Abstract
Background
We present our experience with laparoscopic deroofing of nonparasitic hepatic cysts.
Methods
Laparoscopic deroofing was performed due to a solitary hepatic cyst in 21 patients and polycystic liver in four patients. Laparoscopy was indicated when a cyst was larger than 5 cm (the general size of cysts was 6.9 cm) and caused complaints and was in a superficial position. In eight patients in whom the cyst was larger than 10 cm, omentoplasty was performed.
Results
Intraoperative complications were not detected. Two conversions were performed because of the deep position of the cyst. Postoperative bile leakage was detected in one case that was treated conservatively. The average hospital stay was 4.7 days. Relapse occurred in two patients (8%), but only one of them required a second operation.
Conclusions
We recommend laparoscopic deroofing for treatment of nonparasitic liver cysts. This operation causes only slight discomfort for the patients, the intra- and postoperative morbidity is low, and relapses are rare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nonparasitic hepatic cysts are usually operated on if they cause marked complaints, are approximately 5 cm or larger, cause compression, grow rapidly, or are associated with any complications. Advances in minimally invasive surgical technology have provided an opportunity to treat these cysts laparoscopically. This study analyzes the indications and techniques of laparoscopic treatment for our patients and evaluates the early and late results.
Patients and methods
In the past 10 years, laparoscopic deroofing has been performed in 25 patients at our institution due to nonparasitic hepatic cysts. Nine patients (36%) were male and 16 (64%) were female. The average age was 54.4 years (range, 9–78).
The patients had superior abdominal complaints (pain, discomfort and/or stretching sensation, or meteorism). In five patients, ultrasonic examination revealed hepatic cyst and gallstone simultaneously. CT scan was performed in all cases to assess the characteristics, dimensions, and exact position of the lesion (Fig. 1). Nonparasitic cysts were verified by radiography and were defined to be without calcification in their wall and with no accessorial cysts.
Echinococcus serological test was performed for all patients, and it was negative in all patients undergoing laparoscopy. Liver function tests and blood cell counts were carried out in all patients together with routine laboratory examinations.
The average size of the cysts was 6.9 cm (range, 4.5–20). CT scan verified two cysts in each of two patients and three cysts in one patient. A dominant 10 to 12 cm cyst caused complaints in four patients with polycystic liver disease.
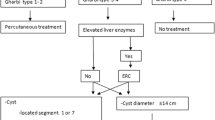
Operation was indicated when the cyst was larger than 5 cm, caused complaints, and grew rapidly. Deroofing of a 4.5 cm cyst was performed due to the simultaneous occurrence of gallstone disease that caused typical complaints. Four patients with polycystic liver disease were underwent deroofing because a large cyst was detected in addition to many small cysts and it caused enlargement of the liver. Laparoscopy was indicated when the cyst was in a superficial position. A cyst positioned in segments VI–VIII of the liver was not a contraindication for the laparoscopic intervention.
We adopted the anterior approach. If the cyst was located in the right lobe of the liver, the position of the four trocars was the same as in the case of a laparoscopic cholecystectomy. When the cyst was located in the left lobe, we used mirror-side trocar positions. The telescope was inserted through the umbilicus port, the two medial ports (10 and 5 mm trocars) were used for the intervention, and a retractor was inserted through the lateral 5 mm port.
In all cases, we resected the complete cyst wall, and it underwent histopathologic examination (Fig. 2). Cysts covered with thin parenchyma can also be fenestrated with the routine use of the ultrasonographic harmonic scalpel (UHS). It is important to examine the cavity of the cyst carefully to preclude both the contingent tissue proliferation and the connection to the biliary ducts. In one patient, eight 3 mm gallstones were found in the cyst, and excision of the cyst was performed by UHS. In all cases, a drain was placed in the cavity of the residual cyst. When the size of the cyst exceeded 10 cm [10 patients (40%)], a flap of omentum was placed in the residual cavity. We also applied Argone plasma coagulation in the cavity of the cyst in cases of extensive cysts. Cholecystectomy was also performed due to associated gallstone disease in five patients.
