Abstract
Background
Immune function is better preserved by laparoscopic versus conventional surgery. Numerous mediators of the systemic trauma response are synthesized and/or regulated by the liver. However, it has been stated that the advantages of laparoscopic surgery are no more obvious when conventional operations are performed via mini-laparotomy. We set out to compare the impact of laparoscopy and mini- and full laparotomy on the hepatic macrophage populations.
Methods
Male Lewis rats were subjected to anesthesia alone (control), mini-laparotomy (1 cm), full laparotomy (7 cm), or laparoscopy for 60 min. Endpoints were the total protein in the peritoneal lavage fluid, hepatic ED-1 cells (recruited monocytes), hepatic ED-2 cells (Kupffer cells), the expression of OX-6 in the liver, and C-reactive protein (CRP) in plasma.
Results
Protein in the peritoneal lavage fluid increased significantly after all interventions. Full laparotomy was accompanied by an enhancement in ED-1-positive monocytes in the liver parenchyma compared to all other groups (p < 0.001). Mini- and full laparotomy led to an increase in ED-2-positive Kupffer cells (p < 0.001). Laparoscopy did not affect the number of monocytes/macrophages. There was no significant alteration of OX-6 expression in either group. No change in the cellular composition in the periportal fields was observed. The CRP plasma levels did not significantly differ between groups.
Conclusions
Laparoscopy completely prevents hepatic macrophage populations from expansion and normal cell disposition is preserved. Laparotomy, irrespective of incision size, increases the number of Kupffer cells. Moreover, full laparotomy, but not mini-laparotomy or laparoscopy, causes an increase in hepatic monocyte recruitment. The regulating pathways after surgery differ from other immunologic challenges, such as sepsis, in which immunocompetent cells accumulate and are stimulated in the periportal fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The response to surgical interventions is initiated by a break in the protective epithelial and endothelial barriers, which results in the activation of numerous pathways of the innate immune system leading to a systemic inflammatory response. Humoral mediators are directly released by the injured tissue or are, to a greater extent, synthesized by the liver [6, 9, 21]. In this regard, Kupffer cells, the resident macrophage population of the liver, play a key role in modeling the local hepatic immune response [5, 14]. Kupffer cells comprise the largest fraction (>50%) of hepatic tissue macrophages. They are predominantly localized in the lumen of hepatic sinusoids and are anchored to the endothelium by long cytoplasmic processes with a pronounced endocytic and phagocytic capacity [3]. They are responsible for the production of potent mediators of the inflammatory response, such as superoxide anions, nitric oxide, interleukin (IL)-6, and tumor-necrosis factor (TNF)-α, and are involved in the regulation of cytokine release by the liver [13].
Numerous studies have demonstrated that laparoscopic surgery leads to less post operative pain and a faster recovery [7, 22]. This was associated with lower serum levels of various cytokines, such as IL-1β, IL-6, and TNF-α [8, 12, 22, 28], and with lower levels of IL-6 in the peritoneal cavity [28]. The underlying mechanisms have not been clarified, and the advantages of laparoscopic surgery have been attributed to a lower degree of surgical trauma [1], effects of carbon dioxide used for pneumoperitoneum [25, 31], and air contamination that takes place during conventional surgery [31]. However, McMahon et al. [18] demonstrated that mini-laparotomy, compared to laparoscopy, similarily preserves immune function and leads to similar clinical results. Therefore, it has been a matter of debate whether the advantages of laparoscopic surgery are still obvious when conventional operations are performed via mini-laparotomy [18].
The aim of this study was to examine the impact of laparoscopy versus mini-laparotomy and full laparotomy on the hepatic microenvironment, particularly the liver-associated macrophage subpopulations.
Materials and methods
The Local Animal Care Ethical Committee Hannover (509c-42502-01/405) approved the protocol of the study.
Study design
Male Lewis rats (250–300 g, Central Animal Laboratory, Hannover Medical School) of specific pathogen-free housing were used. They were given 2 days to recover from transport stress and were kept on a 12-h light/dark cycle in a conventional, nonbarrier rodent housing unit in polycarbonate cages. Water and standard rat laboratory diets (V1324-300, SSNIFF, Soest, Germany) were supplied ad libitum until 12 h before the experiment. To ensure stress-free anesthetic application, an intravenous cannula was implanted 5 days before the experiment started. The catheter was placed as described previously [19]. Briefly, a catheter (Braun, Melsungen, Germany) was subcutaneously tunneled from the top of the head down to the neck and introduced into the right external jugular vein. The animals were randomized into four groups of six animals: anesthesia alone as the control group, laparoscopy, mini-laparotomy (1 cm), or full laparotomy (7 cm) for 60 min. The animals were killed under general anesthesia after 24 h.
