Abstract
Background
The goal of this study was to determine the optimal treatment parameters for the ablation of human esophageal epithelium using a balloon-based bipolar radiofrequency (RF) energy electrode.
Methods
Immediately prior to esophagectomy, subjects underwent esophagoscopy and ablation of two separate, 3-cm long, circumferential segments of non-tumor-bearing esophageal epithelium using a balloon-based bipolar RF energy electrode (BARRX Medical, Inc., Sunnyvale, CA, USA). Subjects were randomized to one of three energy density groups: 8, 10, or 12 J/cm2. RF energy was applied one time (1×) proximally and two times (2×) distally. Following resection, sections from each ablation zone were evaluated using H&E and diaphorase. Histological endpoints were complete epithelial ablation (yes/no), maximum ablation depth, and residual ablation thickness after tissue slough. Outcomes were compared according to energy density group and 1× vs 2× treatment.
Results
Thirteen male subjects (age, 49–85 years) with esophageal adenocarcinoma underwent the ablation procedure followed by total esophagectomy. Complete epithelial removal occurred in the following zones: 10 J/cm2 (2×) and 12 J/cm2 (1× and 2×). The maximum depth of injury was the muscularis mucosae: 10 and 12 J/cm2 (both 2×). A second treatment (2×) did not significantly increase the depth of injury. Maximum thickness of residual ablation after tissue slough was only 35 μm.
Conclusions
Complete removal of the esophageal epithelium without injury to the submucosa or muscularis propria is possible using this balloon-based RF electrode at 10 J/cm2 (2×) or 12 J/cm2 (1× or 2×). A second application (2×) does not significantly increase ablation depth. These data have been used to select the appropriate settings for treating intestinal metaplasia in trials currently under way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Barrett’s esophagus or intestinal metaplasia (IM) is a chronic, predominantly Caucasian male disease state affecting all age groups beyond the fourth decade [2, 5, 7, 9]. Because there is no reliable therapy for this premalignant condition, a patient with Barrett’s esophagus must undergo frequent endoscopy and biopsy to survey for dysplasia and adenocarcinoma [2, 6]. Several medical societies recommend that patients with nondysplastic Barrett’s esophagus undergo endoscopy with biopsy every 2 or 3 years, whereas those with low-grade dysplasia are relegated to endoscopy with biopsy every 6–12 months for the remainder of their lives [8]. For a 40-year old patient, this represents more than 40 endoscopies over the average remaining lifetime with significant attendant health care cost and patient inconvenience/risk. If low-grade dysplasia was eliminated, for example, the need for endoscopy could be reduced from every 6 months to every 2 or 3 years. In the case of nondysplastic Barrett’s esophagus, successful ablation could eliminate the need for surveillance completely.
Patients with Barrett’s esophagus have a significantly increased risk of developing esophageal adenocarcinoma—40–130 times higher than in patients without this condition [2, 6]. This represents a 0.5% risk of developing cancer per patient year of life—a risk very similar to that of a patient with a colon polyp developing adenocarcinoma of the colon [10]. Esophageal adenocarcinoma has the fastest growing incidence rate of all cancers in the United States, increasing 350% since 1970 [6]. At this rate, the number of annual deaths from esophageal adenocarcinoma will equal that of colon cancer within 15 years. Esophageal adenocarcinoma most commonly begins as a premalignant lesion, Barrett’s esophagus, and then typically presents at an advanced stage when therapeutic options are limited and outcomes dismal. Five-year patient survival is estimated at 17% and median survival is 15 months, unless detected at the earliest stages (T1a and T1b).
A safe, tolerable, effective technique to ablate Barrett’s esophagus could reduce the need for surveillance endoscopy and, with further study, could significantly reduce the risk of progression to adenocarcinoma. As the first step in this direction, this pilot study evaluated the histological results of a novel balloon-based bipolar radiofrequency (RF) electrode used to ablate human esophageal mucosa. Previous studies using this probe have demonstrated its effectiveness in a porcine model [3, 4]. This was a dosimetry study to determine the optimal energy density and treatment parameters to achieve complete ablation of the human esophageal epithelium.
