Abstract
Background
Mechanical outflow obstruction and leakage from the exit site of the catheter are two common complications of continuous ambulatory peritoneal dialysis. To lessen these complications and to achieve immediate use of the catheter, we developed a new laparoscopic technique for catheter placement.
Methods
A total of 12 consecutive patients with end-stage renal failure were included in this study between April 2003 and July 2003. The average age of the patients was 42.4 years (range, 37–72). Patients were excluded only if a serious risk for general anesthesia was found. Using two 5-mm ports and a 3.3-mm mini-laparoscope, a peritoneal dialysis catheter was passed through a preperitoneal tunnel before the tip of the catheter was introduced into the pelvis. Routine peritoneal dialysis was started immediately after the operation while the patients were still in the operating room.
Results
The mean operating time was 18.6 min (range, 12–37). There was no operative morbidity. The mean follow-up period was 4.3 months (range, 3–7). No leakage of the dialysate liquid or outflow obstruction was observed during this period.
Conclusion
The advantages of this method include accurate placement, preperitoneal fixation, and immediate use of the catheter for routine peritoneal dialysis. We also believe that because of the preperitoneal fixation of the catheter, this technique will decrease outflow obstruction, which usually occurs due to omental wrapping or displacement of the catheter tip.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Continuous ambulatory peritoneal dialysis (CAPD) is widely used for the treatment of patients with end-stage renal failure [2, 20]. The advantages of this method are lower dialysis costs, simplicity of technique, improved mobility and greater independence for the patient, better control of hypertension, and fewer dietary restrictions [14, 15]. Three main techniques for insertion of the catheter have been described: the open surgical technique, the trocar technique, and the laparoscopic technique [9]. The operative morbidity and complication rates associated with peritoneal dialysis catheter insertion range from 1.0% to 1.5% and vary according to the technique used [6]. To achieve adequate peritoneal dialysis, a functioning catheter should enable unrestricted inflow and outflow of the dialysate liquid from the peritoneal cavity, with an intact peritoneal membrane.
In addition to peritonitis, leakage at the insertion site and mechanical outflow obstruction are common complications [2, 3, 4, 5, 14, 20]. Hemorrhage, visceral organ perforation, and incisional hernia are rare complications, occurring most commonly in patients with visceral and/or peritoneal adhesions due to a previous abdominal operation or recurrent peritonitis. Dialysate leakage, hemorrhage, and visceral organ perforation occur more often with the trocar technique [16, 18].
Surgeons have traditionally placed peritoneal catheters into the abdomen using an open, laparotomy technique. Recent advances in minimally invasive surgery have significantly improved the safe and reliable placement of peritoneal catheters [7, 24]. Indeed, there are numerous reports in the literature that have described the superiority and benefits of the laparoscopic approach, including decreased operating time, less perioperative pain, and fewer complications [8, 12, 23]. Various techniques have been developed, all of them using either two or three laparoscopic ports with diameters of 3 to 10 mm [7, 17, 21, 22, 24].
Leakage at the insertion site and mechanical outflow obstruction prevent the use of the peritoneal catheter for effective peritoneal dialysis. To reduce these complications, some surgeons have made modifications to the peritoneal standard insertion methods [7, 24]. We devised a novel technique for the laparoscopic placement of a peritoneal dialysis catheter through a preperitoneal tunnel using a mini-laparoscope (3.3-mm) and a single 5-mm port. This method offers the advantages of visual confirmation of the catheter’s location, fixation of the catheter with low patient morbidity, and the ability to begin routine peritoneal dialysis as soon as the operation is finished.
Materials and methods
This prospective study was performed after approval from the ethics committee of Zonguldak Karaelmas University Hospital, Zonguldak, Turkey. A total 12 consecutive patients (five female, seven male) with end-stage renal failure were enrolled in this study between April 2003 and July 2003. The mean age of the patients was 42.4 years (range, 37–72). Patients were excluded only if a serious risk for general anesthesia was found. Three patients had had previous upper abdominal operations, and none of the patients had a history of peritonitis.
Operative technique
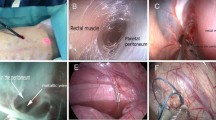
After induction of general anesthesia and gastric decompression, the patient was placed in a supine position. The pneumoperitoneum was established by using a Veress needle placed at the midclaviculer costal margin (Palmer’s point), (Fig. 1). The abdomen was insufflated to 15 mmHg pressure, and a 5-mm nondisposable metal trocar was introduced into the peritoneal cavity at the initial Veress location. The peritoneum was then evaluated using a 3.3-mm, 0° mini-laparoscope (model 26007AA; Karl Storz, Tuttlingen, Germany). A 5-mm left lower quadrant incision was made at the preferred exit site for the peritoneal dialysis catheter. Another 5-mm nondisposable metal trocar was then introduced into the peritoneal cavity for the intraperitoneal manipulations. A 4-mm vertical skin incision was made at a point 2 cm left and inferolateral to the umbilicus (Fig. 1). Under the direct vision, a Veress needle was moved ahead slowly until the tip of needle reached the preperitoneal space without puncturing the peritoneum.