Anesthesia and positioning
Intravenous anesthesia was performed with approximately 2.5 ml of anesthetic solution (0.35 ml ketamine hydrochloride, Ketamin 10%, WDT, Garbsen, Germany, and 0.05 ml medetomidine hydrochloride, Dormitor, Espon, Finland). After induction of anesthesia, the animals were shaved in the abdominal region. They were placed in a supine position on a specially designed small animal operating table with an integrated heated water bath for keeping the continuously measured body temperature constant.
Surgical techniques
All interventions were performed under standard sterile conditions. For laparoscopy, standard reusable equipment was used (Storz, Tuttlingen, Germany). Laparoscopy was performed similarly to the technique described by Gutt et al. [10]. The optic and two working trocars were introduced into the abdominal cavity. The pneumoperitoneum was kept at 5 mmHg and was desufflated after 60 min. The trocar wounds were closed with resorbable sutures.
Midline laparotomy consisted either of mini-laparotomy of 1 cm length or of full laparotomy of 7 cm length. The abdominal cavity was kept open by a spreader without further manipulation for 60 min. The incisions were closed in two layers with resorbable and unresorbable sutures.
Total protein in peritoneal lavage fluids
The amount of protein in the peritoneal lavage fluid was determined using a colorimetric assay (Bio-Rad, Munich, Germany) for protein concentration following the solution of the detergent. The assay was carried out according to the manufacturer’s instructions. The reaction is based on Lowry et al.’s [16] assay. Values were obtained as micrograms of protein per millimeter of peritoneal lavage fluid.
Immunohistochemistry
Liver cryostat sections of 6-μm thickness were air-dried, wrapped in aluminum foil, and stored at −20°C. To determine the macrophage phenotype, the slides were fixed in acetone for 10 min. After rinsing with TBS–Tween (Tris buffered saline supplemented with Tween-20), the sections were incubated with the primary antibodies (monocytes, ED-1 1:1000; Kupffer cells, ED-2 1:200; or MHC-II, OX-6 1:1000; Serotec, Duesseldorf, Germany) for 30 min in a moist chamber at room temperature. They were rinsed with TBS–Tween, followed by incubation for 30 min with the bridging antibody (100 μl of DAKO Z 0259, 1:50 in 5% rat serum in phosphate-buffered saline, rabbit anti-mouse antibody). After washing with TBS–Tween, the APAAP complex (100 μl of DAKO D 0651, 1:50 in TBS–Tween, mouse) was added, and the sections were again incubated for 30 min. The last two steps were repeated for an additional 15 min. To visualize the antibodies, a mixture of APPP substrate (DAKO, Hamburg, Germany) and fast blue (F 3378, Sigma, Darmstadt, Germany) in Tris buffer (pH 8.2) was used for 10 min. Finally, counterstaining was performed with Mayer’s hemalaun (Merck, Darmstadt, Germany) and the sections were then mounted in glycergel medium (DAKO). Method specificity was tested by omission of the primary antibody and by corresponding isotype controls. The antibody-labeled cells were stained blue. Each experimental group was composed of six animals. For quantitative analysis, six visual fields/section (0.8 mm2) for each animal were examined blinded to the treatment conditions.
C-reactive protein in plasma
The C-reactive protein (CRP) was determined by a competitive legend-binding assay (CLBA). All CLBAs were done with enzyme-linked immunosorbent assay (ELISA) plates (microlon, 96 well, high binding, Greiner, Frickenhausen, Germany). All plates were incubated at room temperature on a microtiter plate shaker (400 rpm). The washing buffer was Dulbecco phosphate-buffered saline without Ca2+ and Mg2+ (BBS, pH 7.35) and with 0.1% (v/v) Tween 20 (Sigma-Aldrich, Taufkirchen, Germany, PBST). After the incubation steps, the plates were washed three times with the washing buffer (PBST) using the Nunc Immuno-Washer 12 (Nunc, Wiesbaden, Germany).
