Abstract
Background
Unrecognized laparoscopic bowel injury has a delayed and covert presentation. Differences in monocyte migration and apoptosis between laparoscopic and open bowel injury were determined.
Methods
For this study, 24 rabbits were divided into laparoscopic (n = 9) and open surgical (n = 9) bowel injury groups and a control group (n = 6) without bowel injury. Bowel injury was created using monopolar electrocautery. The animals were killed 1 day, 1 week, and 2 weeks after surgery. Monocyte migration assay was performed across a modified Boyden chamber. Apoptosis was assessed by DNA fluorescent stain H-33342.
Results
In laparoscopy, monocyte apoptosis was decreased (p < 0.001), and migration was increased (p < 0.05), as compared with the open group. Apoptosis increased over time in both study groups, and was higher than in the control group (p < 0.001). Migration was decreased in both study groups as compared with the control group (p < 0.05)
Conclusions
These results suggest decreased immune system priming with laparoscopic bowel injury, which may contribute to the masking of relevant signs and symptoms of peritonitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic surgery has extended its indications and applications in urology. Not only has laparoscopy had an impact on the manner in which surgery for the kidney and adrenal gland is performed; it also has produced a difference in which complications present and are managed. Bowel injury is the second most common complication of laparoscopic urologic surgery, following vascular injuries. The incidence varies between 0.2% and 1.2% depending on whether upper tract or pelvic surgery is performed [11, 20]. In general, the overall complication rate, including bowel injury, is three-to-four fold higher during the learning curve. The mechanism of injury often involves an electrocautery burn during a hemostatic maneuver [11, 20]. Most of these injuries (69%) are unrecognized at the time of surgery, and most importantly, typical signs and symptoms of peritonitis are not reliably present [4]. Patients with unrecognized laparoscopic bowel injury present with low-grade fever, leukopenia, trocar-site pain, and diarrhea [4]. Diagnosis can be delayed for more than 2 weeks.
Monocytes (blood macrophages) play a key role in the initiation and resolution of inflammation and infection processes. This study aimed to investigate blood monocyte migration and apoptosis to determine whether these parameters are different between laparoscopic bowel injury and open bowel injury. We hypothesized that laparoscopy does not stimulate the cellular immune responses to the same degree that open surgery does.
Materials and methods
For this experiment, 18 New Zealand white rabloits weighing 3 to 3.5 kg were used. after approval from the Institutional Animal Care and Use Committee at Long Island Jewish Medical Center. The animals were quarantined at least 7 days before surgery in a light cycle-controlled environment and had access to water and food until the night before surgery. The rabbits were randomized into three groups: two study groups (n = 9 each) and one control group (n = 6). The study groups consisted of laparoscopic and open bowel injuries. The control group was divided in two treatment arms: without bowel injury: pneumoperitoneum creation alone (n = 3) and sham laparotomy (n = 3). All the surgeries required 1 h. The animals were killed 1 day, 1 week, and 2 weeks after surgery.
Surgical technique
For the laparoscopic group, pneumoperitoneum was induced in standard fashion using a Veress needle. Once the intraabdominal pressure reached 15 mm Hg, an 11-mm trocar was placed. A second 5-mm trocar was inserted under direct vision. Pneumoperitoneum was maintained for 1 h. A 30-watt monopolar electrocautery was applied to the serosa of the bowel to create a full-thickness thermal injury. All the injuries required performed at the same location: the hepatic flexure of the ascending colon.
In the open group, a 6-cm midline laparotomy was created, and bowel injury was accomplished in the same way as described earlier for the laparoscopic group. The abdominal incision was closed after 1 h. At the time the animals were killed, the injured bowel segment was harvested for microscopic evaluation. Hematoxylin and eosin staining confirmed full-thickness wall necrosis.
Cell separation
At the time the animals were killed, 40 ml of peripheral blood was collected from each animal in heparinized tubes. Heparinized whole blood was layered on top of a density-gradient material (Histopaque 1119 and 1077 solutions; Sigma, St. Louis, MO) and spun at 800 g for 30 min at 18°C. The mononucleocyte buffy coat layer containing monocytes and lymphocytes was aspirated and transferred to tissue culture plates. Monocytes were separated from lymphocytes by adhesion method after incubation at 37°C for 1 h. Nonadherent lymphocytes were transferred to other plates and adherent monocytes were washed twice and centrifuged.
