Methods
The present study was conducted in a large university referral hospital in New Delhi from January 2002 to March 2003. The first author, the operating surgeon in all cases, was well experienced with TEP. Patients with complicated hernia (in the form of irreducible hernias, strangulated hernia, and obstructed hernia), history of previous lower abdominal surgery, history of radiotherapy, complete (scrotal) inguinal hernia, bleeding and clotting anomalies, local infections, or history of allergy or hypersensitivity to local anesthetics were excluded from the study. All patients were completely investigated for fitness for GA. Patient consent was obtained for surgery under both regional and general anesthesia and by the laparoscopic and open approaches. The study was cleared by the college ethics committee. All patients were made familiar with the visual analog scoring (VAS) pain chart preoperatively. Both unilateral and bilateral repairs were performed.
Anesthesia procedure
In the operating room, peripheral venous access was gained in all cases, and the patient was prehydrated adequately with crystalloids. A lumbar epidural catheter was inserted using the loss of resistance technique. A test dose of 2% lignocaine was given with 1:200,000 adrenaline. Epidural block was established using 2% lignocaine with adrenaline until a sensory level of T6 was reached, and surgery commenced. During the performance of the surgery, intermittent bolus administration of 2% lignocaine with adrenaline was given as and when required to either raise the level up to T4 or to maintain the level of anesthesia. Monitoring of heart rate, blood pressure, oxygen saturation, end-tidal CO2, and ECG changes was done in all cases. Additional intravenous sedation and analgesia were given with injectable pethidine (50 mg) and injectable midazolam (1 mg), as and when required. In cases of poor compliance, failure of anesthesia effect, and incapacitating shoulder-tip pain, the procedure was converted to GA. The event requiring conversion to GA was noted.
Operative procedure
The patients were also instructed to micturate immediately before surgery. For prophylaxis against infection, all patients were given injectable cloxacillin (1g) and injectable gentamycin (80 mg) at induction and at 8 and 16 h postoperatively.
TEP
The TEP repair was performed in the same way as described by Lal et al. [8]. Patients were in the supine position. Epidural anesthesia was used in all patients. Three midline subumbilical trocars were used. A 12–15-mm subumbilical incision was made, after which blunt dissection was performed until the anterior rectus sheath was exposed. A transverse incision was made in the anterior rectus sheath in the midline, and the rectus muscles were separated from each other. Preperitoneal space was created using blunt finger dissection behind the rectus muscle and in front of the peritoneum. Next, a balloon (made of double-finger gloves tied over a no. 20 intercostal drainage tube) was inserted into the preperitoneal space. The balloon was inflated with 180–200 ml of saline and left in place for 5 min for hemostasis. It was then deflated and removed. Thereafter a 10–12 mm canula (without trocar) was placed and positioned at the semicircular line of Douglas and fixed to the skin with horizontal mattress silk sutures. (For the last 2 cases in the series the technique of expanding the preperitoneal space by the inflation balloon was abandoned and the space was expanded by CO2 insufflation only.)
Next, the optical instrument (10 mm 0°/30°) was introduced through the subumbilical canula, and blunt dissection was done with telescope to extend the preperitoneal space in the midline up to the pubic symphysis. The exact site of placement of the two 5-mm ports (the lower one was placed just above the symphysis pubis and the third 5-mm port was placed between the first and second ports) was marked with a needle to confirm their position in the midline. Two 5-mm trocars were then placed under direct vision at the marked sites. Through these ports, two 5-mm dissectors were introduced, and the preperitoneal space was dissected, starting from the symphysis pubis in the midline and proceeding laterally up to the psoas muscle. During this process, the pubic symphysis, superior pubic ramus, cord structures, and inferior epigastric vessels were used as important landmarks. It was ensured that inferior epigastric vessels stayed at the roof of the space created and acted as a guide to the cord structures.