Next, the plates were coated with phosphocholine-conjugated bovine serum albumin (PC-BSA) diluted in 0.1 M NaHCO3 (150 μl/well, 0.08 μg/ml PC-BSA and 2 μg/ml BSA 1 h incubation). After washing, the plates were incubated for 2 h with standard or diluted samples (100 μl/well) and purified human CRP-conjugated biotin (CRP–biotin, 50 μl/well, 0.5 μg/ml). The standard was purified human CRP (Dunn Labortechnik GmBH, Asbach, Germany) and the standard concentration ranged from 2.00 to 0.03 μg/ml. The samples were diluted 1:100 and 1:1000. The assay buffer for dilution of the standard, the plasma samples, and CRP–biotin was 20 mM Tris/HCl (pH 8.0), 0.1% Tween 20 (v/v), 0.1% BSA (w/v), an 5 mM Ca2+. The detection reagent was Avidin conjugated with horseradish peroxidase (Perbio, Bonn, Germany), which was diluted 1:10,000 in assay buffer without Ca2+ (150 ml/well, 0.5-h incubation). The horseradish peroxidase was determined on the solid phase with the substrate 3,3′, 5,5′-tetramethylbenzidine (TMB, 150 μl/well). The substrate reaction was stopped with 1 M H2SO4 (50 μl/well). The optical density was measured with microplate ELISA reader at 450 nm.
For the CLBA, the intraassay variation was less than 10% and the interassay variation less than 15%.
Statistical analysis
Results are presented as mean ± SEM. The Statistical Package for the Social Sciences was used to analyze data. After using analysis of variance on ranks, overall differences between groups were analyzed by the Kruskal-Wallis variance test. In case of a positive result, differences between two groups were analyzed by the Mann-Whitney U test. p < 0.05 was considered significant.
Results
Total protein in peritoneal lavage fluids
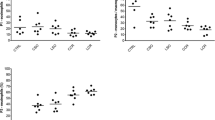
The total protein concentration in peritoneal lavage fluids was significantly enhanced after mini-laparotomy (0.804 ± 0.082 μg/ml), full laparotomy (0.755 ± 0.100 μg/ml), and laparoscopy (0.683 ± 0.064 μg/ml) compared to the control (0.331 ± 0.022 μg/ml) (p < 0.05) (Fig. 1).
Immunohistochemistry
The number of recruited ED-1-positive monocytes in the liver parenchyma was significantly enhanced by full laparotomy (93.8 ± 3.9 cells/mm2) compared to both control (58.7 ± 4.9 cells/mm2) and laparoscopy (58.6 ± 5.4 cells/mm2) (p < 0.001) but not after mini-laparotomy (76.8 ± 5.4 cells/mm) (Fig. 2). ED-1-positive cells were equally distributed in the liver parenchyma of all animals, and no areas of accumulation were detectable by the increased subpopulation (Fig. 3).
ED-2-positive cells, which represent Kupffer cells, were significantly increased after mini-laparotomy (94.6 ± 2.1 cells/mm2) and full laparotomy (96.0 ± 4.2 cells/mm2) compared to control animals (57.4 ± 4.6 cells/mm2) (p < 0.001). There were no significant differences in the number of Kupffer cells in animals after laparoscopy (73.9 ± 3.8 cells/mm2) compared to control animals (Figs. 4 and 5).
ED-2-positive resident hepatic macrophages (Kupffer cells) (mean ± SEM, n = 6)
after anesthesia alone (control), mini-laparotomy, full laparotomy, or laparoscopy. Mini- and full laparotomy caused a statistically significant increase in ED-2-positive macrophages in the liver versus control (p < 0.001).
The number of OX-6-expressing cells in the liver parenchyma was not significantly different between control animals (36.6 ± 8.2 cells/mm2) and animals after mini-laparotomy (38.8 ± 5.8 cells/mm2), full laparotomy (42.7 ± 4.9 cells/mm2), or laparoscopy (29.3 ± 3.7 cells/mm2). Neither morphological changes nor an increase in OX-6-expressing cells were observed in the areas of the periportal fields (Fig. 6).
C-reactive protein in plasma
The level of CRP in plasma of control animals was within the physiological range (497 ± 56 μg/ml). There were no significant differences in the mean plasma CRP levels 24 h after mini-laparotomy (561 ± 16 μg/ml), full laparotomy (582 ± 24 μg/ml), laparoscopy (531 ± 27 μg/ml), or anesthesia (control) (Fig. 7).