Materials and Methods
Study device
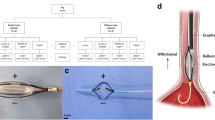
The BARRX system (BARRX, Sunnyvale, CA, USA) is composed of a RF controller (Fig. 1), a series of esophageal sizing balloons, and balloon-based bipolar RF electrodes (outer diameter, 22, 25, 28, 31, and 34 mm). The RF controller delivers a preset amount of energy [Joules per centimeter2 of electrode (J/cm2)] at up to 350 W to the bipolar RF electrode for the ablation of esophageal epithelium. The 3-cm-long bipolar RF electrode balloon is composed of 60 electrode rings (∼250 μm width each), tightly spaced every 500 μm (Fig. 2).
The treatment settings used for this study were determined by previous bench and animal research, which demonstrated that 10 and 12 J/cm2 resulted in complete removal of porcine esophageal epithelium, a maximum depth of ablation extending to the superficial submucosa, and no stricture formation [3, 4].
The device used in this clinical trial received 510(k) clearance from the Food and Drug Administration on December 18, 2001. It is approved for use in the coagulation of bleeding and nonbleeding sites in the gastrointestinal tract including, but not limited to, the esophagus. Indications include esophageal ulcers, Mallory–Weiss tears, arteriovenous malformations, angiomata, Barrett’s esophagus, Dieulafoy lesions, and angiodysplasia. In this study, the BARRX system was utilized within the bounds of its labeling.
Study design
Four clinical centers received institutional review board approval for this study, and informed consent was obtained from all patients prior to enrollment. Eligible patients were older than 18 years of age and were undergoing esophagectomy for adeno- or squamous carcinoma. Patients were excluded if they had undergone any previous ablative or resective esophageal procedure or had an esophageal stricture preventing passage of the endoscope or investigational catheter. Patients were randomized to receive an energy density setting of 8, 10, or 12 J/cm2. After the use of the 8 J/cm2 setting in one patient, we determined that the gross and histological ablation effect at that energy density setting was negligible. As a result, the remaining patients (n = 12) were randomized to receive either 10 or 12 J/cm2.
All procedures were performed in the operating room. After endotracheal intubation and initiation of general anesthesia, upper endoscopy was performed to verify the location of the tumor-bearing portion of the esophagus. A 25-mm sizing balloon was introduced alongside the endoscope, positioned above the tumor, and inflated to 7 psi (0.5 atm). Proper fit was assessed by observing balloon contact with the esophageal wall while rotating or moving the balloon linearly. Additional sizing was performed with larger or smaller balloons to determine esophageal inner diameter.
Thereafter, an appropriately sized bipolar balloon electrode (22–34 mm) was introduced and positioned, under direct visualization, approximately 10 cm above the tumor. Two separate, circumferential ablation zones were planned (zones I and II), each 3 cm in length. Zone I was 10 cm proximal to tumor, whereas zone II was 5 cm proximal to tumor.
An ablation was created by inflating the balloon electrode to 7 psi followed by activation of the RF controller. Power was 300 W. Energy density was preset according to randomization group as 8, 10, or 12 J/cm2 and was automatically controlled. Zone I received one RF application (1×), whereas zone II received two RF applications (2×), with 1 or 2 min between applications.
During the subsequent surgery, the esophageal adventitia and mediastinum were inspected for thermal injury and inflammation. After removal, each esophagectomy specimen was opened longitudinally and photographed. The sloughed mucosa was gently wiped away with a saline-soaked gauze to facilitate more accurate assessment of the maximum ablation depth and thickness of residual ablation. The specimen was rephotographed and fixed in formalin. Sections were cut from each ablation zone (three axial and two longitudinal) and from the nonablated portion of the esophagus, and they were prepared with hematoxylin and eosin (H&E).