Monocyte apoptosis
Morphologic evaluation of monocyte apoptosis was performed by staining cells with H-33342 (Molecular Probes, Portland, OR, USA) and propidium iodide (PI; Sigma-Aldrich, St. Louis, MO). In live cells with intact outer walls, H-33342 stains the nuclei by regular fluorescence, whereas apoptotic cells have increased fluorescence and fragmented nuclei. Propidium iodide enters only cells with damaged cell walls and stains necrosed cells pink.
Cells were prepared from control, laparoscopy, and open surgery groups. After 45 min of incubation, the cells were treated with H-33342 (1 μg/ml) for 10 min and again incubated at 37°C. Subsequently, Propidium iodide at a final concentration of 1 μg/ml was added to each well. The percentages of live, apoptotic, and necrosed cells were recorded in eight random fields by two observers unaware of the experimental conditions. The mean value for the percentage of apoptotic cells was calculated.
Macrophage migration
Aliquots of 200 μl of buffer of macrophage chemoattractant containing Macrophage Chemoattractant Protein 1 (MCP-1) mg/ml were added to the lower compartment of a modified Boyden chamber. Equal numbers of macrophages (1 × 105 cells/ml) in Dulbecco modified Eagle medium were added to the upper compartment of the chamber and kept at 37°C for 1 h. At the end of the incubation period, cells from the upper surface of the filter were wiped off with a cotton swab. The lower surface of the filter was stained with Diff Quick (Baxter, Chicago, IL, USA). The number of migrated macrophages were counted under light microscope in eight randomly selected fields. The mean number of macrophages was calculated.
Statistical analysis
Analysis of variance (ANOVA) was used to assess differences between the three groups. Statistical significance was defined as a p valve less than 0.05.
Results
The control group animals underwent pneumoperitoneum creation and sham laparotomy, respectively, without bowel injury. Considering the small number of animals allowed by the experimental protocol, only one animal was included in each control arm at every time point, for a total of three animals per control arm. The results were similar for both the laparoscopic and open control arms, so they were combined for statistical purposes. Our study was not powered to detect differences between control subjects. However, these differences are unlikely to be clinically significant (e.g., narrow variation, see later). Accordingly, previous studies from our laboratory showed that pneumoperitoneum alone and open midline laparotomy without bowel injury did not produce statistically significant differences in systemic and peritoneal white blood cell counts [9].
Similar macroscopic findings in the laparoscopy and open study groups
The bowel injury contracted over time, and at 1 week, bowel perforation was present in all animals that had fecal spillage contained within dense adhesions. Overall, there were no significant differences in gross pathology between the laparoscopic and open bowel injury groups. In addition, there were no abnormal macroscopic peritoneal findings in either control group. All the animals survived until the scheduled killing date.
Monocyte apoptosis (Table 1)
Monocyte apoptosis increased in both study groups over time. The mean percentage of apoptosis ± standard error of the mean (SEM) in laparoscopic bowel injury group was 7 ± 0.61 at 1 day, 13.92 ± 1.13 at 1 week, and 72.75 ± 3.43 2 weeks (p < 0.001). Similarly, in the open bowel injury group, the mean percentage of apoptosis was 15.62 ± 1.35 at 1 day, 69.12 ± 2.69 at 1 week, and 93.62 ± 1.53 at 2 weeks (p < 0.001). Both study groups had a higher percentage of apoptosis than the control group at each time point (p < 0.001). Control values were not statistically different over time. The mean control percentage of apoptosis was 0.5 ± 0.49 at 1 day, 1 ± 0.32, at 1 week, and 3.62 ± 1.55) at 2 weeks (p was not significant). Importantly, the laparoscopic group demonstrated a lower monocyte apoptosis rate than the open surgery group at each time point.