Discussion
Surgical interventions lead to local vascular dilatation and a break in epithelial and endothelial barriers, which is associated with an increase in total protein in the peritoneal cavity. Our experiments showed that the protein concentration in the peritoneal cavity was elevated twofold after any of the surgical procedures investigated. Thus, no difference in the increased permeability was observed between mini- or full laparotomy and laparoscopy.
The production of acute phase proteins and the activation of the cytokine network by the hepatic microenvironment are essential parts of the systemic inflammatory trauma response [26]. Numerous mediators are released by hepatic cells, particularly Kupffer cells, regulating the early phase of the systemic immune reaction [2]. In our experiments, the hepatic cell compartment was histologically unaffected after laparoscopy. No changes in the number of resident ED-2-positive Kupffer cells and ED-1-positive recruited monocytes compared to controls were detected. Mini-laparotomy and full laparotomy induced a significant increase in the number of Kupffer cells. Full laparotomy had an even greater impact on the hepatic macrophage population since the number of resident macrophages and the number of recruited monocytes were increased. It may be concluded that the immunological advantages of laparoscopic surgery become evident when conventional surgery is performed via mini-laparotomy.
An enhanced expression of OX-6 indicates macrophage activation [24]. In the current study, no enhanced expression of OX-6 was detected after any of the investigated surgical interventions. Therefore, it may be assumed that the trauma associated with a laparoscopic or conventional approach to the abdominal cavity without a subsequent operation is not serious enough to activate hepatic macrophages.
However, under physiological conditions, OX-6 is expressed by only 40–60% of Kupffer cells [15]. With no difference in the number of activated Kupffer cells between the experimental groups in this study, but an enlarged macrophage population after laparotomy, it may be speculated that conventional surgery induces a distinct differentiation pathway compared to laparoscopy. Previous data suggest an immunosuppressive effect of laparoscopy. Our data indicate that laparoscopy does not suppress, but preserves, baseline functions of Kupffer cells in the liver. Furthermore, it has been reported that OX-6 also stains endothelial cells of postcapillary venules and other cells scattered throughout the liver parenchyma [4]. We combined OX-6 staining and cell morphology in our study and confirmed the labeling of macrophages in the sections.
A lack of macrophage activation in our study is in accordance with clinical findings of Nguyen et al. [20]. The authors demonstrated that laparoscopic gastric bypass resulted in transient postoperative elevation of hepatic transaminases but did not adversely alter hepatic function to any greater extent than did conventional gastric bypass. Vittimberga et al. [29] confirmed these findings in an experimental setting. No significant differences in the function of isolated Kupffer cells were detected, and hepatic cytokine production after stimulation with lipopolysaccharide was similar after laparotomy, laparoscopy, and in controls. Vittimberga et al. confirmed no alteration of cytokine release by purified Kupffer cells but found differences in the activation of intracellular pathways. The authors demonstrated that the activation of the p38 mitogen-activated protein kinase, an important intracellular messenger, was more pronounced after laparotomy than after laparoscopy. It appears that immunostimulating mediators are different in composition and quantity after laparotomy and laparoscopy. Our study indicates that such mediators may enter circulation and lead to an expansion of the hepatic macrophage population after laparotomy but not after laparoscopy. The levels of CRP in plasma were not elevated in our experiments. This confirms that hepatic acute phase protein release had not been activated.
The periportal fields and OX-6 expression were not affected by any surgical intervention investigated, as would be expected within the scope of other immunological challenges, such as infection [23]. In addition, the enlarged macrophage population after laparotomy was evenly distributed in the parenchyma without specific areas of accumulation. Many immunologically mediated liver diseases, as well as acute rejection after liver transplantation, are characterized by cell infiltration, especially in the periportal fields. A major increase in OX-6 expression in these areas, as well as an activation of Kupffer cells, was observed [11, 17, 27]. This suggests that the regulating mechanisms of hepatic inflammatory responses are induced differently after infection and surgical trauma.
To our knowledge, this study confirmed for the first time that immunological advantages of laparoscopy are still obvious when compared with mini-laparotomy. Laparoscopy prevents the macrophage population from expanding within the liver and preserves normal cell distribution and baseline function of the hepatic macrophages. Laparotomy, irrespective of the incision size, increases the number of Kupffer cells. Full laparotomy increases hepatic monocyte recruitment. Further studies are required to understand the underlying mechanisms and regulatory pathways that lead to a better preserved immune function after laparoscopy.