At one center (University of Miami), fresh frozen sections from each ablation zone were obtained prior to formalin fixation for diaphorase and H&E staining. Diaphorase is a vital stain for NADPH. Cells with acute, irreversible injury may, theoretically or variably, appear normal on H&E staining. The diaphorase technique will stain only viable cells. By comparing identical sections stained with both diaphorase and H&E, we validated the use of H&E for detecting the “true” depth of injury for this ablation technique.
A single pathologist (PB) reviewed all histopathology slides and was blinded as to treatment group and zone of treatment. There were three primary histopathological endpoints: (1) complete epithelial ablation, (2) maximum depth of ablation, and (3) thickness of residual ablation effect. “Ablation” was defined as any sign of irreversible injury (coagulum, loss of cellular architecture, and loss of nuclei). Because each zone had five sections, “complete epithelial ablation” was confirmed if all epithelium from all five sections was absent (sloughed) or irreversibly injured. Since all specimens were gently wiped of sloughed, injured tissue, the maximum ablation depth was defined as the most superficial, uninjured cell layer present in all sections (described as intraepithelial, lamina propria, muscularis mucosae, submucosa, muscularis propria, or adventitia). If there was a difference in ablation depth between sections, the deepest ablation depth within a zone was read as the maximum ablation depth for the entire zone. “Residual ablation effect” was defined as the thickness of thermally injured cells lying on the surface of the viable cell layer, as defined in endpoint 2.
Outcomes were compared between groups according to energy density setting and 1× vs 2× treatment.
Results
Thirteen male patients were enrolled (age, 49–85 years), each with a diagnosis of adenocarcinoma at or near the gastroesophageal junction. Treatment was completed in 12 of the 13 patients. In one patient, the endoscope and catheter could not be simultaneously introduced into the esophagus due to anatomical constraints, so this patient was not included in the analysis.
Radiofrequency energy delivery time for each ablation zone was <1 sec. Median procedure time was 24 min, with most of the time allocated to initial endoscopy and sizing of the inner diameter of the esophageal target. Electrode sizes used were 28 (10), 31 (2), and 34 mm (1). Visual assessment of the immediate postablation endoscopy images revealed uniform, circumferential ablations (Fig. 3) for those zones treated with 10 J/cm2 (2×) and 12 J/cm2 (1× or 2×), whereas those areas treated with 8 J/cm2 (1× or 2×) and 10 J/cm2 (1×) had less visible effect of ablation and/or less uniformity of ablation.
There were no serious adverse events, such as perforation or transmural thermal injury, and no device malfunctions. One patient, with a history of previous esophageal XRT, had a superficial, 1-cm-long mucosal injury that occurred during sizing, possibly due to reduced esophageal compliance from XRT. In all cases, the esophagectomy procedure was carried out in the usual manner without adverse effect from the study procedure. Upon esophagectomy, the surgeons reported no evidence of pleural effusion, perforation, or transmural thermal injury in any patient. In two patients, including the XRT patient, there was a small amount of periesophageal edema.
The epithelium was easily wiped from the esophageal specimen for zones treated at 10 J/cm2 (2×) and 12 J/cm2 (1× or 2×), revealing a clean ablation zone (Fig. 4). Completeness of ablation, maximum ablation depth, and residual ablation depth were evaluated histologically for each section from each ablation zone. The histological sections from the first patient (8 J/cm2) showed ablation depth as only midepithelium, even at 2×; therefore, all subsequent cases were performed at either 10 or 12 J/cm2.
Complete epithelial ablation (Table 1) was reliably achieved at 10 J/cm2 (2×) and 12 J/cm2 (1× or 2×) (Fig. 5), whereas 8 J/cm2 (1× or 2×) and 10 J/cm2 (1×) only partly ablated the epithelium. In these cases of partial ablation, the epithelium was either unchanged or ablated to midepithelium depth with viable cells persisting near the basement membrane.