The direct hernial sac when present was the first structure to be reduced, starting from the midline laterally, medial to the inferior epigastric vessels. The fundus of the direct sac was separated from the redundant fascia transversalis, which gives the appearance of a “reverse sac,” and then pulled down. At the completion of this dissection, the cord structures were identified at the deep ring. Lateral to the inferior epigastric vessels in cases of indirect hernia, the peritoneal sac was dissected away from the cord structures, both medially and laterally until it was separated completely. The sac was then pulled back completely from the deep ring, in much the same way as a rope is pulled to draw water from a well.
In two cases, the sac was divided and the proximal sac was ligated with a catgut endoloop. In all other cases complete reduction was possible. Furthermore, in cases of direct hernia the cord structures were explored for any synchronous indirect sac, which if present was reduced. The indirect sac in all cases was well separated from the cord posteriorly. After this part of the procedure the lateral space was developed up to the level of the psoas muscle, and the lateral limit was identified by visualization of the genitofemoral nerve.
A 12–14-cm mesh (Prolene mesh, J & J, Bard mesh ) was now rolled up, secured with 2 Vicryl ties, and introduced into the space via the 10-mm telescope port. The mesh was positioned, and the vertical fold of the mesh was tacked to the pubic symphysis in the midline with a 5-mm tacker (ProTack, Autosuture). The mesh was then unrolled over the peritoneum on the floor, covering the hernial defect and the entire musculopectineal orifice (MPO). The preperitoneal space was deflated under vision in all cases. After expressing out all the residual gas from the space, the rectus sheath was closed with 1-0 Vicryl and skin closed with sutures. A gauze roll was strapped over the groin in all cases to prevent seroma formation for the first 24 h.
Intraoperative monitoring
Details of the operative procedure, including operation time and complications such as bleeding, peritoneal breach, nerve injury, vas deferens injury, tearing of inferior epigastric vessels, and major visceral or vascular injury, were recorded.
Anesthetic parameters, including site of epidural administration, amount of drug, additional intermittent bolus administration of epidural local anesthetic drug used, level of sensory block, incidence of shoulder-tip pain, respiratory embarrassment, chest pain, additional intravenous sedation, and any event causing conversion to general anesthesia and time of conversion.
Postoperative monitoring
Postoperative pain charting was done using the VAS pain scoring systems, and a record of oral and parenteral analgesics was kept. Complications such as pneumoscrotum, hematoma, seroma, neuralgia, and wound infection, as well as early recurrence, were noted. A record of length of hospitalization and return to work was kept. All patients were advised to come for follow-up after 6 weeks, 3 months, and 6 months, or earlier if symptoms of wound infection, pain, scar, neuralgia, seroma formation, or short-term recurrence was suspected.
All data were compiled on Microsoft Excel computer program and subjected to appropriate statistical analysis in consultation with a statistician from the Medical College. Descriptive data and their distribution were analyzed by nonparametric tests: the Fischer exact chi square test; the quantitative data were analyzed using Student’s t-test. The statistics were compiled by the software Statistica (1984–1999 Stat Soft Inc).
Results
A total of 22 patients were included in the study. The mean age of the patients in the study group was 33.12 years (median, 31 years; range, 18–52 years). The distribution of the hernias was as follows: 9 direct, 12 indirect, and 1 pantaloon type. There were 2 bilateral hernias, 11 right-sided hernias, and 9 left-sided hernias. All 22 cases were begun with epidural anesthesia, 7 of which were converted to GA (31.9%). The other15 were completed under epidural anesthesia (68.1%).
Operative time
The mean operative time for TEP repair was 67.4 ± 18.9 min (range, 40–110 min). There was no statistical difference between the cases conducted under epidural anesthesia (67.6 ± 23 min) and those converted to GA (69.3 ± 7.3).
Size of hernia
An arbitrary division of the hernias was made depending on the size of the hernia. Since, complete hernias were excluded from the study, hernias were divided into two groups: incomplete, reaching to the neck of the scrotum (n = 12; converted: 2) and incomplete hernias reaching beyond the neck of the scrotum (n = 10; converted: 5). There was no statistically significant difference between the conversion rates of smaller versus larger hernias in this study (p value 0.22).
