Abstract
Background
Patients with esophagogastric malignancies often require nutritional supplementation in the perioperative period, especially in the setting where neoadjuvant therapy may delay tumor resection. A simple technique is described here that can be performed at the time of staging laparoscopy and that has not been described before.
Results
Forty-three patients treated over a 4-year period who had a laparoscopic feeding jejunostomy placed at the time of staging laparoscopy were reviewed. Of these, 35 had preoperative chemotherapy according to a modified MRC OEO2 protocol. In the period between staging and eventual resection, 32% required immediate feeding, and in 14% of those who were thought not to need feeding it later became necessary. More patients gained weight or had a rise in albumin in the group that had jejunal feeding (p < 0.05). The mean time to surgery was 10 weeks. There were no conversions to an open procedure, nor were there any laparotomies for tube-related complications. Dislodgement was recorded in 6 patients; blockage, in 4. In most of these cases a simple bedside replacement of the tube was all that was required. Mean time in the operating room for each procedure was 44 minutes.
Conclusions
Laparoscopic percutaneous feeding jejunostomy is a safe and simple technique that adds little to the morbidity and cost of managing patients with esophagogastric cancers. It facilitates optimization of nutrition in the perioperative period for these patients, especially in those receiving preoperative chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with malignant tumours of the esophagus and stomach are often malnourished at presentation. The tumor promotes a catabolic state and at the same time impairs the person’s ability to eat. Current treatment regimens for these tumors involve multiple modalities such as surgery, chemotherapy, and, at times, radiotherapy, all of which can worsen the patient’s nutritional state.
Many forms of nutritional support have been used in a perioperative setting, including parenteral and enteral routes. Enteric feeding, where possible, avoids the risk of central venous sepsis and pneumothorax, is more physiological, does not require hospitalization, costs less, and has the immunological benefit of directly nitrifying the enterocytes. In the setting of esophagogastric malignancy, feeding into the stomach may not be possible because of tumor occlusion, tumor involving the stomach, or the need for the esophagus after oesophagaltomy to serve as a conduit, making jejunal feeding an ideal method.
Multimodality therapy using combination chemotherapy in a neoadjuvant setting has been shown to improve survival after resection of esophageal and gastric cardia malignancies [11]. Its use will, however, delay surgery for a number of weeks, and may worsen the patient’s ability to eat. It has become the practice in our institution to place a feeding jejunostomy tube in all patients at the time of laparoscopic staging to allow jejunal feeding any time it is required during the preoperative and postoperative periods. Even in inoperable cases, the tube may be left in place for supplemental nutrition during palliative therapy.
Many techniques of laparoscopic jejunal tube placement have been described in the literature. These often require specialized equipment, expensive kits, disposable instruments, or exteriorization of the bowel. This article describes a simple percutaneous technique that adds minimal time and cost to a staging laparoscopy and requires no equipment beyond what should already be available in a center performing laparoscopic surgery.
Methods
Retrospective review
The medical records of all patients who had an esophagectomy or gastrectomy for malignancy by a single surgeon over the 4-year period from 2000 to 2004 were reviewed. Data on all cases where a laparoscopic feeding jejunostomy was placed were recorded, including information on the patient, the tumor, timing of surgery, nutritional status, and any complications that arose.
Operative technique
Under a general anesthesia, with the patient in the supine position, skin prepping and sterile drapes are placed. With the surgeon standing on the patient’s right, a 10-mm port is placed in the umbilicus using the direct puncture technique, and CO2 insufflation. A 10-mm 30-degree laparoscope is positioned in this port. A 5-mm port in the line of a future midline incision is placed inferior to the xiphisternum, and a second 5-mm port is placed just lateral to the left linea semilunaris (at or just below the level of the umbilicus) for the working instruments.