Maximum ablation depth (Table 1) was directly related to energy density setting and, for the 10 J/cm2 sections, to the number of treatments (1× vs 2×). The maximum depth of ablation was lamina propria or muscularis propria (Fig. 5) for all zones treated at 10 J/cm2 (2×) and 12 J/cm2 (1× or 2×), whereas 8 J/cm2 (1× or 2×) was midepithelium and 10 J/cm2 (1×) was either midepithelium or at the lamina propria. There were no sections with evidence of thermal injury to the submucosa, muscularis propria, or adventitia. Each section (axial and longitudinal) within a given ablation zone showed similar ablation depth. Diaphorase and H&E slides were compared side by side. Maximum depth of injury and residual ablation depth correlated for all sections, confirming that H&E is adequate as a technique for determining the histological ablation endpoints for this study (Fig. 6).
Residual ablation depth (Table 1) was minimal in all sections and was not related to energy density setting or 1× versus 2×.
Discussion
A safe, tolerable, effective technique to ablate Barrett’s esophagus could reduce the need for surveillance endoscopy and, with further study, could potentially reduce the risk of progression to adenocarcinoma. As the first step in this direction, we sought to evaluate the histological results of a novel balloon-based bipolar RF electrode (BARRX System) used to ablate human esophageal mucosa.
Radiofrequency energy is used for tissue ablation in many target sites. The heat generated by RF delivery results in tissue vaporization or coagulation. After the BARRX balloon electrode is inflated to contact the esophageal epithelium, RF is delivered in less than 1 sec resulting in a thin, uniform layer of thermal injury (typically 500–700 μm deep). The tight electrode spacing and ultrashort RF “on-time” constrains the electrical field and, therefore, limits the depth of injury.
Ganz et al. [3, 4] reported that this device achieved complete ablation of the squamous epithelium in a porcine model at energy density settings of 8 J/cm2 and higher. In the pig, mild and moderate strictures occurred in approximately 50% of areas treated at 13.3 J/cm2, whereas severe strictures occurred in all areas treated above 22 J/cm2. Ganz et al. also found that the maximum depth of ablation was related to energy density, with 8 and 10 J/cm2 ablating to the muscularis mucosae and 12 J/cm2 ablating to either the muscularis mucosae or the superficial submucosa.
We believe that the ablation depth necessary to completely and reliably eradicate intestinal metaplasia is the muscularis mucosae, and to avoid the risk of stricture formation one must avoid any significant injury to the submucosa or deeper structures. Based on cited animal study results, we selected 8, 10, and 12 J/cm2 as our settings to determine if this “ideal” ablation depth could be achieved.
Technically, the BARRX system was easy to use. In most cases, the esophagus could be quickly sized to allow selection of the appropriately sized balloon electrode. Once the balloon electrode was positioned and inflated, energy delivery was <1 sec, which ultimately translates to patient tolerability and very short balloon inflation time. The shaft of the catheter is small enough to allow a standard endoscope to be placed along side so that treatment can be done under direct vision. Lastly, the procedure is performed under real-time direct endoscopic visualization and the ablated area is sharply defined, allowing the operator to be certain as to which areas are ablated and which are not.
We saw mild periesophageal edema in two patients and a mucosal injury in one patient. This represents three observations in two patients, one of which had previous XRT, which likely created a less compliant esophagus. We believe that these observations were due to excessive stretch, rather than an RF effect.
Complete ablation of epithelium was dependent on energy density as well as the number of treatments (1× vs 2×), with complete ablation occurring at 10 (2×) and 12 J/cm2 (1× or 2×). From these data, it appears that a second treatment “fills in” any partially ablated areas (in the specific case of 10 J/cm2) but does not double the depth of injury as might be expected. Two factors are responsible for the limitation in additional depth with a second treatment. First, the coagulum overlying adequately ablated tissue may insulate that area from further injury, thus diverting the RF to areas that are initially undertreated. Second, heated tissue returns to normal body temperature within seconds of completing ablation, a phenomenon deemed thermal relaxation. RF heats via conduction, so the second application must start heating the tissue from the surface and will penetrate only the initial ablation depth or slightly deeper if the surface has sloughed.