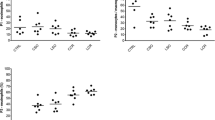
Monocyte migration (Figure 1)
The laparoscopy group demonstrated higher monocyte migration than the open surgery group at each time point. The mean number of macrophages per field was 3.71 ± 0.45 vs 2.25 ± 0.47 at 1 day 10.14 ± 3.28 vs 2.33 ± 0.33, at 1 week and 8 ± 0.82 vs 4.18 ± 0.95, at 2 weeks (p < 0.05).
Monocyte migration was lower in both study groups than in the control group at 1 day and 2 weeks (p < 0.05), and was found only in the open surgery group at 1 week (p < 0.05). Control values were not statistically different over time. The mean number of macrophages per field was 10.25 ± 0.75 at 1 day, 10.92 ± 1.33 at 1 week, and 13.42 ± 1.36 at 2 weeks (p was not significant).
Discussion
Laparoscopic bowel injury has a covert and delayed presentation, as compared with open bowel injury [4]. Animal models of laparoscopic bowel injury have not been developed. Several studies, however, were designed to mimic preexisting intraperitoneal infection and the influence of pneumoperitoneum on abdominal sepsis [19]. These studies tried to address a relevant but different clinical problem regarding the safety of laparoscopy for a previously infected peritoneal cavity (i.e., perforated appendicitis, perforated peptic ulcer disease). We developed the first animal model of laparoscopic bowel injury.
Surgical intervention triggers a series of alterations in the immune system mainly determined by the magnitude of surgical insult [13]. Several studies have proposed that systemic immunity is better preserved after laparoscopy than after open surgery. In our experiment, we demonstrated lower monocyte apoptosis and higher migration in laparoscopic bowel injury group than in the open group. Do these findings correlate with immune system depression or activation after laparoscopic bowel injury? And how would these differences translate clinically?
Monocytes play a major role in both innate and acquired immunity to infections. Monocytes have various functions including phagocytosis, protein expression, cytolysis, and antigen presentation. Apoptosis is the process by which cells die in a controlled manner through the interaction of a death factor and its receptor. Indeed, apoptosis is important in shaping or remodeling tissues to maintain their integrity and specialized functions during development and wound healing. It also contributes to the development of inflammation and/or its resolution after an injury or infection [12]. Immune cells are no exception to this homeostatic process. Programmed cell death (apoptosis) can be induced by a number of physiologic and pathologic factors including Fas-Fas ligand interaction, tumor necrosis factor, ceramide, and reactive oxygen species [14]. On the other hand, soluble factors other than granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor including interleukin-6 inhibit or delay apoptosis [3]. Bacterial infection induces immune cell apoptosis, which is mediated by the direct activation of caspases in the absence of death receptor coupling. Bacterial induction of apoptosis may be attributal to bacterial factors or to the result of host immune responses [8]. In the current animal model, peritoneal bacterial infection induced an increase in monocyte apoptosis over time in both study groups, as expected.
Recent evidence has suggested that cells dying by apoptosis are actively involved in immunosuppression in various circumstances. Apoptotic cells could inhibit the expression of CD69 during T-cell activation [18]. Once phagocytosed, either apoptotic cells or phagocytic cells produce, transforming growth factor β1, prostaglandin E2, or interleukin 10 which can down-regulate proinflammatory cytokines [10]. In addition, apoptotic monocytes lose their inflammatory function, and this loss contributes to the immunomodulation state.
However, although the default outcome of apoptotic cells may be tolerance induction, the consequences may vary depending on the local environment. Several reasons may account for the conflicting results at antigen presentation of apoptotic cells. In an infection model, infected cells may undergo apoptosis at a rate that outpaces the phagocytic ability of scavenging cells, and residual apoptotic cells may develop to a late stage resembling necrotic cells that activate the immune system [18]. In addition, infection itself may be accompanied by a large number of proinflammatory cytokines. Overproduction of these cytokines can promote maturation of dendritic cells or macrophages, which would serve to shift the immune balance to a priming response [1]. Since the optimal number of apoptotic cells for inducing tolerance is unknown, a large number of apoptotic cells may exceed scavenging . capacity and may cause residual apoptotic cells to be seen as “danger signals,” and thus, promoting priming of the immune system [16]. Furthermore, defects in phagocytosis of apoptotic cells may contribute to the pathogenesis of inflammatory states and activation of the immune system [7]. Finally, during infection, immune cell apoptosis can have advantages or disadvantages depending on the pathogen specificity and the cell population affected [21]. This puts our results into a new perspective showing that higher monocyte apoptosis in open bowel injury would prime the peripheral immune system to a larger extent than laparoscopic bowel injury.