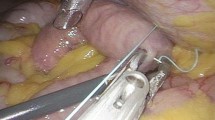
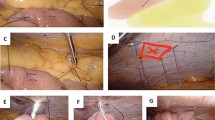
Next, laparoscopic examination is concluded, and any required biopsies are taken. The duodenojejunal flexure is then identified by lifting the transverse mesocolon in a cephalad direction. The apex of the first jejunal loop at 20–30 cm from the ligament of Treitz is brought up and stitched to the anterior abdominal wall in a left subcostal position, using laparoscopic needle holders and a 2/0 Vicryl stitch. The introducer sheath of a Medicut 12 g cannula (Argyle) is then slit longitudinally with a scalpel blade, and its plastic connector is cut off before percutaneous insertion of the entire Medicut cannula through the abdominal wall at the point corresponding to the internal position of the sutured jejunum. Under laparoscopic vision, the needle and sheath of the Medicut cannula are inserted into the lumen of the jejunal loop immediately caudal to the previously placed Vicryl stitch. Use of a grasper to hold the jejunum up 1 cm below the insertion point facilitates accurate placement. The Medicut needle is removed, leaving the sheath in place to allow passage of a 6 F infant feeding catheter (Vygon) down the sheath into the lumen of the jejunum. Intraluminal positioning of the catheter is confirmed by aspiration of bile-colored fluid, or by inflating air into the lumen via the new catheter. The sheath is then easily removed because of the longitudinal slit made in it. A second 2/0 Vicryl laparoscopic stitch is placed just caudal to the catheter insertion site, using the length remaining from the first stitch. The needle is then removed from the abdomen, and a 2/0 silk stitch is used to secure the tube externally to skin.
Results
A total of 121 patients underwent esophageal/gastric resection between January 2000 and March 2004. Of these, 43 patients (30 men and 13 women) with an esophagogastric malignancy had a laparoscopic feeding jejunostomy tube placed at the time of their staging laparoscopy. Mean patient age was 66.0 years (range: 42–82 years). In addition, 35 of these patients had preoperative chemotherapy and the remaining 8 did not, because it was deemed inappropriate (in-situ disease, high-grade dysplasia, sarcoma, medical contraindications, patient preference). In 16 patients jejunal feeding commenced immediately. Of the remaining 27 patients who did not require feeding initially, 4 needed jejunal feeding later in the pre-resection period. The mean time between jejunostomy and tumor resection was 10.4 weeks (SD 1.6). Average operating time for the staging laparoscopy with feeding jejunostomy was 44 minutes (5.6), from entering the operating room to leaving.
Of the 20 patients who received preoperative jejunal feeding, 14 (70%) maintained or gained weight, and 55% showed a rise in serum albumin (Table 1). Of the patients who did not receive jejunal feeding, 8 of 23 (35%) maintained or gained weight, and 30% showed a rise in serum albumin. The decision to feed in each case was made in conjunction with a dietician, using 10% weight loss as the main indication to commence feeding.
There was no peritoneal leakage or bowel obstruction from the feeding tubes, conversion to an open procedure was never required for tube placement, and there were no laparotomies required for tube-related complications. Dislodgement occurred in 6 patients (20%) and blockage occurred in 4 (13%). One of these patients required laparoscopic replacement and the others either simple reinsertion of the tube at the bedside or replacement at the time of resection. Connector breakage occurred in 2 (7%).
Discussion
The first option for patients requiring long-term enteral nutrition who are unable to eat or eat adequately is nasoenteric feeding. This has many disadvantages including discomfort, unsavory appearance, liability to clogging, risk of reflux and aspiration if gastric motility or emptying is impaired, and in some patients the tumor itself may prevent passage of a tube [8]. Percutaneous endoscopic gastrostomy (PEG) is the most popular method of prolonged enteric feeding, and was first described by Ponsky and Gauderer in 1980 [7, 15]. Ponsky also described the technique of percutaneous endoscopic jejunostomy (PEJ) [14], where a feeding tube traversed the gastric lumen through the pylorus and into the jejunum. Subsequently a method of direct percutaneous endoscopic jejunostomy (DPEJ) was also described [17, 18], where the feeding tube was passed into the jejunum without traversing the stomach. All of these techniques were associated with problems, including silent aspiration, hemorrhage, tube failure, and reflux of the jejunal tube contents into the stomach [2, 19], and all required the endoscopist to pass a tumor with an endoscope.