Then 10 ml of isotonic saline solution was infused into this space to obtain the initial dissection (Fig. 2). After removal of the Veress needle, the subcutaneous tissue, rectus abdominis sheath, and muscles were dissected blindly with a Kelly clamp until the tip of clamp was inserted into the preperitoneal space. From this incision, a Tenckhoff peritoneal dialysis catheter was inserted with a metal guide wire until the tip of catheter was introduced into the preperitoneal space. It was then directed to the pubic symphysis through the preperitoneal space parallel to the peritoneum (Fig.3). Because the preperitoneal space is filled with avascular fat tissue and loose areolar tissue, we performed this procedure very easily; it took only a few seconds to create a preperitoneal tunnel.
At the level of the pubic symphysis, a 3–4-mm incision was made on the peritoneum overlying the superior border of the bladder with the help of endoscopic scissors, and the peritoneal dialysis catheter was passed through this opening (Fig.3). After placement of the catheter tip into the retrovesical pouch, the guide wire was removed. We did not place any sutures into the fascia at the insertion site of the catheter to obviate the possibility of leakage. After the camera was removed, the pneumoperitoneum was deflated. Neither the fascia at the camera port nor the fascia at the second port was sutured. Thus, after the body of the catheter was passed through the preperitoneal tunnel, which was 25–30 cm in length, only the part of the catheter tip with multiple holes was left inside the pelvis (Figs. 3 and 4).
Placement of the catheter for continuous ambulatory peritoneal dialysis (CAPD) through a preperitoneal tunnel. Care must be taken not to penetrate the peritoneum during this procedure. Almost the entire length of the catheter was placed into the preperitoneal space, except for the part of the catheter tip with multiple holes.
A subcutaneous tunnel was created from the last incision to the incision located in the left lower quadrant (catheter exit site) with the help of a specially designed L-shaped trocar. The outer end of the peritoneal dialysis catheter, which was connected to the bottom of the L-shaped trocar, was pulled outside the pelvis from the left lower quadrant trocar incision after being passed through this tunnel (Fig.4). Finally, all of the skin incisions were sutured.
The peritoneal cavity was flushed with 2,000 ml of peritoneal dialysis solution to check for gross bleeding or leakage. Routine dialysis was started as soon as the operation was finished.
Results
All patients did well after the procedure. The mean operating time was 18.6 min (range, 12–37). No operative morbidity was seen. Routine peritoneal dialysis was started on the day of operation in all patients. There was no catheter site leakage in the early postoperative period or during the follow-up. Mean follow up was 4.3 months (range, 3–7). No mechanical outflow obstruction was detected during this period.
Discussion
The laparoscopic placement of peritoneal dialysis catheters offers many advantages, especially in patients with abdominal adhesions. Because the operation is performed under direct visualization, it has lower rates of operative and postoperative complications, such as hemorrhage, organ damage, and incisional hernia, when compared with the open technique and the trocar technique [10, 14, 16, 18]. The laparoscopic technique has also the advantages of enabling complete exploration of the intraperitoneal cavity, adhesiolysis, and management of the concomitant diseases, such as cholelithiasis or hernia, if needed. For these reasons, we prefer to use the laparoscopic technique for the placement of peritoneal dialysis catheters in our clinic. The sole disadvantage of this procedure is that it requires general anesthesia, whereas the open surgical procedure is generally done under local anesthesia.
Even though catheter-related peritonitis is the most frequent complication of CAPD, obstruction of the catheter outflow and leakage of the dialysis fluid are still common and serious catheter-related early complications that prevent effective use of the catheter for peritoneal dialysis [1, 11, 13]. Outflow obstruction results in a nonfunctioning catheter and necessitates an additional operation either to restore its function or to completely remove the catheter, whereas leakage of the dialysate fluid prevents the peritoneal catheter from being used for some time.
Proper functioning of peritoneal dialysis catheters can be restricted by catheter malposition, kinking, catheter tip migration, a fibrin clot, or omental wrapping [3, 4, 5, 14, 15, 19]. Mechanical catheter obstruction has been documented in ≤60% of patients [4, 14]. Catheter tip migration, resulting in poor return of the dialysate, has been reported in 20% of cases [10]. Furthermore, this complication can also promote omental wrapping. Different techniques aimed at lessening the catheter tip migration, malposition, and omental wrapping have been described. Evangelos et al. reported that fixing the catheter tip into the pelvis with nonabsorbable sutures prevented catheter migration [10]. We agree that this technique could prevent catheter dislocation. However, because this procedure creates a catheter loop, which is fixed from its two points, it constitutes a potential risk for mechanical intestinal obstruction. On the other hand, if the catheter needs to be removed for any reason, an additional intraperitoneal operation may be required.