The ability of monocytes to be chemotactically attracted to the site of initial microbial invasion or to an inflammatory focus is fundamental for the full activation of the immune response that follows [17]. This study reports for the first time that in vitro monocyte migration ability is less after open bowel injury than after laparoscopy. Many cytokines mediate or contribute to the inhibitory effect on macrophage migration, such as interleukin-4, interferon-?, and most importantly macrophage migration inhibitory factor (MIF). As an important proinflammatory cytokine, MIF is proposed to be the physiologic counterregulator of the glucocorticoid immunosuppressive action [2]. The term MIF was based on the ability of this factor to inhibit the random migration of cultured guinea pig peritoneal exudate macrophages in capillary tubes [5]. MIF was subsequently found to correlate with general macrophage activation functions, including adherence, spreading, phagocytosis, and enhanced tumoricidal activity [15]. Also, MIF was discovered to be a pituitary mediator of systemic stress response [6]. In addition, inferleukin-4 and interferon-γ are known proinflammatory cytokines. Therefore, cytokine-mediated migration inhibition may be accompanied by global activation of the immune system. Consequently, a higher level of immune activation is expected in the case of open bowel injury.
In conclusion, laparoscopic bowel injury does not appear to prime systemic cellular immunity sufficiently, which probably is a critical factor in the development of typical signs and symptoms of peritonitis after bowel injury.
References
ML Albert B Sauter N Bhardwaj (1998) ArticleTitleDendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs Nature 392 86–89 Occurrence Handle10.1038/32183 Occurrence Handle1:CAS:528:DyaK1cXhvVOntrw%3D Occurrence Handle9510252
JA Baugh R Bucala (2002) ArticleTitleMacrophage migration inhibitory factor Crit Care Med 30 S27–S35 Occurrence Handle10.1097/00003246-200201001-00004 Occurrence Handle1:CAS:528:DC%2BD38XhtFWns7Y%3D
WL Biffl EE Moore FA Moore CC Barnett SuffixJr VS Carl VN Peterson (1996) ArticleTitleInterleukin-6 delays neutrophil apoptosis Arch Surg 131 24–29 Occurrence Handle1:CAS:528:DyaK28Xotl2kuw%3D%3D Occurrence Handle8546573
JT Bishoff ME Allaf W Kirkels RG Moore LR Kavoussi F Schroder (1999) ArticleTitleLaparoscopic bowel injury: incidence and clinical presentation J Urol 161 887–890 Occurrence Handle10.1097/00005392-199903000-00039 Occurrence Handle1:STN:280:DyaK1M7ksFejtg%3D%3D Occurrence Handle10022706
BR Bloom B Bennett (1966) ArticleTitleMechanism of a reaction in vitro associated with delayed-type hypersensitivity Science 153 80–82 Occurrence Handle1:CAS:528:DyaF28XksVWmt7g%3D Occurrence Handle5938421
R Bucala (1996) ArticleTitleMIF rediscovered: cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response FASEB J 10 1607–1613 Occurrence Handle1:CAS:528:DyaK2sXmslKmuw%3D%3D Occurrence Handle9002552
L Dini P Pagliara EC Carla (2002) ArticleTitlePhagocytosis of apoptotic cells by liver: a morphological study Microsc Res Tech 57 530–540
DH Dockrell (2001) ArticleTitleApoptotic cell death in the pathogenesis of infectious diseases J Infect 42 227–234 Occurrence Handle10.1053/jinf.2001.0836 Occurrence Handle1:STN:280:DC%2BD3Mvps1eisA%3D%3D Occurrence Handle11545564
El-Hakim A, Chiu Ky, Sherry B, Bhuiya T, Smith AD, Lee BR (2004) Peritoneal and systemic inflammatory mediators of laparascopic bowel injury in a rabbit model. J Urol 172: 1515–1519