In the presence of an esophagogastric malignancy, feeding is ideally done distal to the pylorus because of possible involvement of the stomach with tumor, and to allow esophagus after oesophagaltomy to be used as a conduit after esophageal resection. In our institution a laparoscopic examination is done to complete staging of these malignancies, and in accordance with the findings of a multicentre Medical Research Council trial [11], operable candidates go on to have neoadjuvant chemotherapy, and patients with inoperable tumors progress to palliative chemotherapy or radiotherapy as appropriate. Because neoadjuvant therapy will delay surgery for 6–10 weeks, it has become routine here to place a feeding jejunostomy tube laparoscopically during the staging procedure so that enteral feeding can be begun if necessary. Our study notes that 27% of patients deemed not to require immediate jejunal feeding at the time of staging came to need it later in the preoperative period.
The first published technique of laparoscopic jejunostomy [13] used a Foley catheter inserted through a left upper abdominal stab incision, and the jejunum was secured to the abdominal wall with transabdominal nylon sutures. Several authors have since described the technique of exteriorizing a loop of jejunum to place the jejunostomy [4–6, 8, 10, 16], usually by grasping and withdrawing the loop through an upper abdominal 10-mm2 [6], 11-mm2 [10], or 18-mm2 port site, or through a separate incision [5]. The feeding catheter would then be secured at its entry into the jejunum with a pursestring suture [16], in a Stamm [10] inverting style, or with a Witzel tunnel [5]. The port site often had to be enlarged to facilitate securing the jejunal serosa to the fascial edges of the port site.
Laparoscopic percutaneous techniques did not require exteriorizing the bowel, but in all the published articles, a percutaneous jejunostomy kit was required [1, 3, 9, 12]. The method of securing the jejunum to the anterior abdominal wall usually entailed transabdominal wall sutures, which required bolsters on the external skin edge to reduce the incidence of skin necrosis. One author advocated the use of T-fasteners [3] to avoid having to place intracorporeal stitches, but these still required securing externally over bolsters. The article also suggested inflating air into the bowel lumen via a nasogastric tube to facilitate puncture of the jejunal wall with the catheter needle. A more recent article described total intracorporeal suturing to secure the jejunum, which required an Endostitch suturing device [12].
The technique described in this article is a totally intracorporeal, percutaneous one that requires no special kit,; reusable laparoscopic needle holders are the most sophisticated piece of equipment required. It does entail placing two intracorporeal knots, but most trainees experienced in laparoscopic procedures would accomplish this swiftly. The cost of the disposable equipment needed is negligible. Medicut needle = £0.51, umbilical feeding catheter = £0.49. The average time of 44 minutes in our series was the operating room entry to exit time; the actual procedure was much shorter. This the procedure added little to the staging laparoscopy in terms of both time and cost.
The complication rate in this study was comparable to other reports. Hotokezakaet al., in a study of similar size [9], reported a 25% major complication rate (including displacement, dislodgment, and aspiration) and a 25% minor complication rate. Although our series had a significant dislodgement rate, in most cases this required sliding a new feeding catheter into the track; in only one patient was a laparoscopic replacement required.
The fine-bore tube used is liable to blocking, and does require using thin proprietary feeds, but with a controlled rate pump and attention to flushing the tube, one could last up to several months. Even well crushed tablets have been successfully delivered through these tubes, but this is not recommended. As with dislodged tubes, a blocked tube can also be easily replaced at the bedside.
There were no cases of intraperitoneal leakage in our series, and we feel it is unnecessary with a fine-bore catheter to place complex pursestring or tunnelled sutures. In fact, creating a Witzel type tunnel would prevent the easy replacement of catheters that a direct puncture track allows.