In our novel technique, the preperitoneal tunnel achieves the extraperitoneal fixation of the peritoneal catheter without suturing. Creation of the preperitoneal tunnel is an easy procedure that takes only a few seconds. We believe that because this technique is effective in preventing catheter migration, it will reduce the frequency of omental wrapping, catheter kinking, and malposition.
Leakage of the dialysate fluid is another early complication of CAPD, and it results in the interruption of routine peritoneal dialysis for some time. It is generally recommended that the catheter not be used for routine peritoneal dialysis for 14–21 days after the operation. Otherwise, the frequency of leakage from the exit site of the catheter increases. A number of technical modifications that can decrease the frequency of this complication and shorten the time needed before routine dialysis can be started have been described. Dalgiç et al. [7] reduced the number and diameter of the trocars. Despite this modification, they reported that routine dialysis could not be started until the 3rd postoperative week, and even with this waiting period, the frequency of leakage was reported to be 12.5%. Yun et al. [24] used a 2.7-mm microlaparoscope to obtain direct visualization and placed the catheter obliquely through the abdominal wall, creating a small tunnel to minimize the risk of dialysate leakage and to enable routine dialysis to be started earlier. They reported a leakage rate of 0% and a catheter failure rate of 2.6%. Their patients were able to use the peritoneal catheter for routine dialysis as early as 2 days after the operation. Thus, this technique for oblique placement of the peritoneal catheter expedites the initiation of routine dialysis. However, because almost the entire length of catheter is placed into the peritoneal cavity without any fixation, this technique does not reduce the rate of catheter failure due to dislocation.
Our method uses only two 5-mm trocars, and the catheters are introduced into the peritoneal cavity after being passed through a long preperitoneal tunnel. Thus, most of the catheter is fixed extraperitonealey by this tunnel, without any need for sutures. It is clear that this modification will be effective in preventing catheter dislocation. Although there was no control group and the mean follow-up period was not long enough to draw definitive conclusions, the early results are promising, and we have not yet encountered any catheter failures among our patients. Furthermore, we have started to use the peritoneal catheter for routine peritoneal dialysis on the same day as the operation in all patients, and no early or late leaks have been detected.
During the follow-up period, there were no cases of peritonitis among our patients. This early result is also promising. We believe that the incidence of peritonitis will not be any greater than it is with the other methods. However, further studies with a greater number of patients and a longer follow-up period are needed to confirm our preliminary findings.
Overall, it is well known that the laparoscopic technique enables the management of adhesions, if present, and the precise placement of the catheter under direct vision without any increase in operative complexity or perioperative complications. In addition to these widely recognized advantages of the laparoscopic method, our technique also has several other important advantages, including the ability to use peritoneal catheter for routine peritoneal dialysis immediately after the operation, prevention of the leakage of the dialysate fluid and a reduction of catheter failures due to dislocation.
References
M Allon JM Soucie EJ Macon (1988) ArticleTitleComplications with permanent dialysis catheters: experience with 154 percutaneously placed catheters Nephron 48 8–11 Occurrence Handle1:STN:280:BieC383oslM%3D Occurrence Handle3340260
GR Bailie G Eisele (1992) ArticleTitleContinuous ambulatory peritoneal dialysis: a review of its mechanics, advantages, complications, and areas of controversy Ann Pharmacother 26 1409–1420 Occurrence Handle1:STN:280:ByyC38%2FmvFE%3D Occurrence Handle1477448
CP Brandt ES Ricanati (1996) ArticleTitleUse of laparoscopy in the management of malfunctioning peritoneal dialysis catheters Adv Perit Dial 12 223–236 Occurrence Handle1:STN:280:ByiD3MbjvFM%3D Occurrence Handle8865908