Amount of anesthetic agent used
The mean amount of injectable lignocaine with adrenaline used in the epidural space was 26.27 ± 3.86 ml. There was no statistical difference in the amount of lignocaine used in the epidural (26.13 ± 3.81 ml) versus the converted cases (26.57 ± 4.27 ml).
Sensory level achieved
The sensory level achieved at the start of the surgery was noted and statistically analyzed. A sensory level of T6 was obtained in 15 cases, T7 in 4 cases, and T8 in 3 cases. The cases were divided into two groups, one in which the sensory level was up to T6 and above (15 cases; 2 converted: conversion rate 13.3%) and another in which the sensory level was below T6 (7 cases; 5 converted: conversion rate 71.4%). There is a statistically significant difference between the number of cases converted to GA with a sensory level below T6 and the number of cases converted with a sensory level of T6 (p = 0.014).
Supplemental intravenous sedation
Intravenous sedation and analgesia were given at the start of surgery in the form of injectable pethidine or injectable midazolam. Sedation and analgesia were given at the start of the procedure in 14 patients, of which 8 were given injectable pethidine 50 mg and 6 were given injectable midazolam, 1 mg. There was no significant difference in the conversion rates between those given intravenous sedation at the start of surgery (14 cases of which 3 converted: 21.4%) and those not given intravenous sedation (8 cases, 4 of which were converted: 50%) (p = 0.18, p > 0.05; Fisher’s exact and chi square test). All patients were eventually given supplemental intravenous sedation/analgesia depending on the symptoms of intraoperative shoulder-tip pain, straining, and discomfort. Statistical analysis for the above parameters could not be done because of the multiplicity of agents used and the different times of intravenous sedation/analgesia administration.
Other anesthetic parameters
Others parameters like site of epidural puncture and intraepidural catheter length were noted. Ten cases were conducted with a catheter length of 2–3 cm; 6 (60%) were converted to GA. Another 12 cases where a catheter length of 4–5 cm was used resulted in conversion of only 1 (8.3%). On statistical analysis, this revealed a statistically significant difference (p = 0.015). No significant statistical relation to conversion could be inferred from the site of catheter insertion (p = 0.06, p > 0.05).
Intraoperative parameters
During the course of the operation pneumoperitoneum was seen in 10 cases, shoulder-tip pain (due to phrenic nerve irritation by free intraperitoneal gas in pneumoperitoneum) in 9 cases, chest pain in 4, intraoperative discomfort (not attributable to shoulder-tip or chest pain) in 7, intraoperative straining by the patient in 6, and inadequate operative space (preperitoneal space) as assessed by the surgeon in 9 cases (Table 1).
The incidence of conversion to GA in the cases with the above parameters was statistically analyzed for a relation between these parameters and the success of the procedure under epidural anesthesia. A statistically significant difference was sought between the presence of these parameters in the cases completed under epidural anesthesia and those converted to GA.
Severe shoulder-tip pain
Of the 9 patients with severe shoulder-tip pain, 6 were converted to GA. The incidence of conversion to GA was significantly higher among patients with shoulder-tip pain than those without.
Pneumoperitoneum
Ten cases of pneumoperitoneum were seen, 6 of which were converted to GA. This represented a significant difference in the conversion rates in the group with pneumoperitoneum and the one without.
Intraoperative straining
The majority of patients with intraoperative straining required conversion to GA (5 of 6 cases; 83.3%).
Inadequate space
Inadequate space was seen in 9 cases, most of which (6 out of 9; 66.6%) were converted to GA.
Chest pain
Four cases of chest pain were noted of which 2 (50%) needed conversion to GA.
Intraoperative discomfort
There were 7 patients with intraoperative discomfort not related to shoulder-tip or chest pain, of which 4 (57.1%) were converted to GA.
An interesting observation was that shoulder-tip pain was seen in most patients that had pneumoperitoneum (7/9; 77.7%) and intraoperative straining (5/6; 83.3%). The risk of intraoperative loss of space was significantly higher in patients with intraoperative straining (6/6) and with shoulder-tip pain (8 of the 9 cases; 88.9%).