Tube flushing with water twice a day is started on the day of operation, and tube feeding is permitted within 24 h. Patients are educated in the use of a take-home pump by a dietician and a nurse prior to discharge, and home visits by another dietician are made when necessary to ensure adequate supervision.
Conclusions
Laparoscopic percutaneous feeding jejunostomy is a safe and simple technique that adds little cost and morbidity to the management of a patient with an esophagogastric malignancy. Routine placement of a feeding tube at the time of the staging laparoscopy ensures optimization of nutritional status, even in patients who initially do not require supplemental feeding.
References
Allbrink MH, Foster J, Rosemurgy AS, Carey LC (1992) Laparoscopic feeding jejunostomy: also a simple technique. Surg Endosc 6: 259–260
Di Sario JA, Foutch PG, Sanowski RA (1990) Poor results with percutaneous endoscopic jejunostomy. Gastrointest Endosc 36: 257–260
Duh QY, Senokozlieff-Englehart AL, Siperstein AE, Pearl J, Grant JP, Twomey PL, Gadacz TR, Prinz RA, Wolfe BM, Soper NJ, et al. (1995) Prospective evaluation of the safety and efficacy of laparoscopic jejunostomy. West J Med 162: 117–122
Duzgun SA, Bozer M, Coskun A, San I, Uzunkoy A, Akinci OF, Canbeyli B (2002) A simplified laparoscopic technique for enteral access in cancer patients. Hepato-Gastroenterology 49: 1002–1005
Ellis LM, Evans DB, Martin D, Ota DM (1992) Laparoscopic feeding jejunostomy tube in oncology patients. Surg Oncol 1: 245–249
Eltringham WK, Roe AM, Mountford RA, Espiner HJ (1993) A laparoscopic technique for full thickness intestinal biopsy and feeding jejunostomy. Gut 34: 122–124
Gauderer MWL, Ponsky JL, Izant RJ (1980) Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Paediatr Surg 15: 872–875
Gedaly R, Briceno P, Ravelo R, Weisinger K (1997) Laparoscopic jejunostomy with an 18-mm Trocar. Surg Laparosc Endosc 7: 420–422
Hotokezaka M, Adams RB, Miller AD, McCallum RW, Schirmer BD (1996) Laparoscopic percutaneous jejunostomy for long term enteral access. Surg Endosc 10: 1008–1011
Morris JB, Mullen JL, Yu JC, Rosato EF (1992) Laparoscopic-guided jejunostomy. Surgery 112: 96–99
MRC Oesophageal Cancer Working Party, Girling DJ, et al. (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359: 1727–1733
Nguyen NT, Schauer PR, Wolfe BM, Ho HS, Luketich JD (2000) Laparoscopic needle catheter jejunostomy. Br J Surg 87: 482–483
O’Regan PJ, Scarrow GD (2002) Laparoscopic jejunostomy. Endoscopy 22: 39–40
Ponsky JL, Gauderer MWL (1981) Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc 27: 9–11
Ponsky JL, Aszodi A (1984) Percutaneous endoscopic jejunostomy. Am J Gastroenterol 79: 113–116
Ramesh S, Dehn TCB (1996) Laparoscopic feeding jejunostomy. Br J Surg 83: 1090
Shike M, Latkany L, Gerdes H, Bloch AS (1966) Direct percutaneous endoscopic jejunostomies for enteral feeding. Gastrointest Endosc 44: 536–540
Shike M, Shroy P, Ritchie MA, Lightdale CJ, Morse R (1987) Percutaneous endoscopic jejunostomy in cancer patients with previous gastric resection. Gastrointest Endosc 33: 372–374
Wolfsen HC, Kozarek RA, Ball TJ, Patterson DJ, Botoman VA (1990) Tube dysfunction following percutaneous endosc opic gastrostomy and jejunostomy. Gastrointest Endosc 36: 261–263
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jenkinson, A.D., Lim, J., Agrawal, N. et al. Laparoscopic feeding jejunostomy in esophagogastric cancer. Surg Endosc 21, 299–302 (2007). https://doi.org/10.1007/s00464-005-0727-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0727-z