Results
Intraoperative complications were not detected in our patients. There was no significant bleeding during deroofing of the cyst, which was related to the routine use of UHS. We performed conversion in two patients. In one patient, the cyst strongly adhered to the right diaphragm, and in the second patient the cyst was positioned posterior (segment VII) and it was technically impossible to assess it with laparoscopy. One patient had a fever in the postoperative period that diminished spontaneously. Bile leakage from the cystic cavity occurred in one patient, but no reoperation was performed because the leak was drained through the drain left in the cavity of the cyst, and it ceased a few days following the initial operation. The patient recovered and was discharged on day 37 after the operation. The average hospital stay was 4.7 days (range 3–37).
Patients were followed up regularly. The median follow-up time was 48.7 months (range, 20–127). All patients were free from complaints during the follow-up examinations. The possibility of relapse was routinely controlled with ultrasonography 6 weeks after the surgical intervention and then every 3 months during the first year and once a year thereafter.
We detected relapse of the cyst in two patients, but one of them did not require reoperation since there were no complaints. The other relapsed cyst was treated with another laparoscopic operation. This patient was free of complaints following the operation.
Discussion
The prevalence of hepatic cysts is 4–7% and it increases with age [2, 3, 8–10]. Most hepatic cysts are nonparasitic. They can be solitary or multiplex, depending on the appearance (hepar policysticum) [2, 4, 7–9]. Part of the nonparasitic cysts of the liver is of embryonic origin, and they can evolve in accordance with the abnormal development of the biliary system. In these cases, the cysts often communicate with one of the biliary ducts [2–4, 6, 8, 12]. In two patients, we found that the cyst communicated with the biliary duct, and there were many gallstones inside one cyst.
Hepatic cysts usually do not cause complaints and are often detected by chance with ultrasonography or other radiographic (CT or MRI) examinations [1, 3, 8–10, 12]. They can cause a variety of clinical symptoms when they are sufficiently large. Compression and jaundice can develop depending on their position or size and when they are associated with complications [1–4, 6, 7, 9, 10, 12, 13].
Symptoms include upper abdominal pain, meteorism, nausea, vomiting, and, sometimes, dyspnea. Complications such as bleeding, rupture of the cyst wall, contamination, or torsion can manifest as intense abdominal pain [1–4, 6, 8–13]. The cyst as an elastic mass or the enlarged liver can be palpated during physical examination. This is particularly frequent in cases of polycystic liver disease [1, 7, 10].
Ultrasonography is especially suitable to detect the cysts, but a CT scan is essential as a preoperative examination. The CT scan is suitable for determining the dimensions and position of the cyst(s), and it gives information to clarify the parasitic origin. The nonseptal appearance and the noncalcificated cyst wall (ultrasonography, CT, or MR) indicate the nonparasitic origin of the cyst [1, 3, 4, 6–10, 12, 13]. Among the laboratory findings, eosinophilia and positive Echinococcus serology relate to the parasitic origin [6–8, 10, 13].
Operation is indicated when the cyst causes complaints and the diameter is at least 5 cm or rapid growth is observed. The complications of the cysts are also an indication for operation [3, 4, 6, 8–10, 12, 13].
Deroofing and extirpation of the cyst or resection of the cyst wall are possible surgical treatments for hepatic cysts [4, 5, 8, 10]. Conservative treatment (aspiration, sclerotherapy, and percutaneous drainage) is not often recommended because of the frequent relapses [1, 2, 3, 9, 12, 13].
Increasingly more authors perform laparoscopic deroofing of the cysts [3–9, 12], and use of UHS and Argone plasma increases the safety of deroofing [4, 13]. We routinely perform UHS dissection, which makes fenestration safe without the risk of bleeding. We focus on excising the largest possible part of the cyst wall, and the cavity of the cyst must be examined carefully following fenestration to detect possible communication between the cyst and the biliary tract [3, 4, 6, 8–10, 13]. In all cases it is recommended to examine the excised cyst wall histologically to preclude the possibility of malignancy [2, 3, 7–9, 13]. In the case of large cysts, some authors recommend omentoplasty or Argone plasma coagulation to avoid relapse in the residual cavity [3, 4, 6, 9].
Many authors place a drain into the cavity of the residual cyst at the end of the operation [2–4, 6, 8, 10, 13]. We placed a drain in the cystic cavity in all our patients, and when the cyst was large (>10 cm) we performed an omentoplasty. We also routinely use the Argone plasma coagulator.
Other laparoscopic operations such as cholecystectomy can be performed at the same time as deroofing [4, 8, 10, 13]. We performed laparoscopic cholecystectomy simultaneously in five patients.