Postoperative complications
Postoperative parameters were noted at discharge and also at the first two postoperative follow-up visits in the outpatient clinic. The postoperative complications seen in this study were pneumoscrotum in 4 cases, subcutaneous emphysema in 4 patients and PONV in 2 cases. No cases of seroma, hematoma, paraesthesia, or inguinodynia were seen in the study.
Hospital stay
All patients were discharged on the first postoperative day, in keeping with our present policy regarding TEP. However, all patients were encouraged to begin early mobilization.
Recurrence
The recurrence rate over a follow-up period of 20–36 months (i.e., incidence of early recurrence) in this study was nil.
Return to work
The average time of return to light routine or activities of daily living was 2.3 days (range, 1–6 days) and the mean time before return to work was 10 days (range, 4–21 days). The parameter of return to work could not be gauged in two unemployed patients. The return to work in the Job type 1 group (manual laborers) was 11.2 ± 4.1 days, and that in the Job type 2 (sedentary workers) was 7.7 ± 3.1 days.
Discussion
All 22 procedures included in the study were started using epidural anesthesia, of which 15 (68.1%) were successfully completed under epidural anesthesia and 7 (31.9%) were converted to GA. There were no conversions to open hernia repairs.
An arbitrary division of hernias according to the size of the hernia was done in this study. This was done because a large complete sac reaching up to the scrotum requires a considerable amount of manipulation in order to be reduced, and this would increase the chances of an inadvertent peritoneal rent and pneumoperitoneum leading to shoulder-tip pain, the leading cause of conversion to GA. A statistically significant difference was sought between large hernias converted to GA and large hernias done successfully under epidural anesthesia. However no such significant difference was seen between the two groups. We explain this situation by the increasing experience of the operating surgeon and the small number of the study group. We also infer from our experience that with increasing operator skill, large hernia sacs can be handled under epidural anesthesia.
The mean operative time recorded in this study was 67.4 min, with a range of 40–110 min [2, 5]. The only intraoperative complication (surgically) was peritoneal breach and resultant pneumoperitoneum (40.9%). This value is higher than that reported in most studies in world literature, which ranged from 9% to 40% [2]. However, this can be explained because small peritoneal rents in cases done under GA would pass unnoticed but those that occur during procedures using epidural anesthesia in an awake patient lead to immediate recognition. The fact that there was no other significant surgical complication, such as bladder, nerve, or vascular injury, is favorable as compared to published literature.
The postoperative complications seen in the study were 4 cases each of self-limiting pneumoscrotum and subcutaneous emphysema (18%) and 2 cases of PONV (9%). All the cases of pneumoscrotum and subcutaneous emphysema resolved spontaneously in the first 6 postoperative hours. Thus, these complications were non-morbid and self-limiting in nature. There was no incidence of seroma, hematoma formation, or inguinodynia [5, 10]. We attribute this to the strict adherence to protocol, such as asking the patient to void before surgery; proceeding further only after identification of the inferior epigastric artery; directed dissection around the spermatic cord; and avoiding use of tacks laterally to avoid nerve entrapment.
The anesthetic agent used in the epidural space was injectable lignocaine with adrenaline. This was similar to that used in another study on epidural anesthesia in TEP [2]. The use of lignocaine is recommended for its faster onset of action and safety of the drug. However the option of the use of epidural narcotics and fentanyl alone or in conjunction with other anesthetic agents needs to be explored.
The aim in terms of sensory level of anesthesia in the study was T6 or higher as the need required. This goal was achieved in the majority of cases, and there was a significant association between a level of T6 sensory block or higher and the success of the procedure under epidural anesthesia. This was in contrast to a similar study [2] that cites a sensory level of T4 and above for the success of TEP under epidural block. The reason for recommending a sensory level above T6 is because gas insufflation of the preperitoneum leads to distention and subcutaneous migration of gas up to the level of the epigastrium. A sensory level of T6 and above should adequately counter this problem. The sensory level of T6, however, does not protect against shoulder-tip pain, which is mediated by the phrenic nerve, a cervical spinal nerve.