References
JD Allendorf M Bessler RL Whelan M Trokel DA Laird MB Terry MR Treat (1997) ArticleTitlePostoperative immune function varies inversely with the degree of surgical trauma in a murine model Surg Endosc 11 427–430 Occurrence Handle10.1007/s004649900383 Occurrence Handle9153168
A Ayala PJ O’Neill SA Uebele CD Herdon ICH Chaudry (1997) ArticleTitleMechanism of splenic immunosuppression during sepsis: key role of Kupffer cell mediators J Trauma 42 882–888 Occurrence Handle9191670
L Bouwens M Baekeland R Zanger ParticleDe E Wisse (1986) ArticleTitleQuantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver Hepatology 6 718–722 Occurrence Handle3733004
DS Collier JA Pain DG Wight P Lovat ME Bailey (1986) ArticleTitleThe Kupffer cell in experimental extrahepatic cholestasis in the rat — a light microscopy, immunohistochemical and electron microscopy study J Pathol 150 187–194 Occurrence Handle10.1002/path.1711500307 Occurrence Handle3543274
T Decker ML Lohmann-Matthes U Karck T Peters K Decker (1989) ArticleTitleComparative study of cytotoxicity, tumor necrosis factor, and prostaglandin release after stimulation of rat Kupffer cells, murine Kupffer cells, and murine inflammatory liver macrophages J Leukoc Biol 45 139–146 Occurrence Handle2783725
JF Dhainaut N Marin A Mignon C Vinsonneau (2001) ArticleTitleHepatic response to sepsis: interaction between coagulation and inflammatory processes Crit Care Med 29 IssueIDsuppl S42–S47 Occurrence Handle10.1097/00003246-200107001-00016 Occurrence Handle11445733
M Garcia-Caballero C Vara-Thorbeck (1993) ArticleTitleThe evolution of postoperative ileus after laparoscopic cholecystectomy. A comparative study with conventional cholecystectomy and sympathetic blockade treatment Surg Endosc 7 416–419 Occurrence Handle10.1007/BF00311733 Occurrence Handle8211620
F Glaser GA Sannwald HJ Buhr C Kuntz H Mayer F Klee C Herfarth (1995) ArticleTitleGeneral stress response to conventional and laparoscopic cholecystectomy Ann Surg 221 372–380 Occurrence Handle7726672
Grande M, Tucci GF, Adorisio O, Barini A, Rulli F, Neri A, Franchi F, Farinon AM (2002) Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc, DOI: 10.1007/s0046400190425, November 12
CN Gutt V Riemer C Brier R Berguer V Paolucci (1998) ArticleTitleStandardized technique of laparoscopic surgery in the rat Dig Surg 15 135–139 Occurrence Handle10.1159/000018606 Occurrence Handle9845575
K Imada Y Fukuda Y Koyama I Nakano M Yamada Y Katano T Hayakawa (1997) ArticleTitleNaive and memory T cell infiltrates in chronic hepatitis C: phenotypic changes with interferon treatment Clin Exp Immunol 109 59–66 Occurrence Handle10.1046/j.1365-2249.1997.4281327.x Occurrence Handle9218825
T Kloosterman BM Blomberg Particlevon P Borgstein MA Cuesta RJ Scheper S Meijer (1994) ArticleTitleUnimpaired immune functions after laparoscopic cholecystectomy Surgery 115 424–428 Occurrence Handle8165532
Z Kmiec (2001) ArticleTitleCooperation of liver cells in health and disease Adv Anat Embryol Cell Biol 161 1–151
PA Knolle G Gerken (2000) ArticleTitleLocal control of the immune response in the liver Immunol Rev 174 21–34 Occurrence Handle10.1034/j.1600-0528.2002.017408.x Occurrence Handle10807504
I Lauterschlager (1984) ArticleTitleCharacteristic of the strongly Ia-positive cells in rat liver Scand J Immunol 20 333–338 Occurrence Handle6390667
OH Lowry NJ Rosebough AL Farr RJ Randall (1951) ArticleTitleProtein measurement with the Folin phenol reagent J Biol Chem 193 265–275 Occurrence Handle14907713
B Luettig L Pape U Bode EB Bell SM Sparshott S Wagner J Westermann (1999) ArticleTitleNaive and memory T lymphocytes migrate in comparable numbers through normal rat liver: activated T cells accumulate in the periportal field J Immunol 163 4300–4307 Occurrence Handle10510369