Intraoperative complications, such as bleeding and injury of the biliary tract, are infrequent when laparoscopic hepatic cyst fenestration is performed. Early postoperative complications, such as ascites, bile leakage, and pleural effusion, are rare and they may be treated conservatively [3, 4, 8, 9].
In cases of deroofing, the mean hospital stay was 4.7 days (range, 3–7). The late results were good. The majority of patients were free of complaints following the operation, and the relapse rate was negligible. Relapse occurs mainly in patients with polycystic liver disease [3, 4, 8, 9, 13].
In summary, surgery of nonparasitic hepatic cysts is indicated when the cysts are larger than 5 cm in diameter and cause complaints or they are associated with complications. It is important to resect the largest part of the cyst wall and to carefully examine the residual cavity. The application of UHS and the Argone plasma coagulator increases the safety of the operation. Based on the good early and late results, laparoscopic deroofing is recommended for the treatment of nonparasitic liver cysts.
References
Ammori BJ, Jenkins BL, Lim PCM, Prasad KR, Pollard SG, Lodge PJ (2002) Surgical strategy for cystic diseases of the liver in a Western hepatobiliary center. World J Surg 26: 462–469
De Simone M, Cioffi U (1998) Laparoscopic Lin operation for the treatment of polycystic liver disease. Hepato-Gastroenterol 45: 1846–1848
Diez J, Decoud J, Gutierrez L, Suhl A, Merello J (1998) Laparoscopic treatment of symptomatic cysts of the liver. Br J Surg 85: 25–27
Fiamingo P, Tedeschi U, Veroux M, Cillo U, Brolese A, Da Rold A, Madia C, Zanus G, D’Amico DF (2003) Laparoscopic treatment of simple hepatic cysts and polycystic liver disease. Surg Endosc 17: 623–626
Gamal EM, Kiss J (1993). The role of laparoscopy in the diagnosis of focal hepatic diseases. In: Ihász M, Fazekas T, Todua FI (eds) The diagnostic problems of hepatic focal diseases. Akadémiai kiadó, Budapest
Gigot JF, Metairie S, Etienne J, Horsmans Y, van Beers BE, Sempoux C, Deprez P, Materne R, Geubel A, Glinuer D, et al (2001) The surgical management of congenital liver cysts. The need for a tailored approach with appropriate patient selection and proper surgical technique. Surg Endosc 15: 357–363
Giuliante F, D’Acapito F, Vellone M, Giovanni I, Nuzzo G (2003) Risk for laparoscopic fenestration of liver cysts. Surg Endosc 17: 1735–1738
Kanai T, Kenmochi T, Takabayashi T, Hangai N, Kawano Y, Suwa T, Yonekawa H, Miyazawa N (1999) Obstructive jaundice caused by a huge liver cyst riding on the hilum: report of a case. Surg Today Jpn J Surg 29: 791–794
Kitajima Y, Okayama Y, Hirai M, Hayashi K, Imai H, Okamoto T, Aoki S, Akita S, Gotoh K, Ohara H, et al. (2003) Intracystic hemorrhage of a simple liver cyst mimicking a biliary cystadenocarcinoma. J Gastroenterol 38: 190–193
Martin IJ, McKinley AJ, Currie EJ, Holmes P, Garden OJ (1998) Tailoring the management of nonparasitic liver cysts. Ann J Surg 228: 167–172
Payatake AH, Kakkos SK, Solomou EG, Tepetes KN, Karavias DD (1999) Surgical treatment of non-parasitic hepatic cysts: report of 12 cases. Eur J Surg 165: 1154–1158
Szentkereszty Zs, Vágvölgyi A, Kollár S, Takács I, Sápy P (2000) Laparoscopic fenestration of nonparasitic cysts of the liver and spleen. Endoscopia 3: 21–23
Tocchi A, Mazzoni G, Costa G, Cassani D, Bettelii E, Agostini N, Miccini M (2002) Symptomatic nonparasitic hepatic cysts. Options for and results of surgical management. Arch Surg 137: 154–158
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasi Szabó, L., Takács, I., Árkosy, P. et al. Laparoscopic treatment of nonparasitic hepatic cysts. Surg Endosc 20, 595–597 (2006). https://doi.org/10.1007/s00464-005-0206-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0206-6