The interspaces of lumbar epidural puncture used were the first three lumbar interspaces. The length of the epidural catheter kept inside the epidural space was 2–5 cm. There was a significant association between an intraepidural space catheter length of 4–5 cm with the successful conclusion of the procedure under epidural anesthesia. We recommend that a sensory level of T6 or above be achieved using a higher lumbar interspace needle insertion (L1–L2 or T12–L1) and an intraepidural catheter length of 4 cm or less.
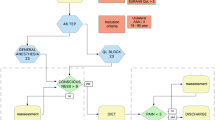
Addition of supplemental intravenous sedation at the start of the surgery was done in the form of injectable pethidine and injectable midazolam. The operations on a majority of patients given sedation and analgesia at the start of surgery were completed successfully under epidural anesthesia. However, these data did not have statistical significance. We recommend additional intravenous sedation and/or analgesia for the benefit of patient compliance and pre-emptive analgesia. Similar studies mentioning the role of intravenous sedation and analgesia have not been reported in published literature. The use of sedation and analgesia in the management of shoulder-tip pain in this study during TEP in 13 cases is outlined in Figure 1.
Various intraoperative parameters were noted and statistically analyzed to ascertain their relation to the successful conduct of the surgery under epidural anesthesia. There were 10 cases of overt pneumoperitoneum and 13 cases of shoulder-tip pain, of which 9 were severe and 4 moderate. There was a significant association between the incidence of pneumoperitoneum and shoulder-tip pain. This parameter has also not found mention in previous studies. With regard to operative time, there was no significant difference between the times in cases with pneumoperitoneum and shoulder-tip pain as compared with cases without either complication. There was a highly statistically significant statistical association between severe shoulder-tip pain and conversion to GA in our study. When shoulder-tip pain was mild, the procedure was successfully completed with conservative management.
There were 6 cases of intraoperative straining in the study, of which 5 needed to be converted to GA and only one could be managed under epidural anesthesia. There were 9 cases of inadequate space, 6 of which had to be converted to GA. These parameters have not been mentioned in previous studies. The reason for intraoperative straining may have been that the level of motor blockade in epidural anesthesia is 2–5 spinal interspaces lower than that of sensory block. This, compounded with distressing pneumoperitoneum and intraoperative subcutaneous emphysema in “awake” patients, causes the patient to strain. Straining has a twofold effect: first is a loss of space and second, increasing apprehension of the patient, increasing the chance of accidental injury. There was a significant association between shoulder-tip pain and the incidence of intraoperative straining. There was a statistically significant association between inadequate space and straining, pneumoperitoneum, and shoulder-tip pain, which depicts a causative relation of these parameters.
There were 4 instances of intraoperative noncardiogenic, nonpulmonary chest pain. A possible explanation is the epigastric and lower chest migration of subcutaneous gas, which may lead to a feeling of chest pain and congestion. All patients with the above symptoms were given an ECG and chest x-ray, and all studies were normal. This uncharacteristic finding is peculiar to this study and is not mentioned in published literature.
All patients were discharged on the first postoperative day, in keeping with our present policy regarding TEP. The average time to return to work after TEP in employed patients was 10 days.
There was no incidence of short-term recurrence in the study during a follow-up period of 29 months (range, 20–36 months). The zero recurrence rate in the present study may be attributed to adequate dissection laterally, followed by the introduction of a large mesh in the preperitoneal space. The mesh was fixed to the pubic bone medially, thereby precluding the chance of mesh migration. The cord structures were thoroughly examined in every case of direct hernia to look for any indirect sac. Lastly, the peritoneum was separated proximally so that the mesh further lateralized the cord. The operating surgeon gave priority to a safe and recurrence-free conduct of the surgery, even if that meant extension of the operating times. All of these parameters have been reported in world literature to be possible causes of recurrence, which is why stringent preventive measures were taken in all cases in our series.
Shoulder-tip pain in cases of pneumoperitoneum was managed with injectable midazolam, injectable pethidine, and injectable morphine. All cases of mild shoulder-tip pain (4) responded. Of the 9 cases of severe shoulder-tip pain, 6 were converted to GA. We recommend the use of 1–1.5 mg of injectable midazolam and 50 mg of injectable pethidine for management of shoulder-tip pain. Morphine may be used instead of pethidine. The use of drugs such as ketamine and fentanyl may be associated with a better response. The management of severe shoulder-tip pain needs further study.