VA Fadok DL Bratton A Konowal PW Freed JY Westcott PM Henson (1998) ArticleTitleMacrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF J Clin Invest 101 890–898 Occurrence Handle1:CAS:528:DyaK1cXht1Wrt70%3D Occurrence Handle9466984
D Fahlenkamp J Rassweiler P Fornara T Frede SA Loening (1999) ArticleTitleComplications of laparoscopic procedures in urology: experience with 2,407 procedures at 4 German centers J Urol 162 765–770 Occurrence Handle10.1097/00005392-199909010-00038 Occurrence Handle1:STN:280:DyaK1MzosFGntg%3D%3D Occurrence Handle10458362
NC Franc (2002) ArticleTitlePhagocytosis of apoptotic cells in mammals, caenorhabditis elegans, and Drosophila melanogaster: molecular mechanisms and physiological consequences Front Biosci 7 1298–1313
JE Hartley BJ Mehigan JR Monson (2001) ArticleTitleAlterations in the immune system and tumor growth in laparoscopy Surg Endosc 15 305–313 Occurrence Handle10.1007/s004640000240 Occurrence Handle1:STN:280:DC%2BD38%2FgvFyitg%3D%3D Occurrence Handle11344435
S Maher D Toomey C Condron D Bouchier-Hayes (2002) ArticleTitleActivation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack, Inmunol Cell Biol 80 131–137 Occurrence Handle10.1046/j.1440-1711.2002.01068.x Occurrence Handle1:CAS:528:DC%2BD38XjtVGgtL8%3D
CF Nathan HG Remold JR David (1973) ArticleTitleCharacterization of a lymphocyte factor which alters macrophage functions J Exp Med 137 275–290 Occurrence Handle10.1084/jem.137.2.275 Occurrence Handle1:CAS:528:DyaE3sXpsF2htQ%3D%3D Occurrence Handle4119587
P Rovere C Vallinoto A Bondanza MC Crosti M Rescigno P Ricciardi-Castagnoli C Rugarli AA Manfredi (1998) ArticleTitleBystander apoptosis triggers dendritic cell maturation and antigen-presenting function J Immunol 161 4467–4471 Occurrence Handle1:CAS:528:DyaK1cXntVWhurk%3D Occurrence Handle9794367
E Schiffmann (1982) ArticleTitleLeukocyte chemotaxis Annu Rev Physiol 44 553–568 Occurrence Handle10.1146/annurev.ph.44.030182.003005 Occurrence Handle1:CAS:528:DyaL38XhsF2kt7w%3D Occurrence Handle7041804
EW Sun YF Shi (2001) ArticleTitleApoptosis: the quiet death silences the immune system Pharmacol Ther 92 135–145 Occurrence Handle10.1016/S0163-7258(01)00164-4 Occurrence Handle1:CAS:528:DC%2BD38Xitlejs7w%3D Occurrence Handle11916534
EM Targarona C Balague MM Knook M Trias (2000) ArticleTitleLaparoscopic surgery and surgical infection Br J Surg 87 536–544 Occurrence Handle10.1046/j.1365-2168.2000.01429.x Occurrence Handle10792307
G Vallancien X Cathelineau H Baumert JD Doublet B Guillonneau (2002) ArticleTitleComplications of transperitoneal laparoscopic surgery in urology: review of 1,311 procedures at a single center J Urol 168 23–26 Occurrence Handle10.1097/00005392-200207000-00007 Occurrence Handle1:STN:280:DC%2BD38zgvVahtg%3D%3D Occurrence Handle12050484
E Zuniga E Acosta Rodriguez C Montes A Gruppi (2002) ArticleTitleLymphocyte apoptosis associated to infections Medicina (Buenos Aires) 62 189–196 Occurrence Handle1:CAS:528:DC%2BD38XovVWktbc%3D
Acknowledgment
This work was supported by a grant from the New York Academy of Medicine, The Ferdinand C. Valentine Award.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Hakim, A., Aldana, J.P., Reddy, K. et al. Laparoscopic bowel injury in an animal model: monocyte migration and apoptosis. Surg Endosc 19, 484–487 (2005). https://doi.org/10.1007/s00464-004-8152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-004-8152-2