The maximum depth of ablation achieved was never deeper than the muscularis mucosae. Ablating to, but not through, the muscularis mucosae was achieved with 10 (2×) and 12 J/cm2 (1× or 2×). Because the goal of ablation is to ablate the surface without injury to the submucosa, future studies, if necessary, may find that energy density settings higher than 12 J/cm2 are also safe.
The residual ablation thickness was minimal, only up to four cells thick or 35 μm. Unlike many RF heating modalities that employ long durations of RF “on-time” resulting in deep propagation of the heating effect, the RF on-time for this electrode is <1 sec. This ultrashort burst of energy heats the surface but does not propagate deeply, as evidenced by the immediate slough of tissue and a maximum of 35 μm of residual ablation thickness.
Although this study provides objective histopathological data regarding the ablative effects of this device on human esophageal squamous epithelium, the intent is to use the data as a guide for selecting treatment settings for esophageal IM for future studies. One potential criticism is that there may be a difference between ablating squamous epithelium in this study and ablating IM in subsequent studies. This criticism is twofold. First, does squamous epithelium respond to RF similarly to IM? We do not yet know the answer. More than 150 patients with IM have been treated with this device in U.S. clinical trials, with complete resolution of all IM in more than 50% of patients who were treated at 10 J/cm2 (2×). Therefore, there may be some differences in RF response between squamous and IM, although the differences may be small and managed with additional applications. Second, are squamous epithelium and IM of similar thickness? Ackroyd et al. [1] reported that, indeed, they are similar, with each being 500 μm thick on average and each having very little inter- or intrapatient variability. Although squamous epithelium may not be the perfect surrogate ablation target for RF, the BARRX IM ablation data and cited pathology data support the applicability of this trial’s results to patients with IM.
In summary, this study demonstrates that this device can remove the full thickness of esophageal epithelium without injury to the submucosa or deeper structures after a single session. Furthermore, the procedure is rapid in its effect and obviates the need for a photosensitizing drug, as used in photodynamic therapy. These data have been used to guide the selection of treatment settings for clinical trials that are currently evaluating this device for ablation of intestinal metaplasia, with and without low-grade dysplasia.
References
Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MWR (1999) Ablation treatment for Barrett oesophagus: what depth of tissue destruction is needed? J Clin Pathol 52: 509–512
Eisen GM (2003) Ablation therapy for Barrett’s esophagus. Gastrointest Endosc 58: 760–769
Ganz RA, Utley DS, Stern RA, Jackson J, Batts KP, Termin P (2004) Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc 60: 1002–1010
Ganz RA, Zelickson B, Stern R, Termin P, Coles C (2003) A method of endoscopic esophageal mucosal ablation using a novel RF energy balloon catheter (BARRx): response characteristics. Gastrointest Endosc 57: M1767
Gerson LB, Shetler K, Triadafilopoulos G (2002) Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 123: 636–639
Reid BJ (1991) Barrett’s esophagus and adenocarcinoma. Gastroenterol Clin North Am 20: 817–834
Rex DK, Cummings OW, Shaw M, et al (2003) Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 125: 1670–1677
Sampliner RE (2002) Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 97: 1888–1895
Spechler SJ (2002) Barrett’s esophagus. N Engl J Med 346: 836–842
Winawer SJ, Zauber AG, Ho MN, et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 329: 1977–1981
Acknowledgments
We thank Robert Shebert, MD, Professor of Medicine. Department of Medicine, Division of Neurology, University of Miami, School of Medicine, for his assistance in providing his knowledge and expertise in the preparation and interpretation of diaphorase stained esophageal sections for this study. This study was supported by a research grant from BARRX medical, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dunkin, B.J., Martinez, J., Bejarano, P.A. et al. Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device. Surg Endosc 20, 125–130 (2006). https://doi.org/10.1007/s00464-005-8279-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-8279-9