Conclusions
From the present study, it is clear that TEP is possible under epidural anesthesia, provided a minimal sensory level of T6 is achieved. To achieve the same (T6 sensory level), an appropriate higher site for catheter insertion and/or adequate intraepidural catheter length needs specific attention. Pneumoperitoneum, shoulder-tip pain, intraoperative straining, and inadequate preperitoneal space are factors whose interplay leads to conversion to GA. The size of the hernia is not related to pneumoperitoneum or conversion to GA.
References
Amid PK, Shulman AG, Lichtenstien IL (1995) An analytical comparison of laparoscopic hernia repair with open “tension free” hernioplasty. Int Surg 80: 9–17
Azurin DJ, Go LS, Cwik JC, Schuricht AL (1996) The efficacy of epidural anaesthesia for endoscopic preperitoneal herniorraphy: a prospective study. J Laparoendosc Surg 6: 369–373
Champault GG, Rizk N, Catheline JM (1997) Inguinal hernia repair: totally preperitoneal approach vs Stoppas operation, a randomized trial of 100 cases. Surg Laparosc Endosc 7: 445–450
Collins VJ (1993) Epidural anaesthesia. In Principles of Anaesthesiology: General and Regional Anaesthesia, vol. 2, 3rd edition, edited by Library of Congress Press. Pennsylvania, pp. 1571–1610
Hiekkinen TJ, Haukiporo K, Koivukangas P, Hulkko A (1998) A prospective randomized outcome and cost comparison of totally extra peritoneal endoscopic hernioplasty versus Lichtenstien hernia operation among employed patients. Surg Laparosc Endosc 8: 338–344
Johannson B, Hallerback B, Glise H (1999) Laparoscopic mesh vs. open preperitoneal mesh vs conventional anterior technique: a multicentric randomized trial. Ann Surg 230:225
Joris JL (1994) Anaesthetic management of laparoscopy. In Anaesthesia, vol 2, 4th edition, edited by Miller RD. Churchill Livingstone, New York, pp. 2011–2027
Lal P, Kajla RK, Chander J, Ramteke VK (2004) Laparoscopic total extraperitoneal (TEP) inguinal hernia repair: overcoming the learning curve. Surg Endosc 18: 642–645
Lal P, Kajla RK, Chander J, Saha R, Ramteke VK (2003) Randomized controlled study of laparoscopic total extraperitoneal versus open Lichtenstein’s repair. Surg Endosc 17: 850–856
Liem MS, Graaf YVD, Steensel CJV, Boelhouwer RU, Clevers G-J, Meijer WS, Stassen LPS, Vente JP, Weidema WF, Schrivers AJP, van Vroonhoven TJMV (1997) Comparison of conventional anterior surgery and laparoscopic surgery for inguinal hernia repair. N Engl J Med 336: 1541–1547
Memom MA, Fitzgibbons RJ Jr (1998) Assessing risks, costs, and benefits of laparoscopic hernia repair. Annu Rev Med 49: 95–109
Pullyblank AM, Carney L, Brandon F, Dixon AR (2002) Laparoscopic inguinal hernia repair: A NICE operation. J R Coll Edinb 47: 630–633
Spivak H, Nudelman I, Fuco V, Rubin M, Raz P, Peri A, Lelcuk S, Eidelman LA (1999) Laparoscopic extraperitoneal inguinal hernia repair with spinal anaesthesia and nitrous oxide insufflation. Surg Endosc 13: 1026–1029
Watkin D (2002) Why NICE does not recommend laparoscopic herniorraphy? BMJ 325:339
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lal, P., Philips, P., Saxena, K.N. et al. Laparoscopic total extraperitoneal (TEP) inguinal hernia repair under epidural anesthesia: a detailed evaluation. Surg Endosc 21, 595–601 (2007). https://doi.org/10.1007/s00464-006-9050-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-006-9050-6