AJ McMahon PJ O’Dwyer AM Cruikshank DC McMillan DS O’Reilly GD Lowe A Rumley RW Logan JN Baxter (1993) ArticleTitleComparison of metabolic responses to laparoscopic and minilaparotomy cholecystectomy Br J Surg 80 1255–1258 Occurrence Handle8242291
H Nave S Hoersten Particlevon F Helfritz S Kuhlmann J Ballof J Drube R Pabst (1998) ArticleTitleIntravenous cannulation of the freely behaving rat: frequent blood sampling and volume-dependent effect on blood leukocyte counts J Exp Anim Sci 39 67–77
NT Nguyen S Braley NW Fleming L Lambourne R Rivers BM Wolfe (2003) ArticleTitleComparison of postoperative hepatic function after laparoscopic versus open gastric bypass Am J Surg 186 40–44 Occurrence Handle10.1016/S0002-9610(03)00106-5 Occurrence Handle12842747
BH Pannen JL Robotham (1995) ArticleTitleThe acute-phase response New Horizons 3 183–197 Occurrence Handle7583160
HP Redmond RW Watson T Houghton C Condron RG Watson D Bouchier-Hayes (1994) ArticleTitleImmune function in patients undergoing open vs laparoscopic cholecystectomy Arch Surg 129 1240–1246 Occurrence Handle7986152
AB Rogers JG Fox (2004) ArticleTitleInflammation and cancer. I. Rodent models of infectious gastrointestinal and liver cancer Am J Physiol Gastrointest Liver Physiol 286 G361–G366 Occurrence Handle10.1152/ajpgi.00499.2003 Occurrence Handle14766534
AS Rosenthal (1978) ArticleTitleDeterminant selection and macrophage function in genetic control of the immune response Immunol Rev 40 136–152 Occurrence Handle89077
C Sietses ME Blomberg Particlevon QA Eijsbouts RH Beelen FJ Berends MA Cuesta (2002) ArticleTitleThe influence of CO2 versus helium insufflation or the abdominal wall lifting technique on the systemic immune response Surg Endosc 16 525–528 Occurrence Handle10.1007/s00464-001-0063-x Occurrence Handle11928041
AF Suffredini G Fantuzzi R Badolato JJ Oppenheim NP O’Grady (1999) ArticleTitleNew insights into the biology of the acute phase response J Clin Immunol 19 203–214 Occurrence Handle10.1023/A:1020563913045 Occurrence Handle10471974
H Tsutsui K Adachi E Seki K Nakanishi (2003) ArticleTitleCytokine-induced inflammatory liver injuries Curr Mol Med 3 545–559 Occurrence Handle10.2174/1566524033479618 Occurrence Handle14527086
BM Ure TA Niewold NM Bax M Ham DC Zee Particlevan der GJ Essen (2002) ArticleTitlePeritoneal, systemic, and distant organ inflammatory responses are reduced by a laparoscopic approach and carbon dioxide versus air Surg Endosc 16 836–842 Occurrence Handle10.1007/s00464-001-9093-7 Occurrence Handle11997833
FJ Vittimberga B Nolan RA Perugini L Spector MP Gallery (2000) ArticleTitleLaparoscopic surgery and Kupffer cell activation Surg Endosc 14 1171–1176 Occurrence Handle10.1007/s004640010065 Occurrence Handle11148792
RW Watson HP Redmond J McCarthy PE Burke D Bouchier-Hayes (1995) ArticleTitleExposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model Br J Surg 82 1060–1065 Occurrence Handle7648154
MA West DJ Hackam J Baker JL Rodriguez J Bellingham OD Rotstein (1997) ArticleTitleMechanism of decreased in vitro murine macrophage cytokine release after exposure to dioxide: relevance to laparoscopic surgery Ann Surg 226 179–190 Occurrence Handle10.1097/00000658-199708000-00010 Occurrence Handle9296512
Acknowledgments
The excellent technical assistance of Karin Westemann is very much appreciated. This research was supported by a grant (HiLF) from the Hannover Medical School, Hannover, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jesch, N., Vieten, G., Tschernig, T. et al. Mini-laparotomy and full laparotomy, but not laparoscopy, alter hepatic macrophage populations in a rat model. Surg Endosc 19, 804–810 (2005). https://doi.org/10.1007/s00464-004-2189-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-004-2189-0