CP Cacho MJ Tessman LN Newman MA Friedlander (1995) ArticleTitleInflow obstruction due to kinking of coiled catheters during placement Perit Dial Int 15 276–278 Occurrence Handle1:STN:280:BymD3sblsF0%3D Occurrence Handle7578511
SH Chao TJ Tsai (1993) ArticleTitleLaparoscopic rescue of dysfunctional Tenckhoff catheters in continuous ambulatory peritoneal dialysis patients Nephron 65 157–158 Occurrence Handle1:STN:280:ByuD3MbpvVc%3D Occurrence Handle8413779
JH Crabtree A Fishman (1999) ArticleTitleLaparoscopic omentectomy for peritoneal dialysis catheter flow obstruction: a case report and review of the literature Surg Laparosc Endosc Percutan Techn 9 228–233 Occurrence Handle1:STN:280:DC%2BD3c3mtFWmtg%3D%3D
A Dalgiç E Ersoy ME Anderson J Lewis A Engin AM D’Alessandro (2002) ArticleTitleA novel minimally invasive technique for insertion of peritoneal dialysis catheter Surg Laparosc Endosc Percutan Tech 12 252–254 Occurrence Handle12193820
B Draganic A James M Boot JS Gani (1998) ArticleTitleComparative experience of a simple technique for laparoscopic ambulatory peritoneal dialysis catheter placement Aust N Z J Surg 68 735–739 Occurrence Handle1:STN:280:DyaK1cvjvFGnug%3D%3D Occurrence Handle9768612
BH Eklund (1995) ArticleTitleSurgical implantation of CAPD catheters: presentation of midline incision–lateral placement method and review of 110 procedures Nephrol Dial Transplant 10 386–390 Occurrence Handle1:STN:280:ByqA3M3isFU%3D Occurrence Handle7792036
T Evangelos S Philipos G George T Chrysoula S George P Michael M Adamandia (2000) ArticleTitleLaparoscopic placement of the Tenckhoff catheter for peritoneal dialysis Surg Laparosc Endosc Percutan Tech 10 218–221 Occurrence Handle10961749
DM Francis PK Donelly PS Veitch G Proud RM Taylor JM Ramos MK Ward et al. (1984) ArticleTitleSurgical aspects of continuous ambulatory peritoneal dialysis-3 years experience Br J Surg 71 225–229 Occurrence Handle1:STN:280:BiuC38bisVI%3D Occurrence Handle6697131
MF Gadallah A Pervez MA El-Shahaway D Sorrells G Zibari J McDonald J Work (1999) ArticleTitlePeritoneoscopic versus surgical placement of peritoneal dialysis catheters: a prospective randomized study on outcome Am J Kidney Dis 33 118–122 Occurrence Handle1:STN:280:DyaK1M7ht1CrsA%3D%3D Occurrence Handle9915276
KM Hiltunen M Viranta (1985) ArticleTitleOne-way obstruction during continuous ambulatory peritoneal dialysis (CAPD) with Tenckhoff catheter: management by a simple operation Scand J Urol Nephrol 19 67–68 Occurrence Handle1:STN:280:BiqB2s3ksVE%3D Occurrence Handle4023651
FM Kimmelstiel RE Miller BM Molinelli JA Lorch (1993) ArticleTitleLaparoscopic management of peritoneal dialysis catheters Surg Gynecol Obstet 176 565–570 Occurrence Handle1:STN:280:ByyA3M%2FmtVA%3D Occurrence Handle8322130
DS Kittur PM Gazaway MR Abidin (1991) ArticleTitleLaparoscopic repositioning of malfunctioning peritoneal dialysis catheters Surg Laparosc Endosc 1 179–182 Occurrence Handle1:STN:280:ByuD1c%2FkvVQ%3D Occurrence Handle1669399
G Mellotte C Ho SH Morgan MR Bending AJ Eisinger (1993) ArticleTitlePeritoneal dialysis catheters: a comparison between percutaneous and conventional surgical placement techniques Nephrol Dial Transplant 8 626–630 Occurrence Handle1:STN:280:ByyA1c3mt1c%3D Occurrence Handle8396747
PH Nijhuis JF Smulders JJ Jakimowicz (1996) ArticleTitleLaparoscopic introduction of continuous ambulatory peritoneal dialysis (CAPD) catheter by a two-puncture technique Surg Endosc 10 676–679 Occurrence Handle1:STN:280:BymB2s%2FitlM%3D Occurrence Handle8662414
A Nissenson D Gentile RE Soderblow DF Oliver C Brax (1986) ArticleTitleMorbidity and mortality of continuous ambulatory peritoneal dialysis: regional experience and long-term prospects Am J Kidney Dis 6 227–233
DW Smith RA Rankin (1989) ArticleTitleValue of peritoneoscopy for nonfunctioning continuous ambulatory peritoneal dialysis catheters Gastrointest Endosc 35 90–92 Occurrence Handle1:STN:280:BiaB3s3mtFw%3D Occurrence Handle2523835
S Steinberg S Cutler KD Nolph JW Novak (1985) ArticleTitleA comprehensive report on the experience of patients on continuous ambulatory peritoneal dialysis for the treatment of end-stage renal disease Am J Kidney Dis 4 233–239
JY Wang JS Hsieh FM Chen CH Chuan HM Chan TJ Huang (1999) ArticleTitleSecure placement of continuous peritoneal dialysis catheters under laparoscopic assistance Am Surg 65 247–249 Occurrence Handle1:STN:280:DyaK1M7ntlKhtg%3D%3D Occurrence Handle10075302
DI Watson D Peterson K Bannister (1996) ArticleTitleSecure placement of peritoneal dialysis catheters using a laparoscopic technique Surg Laparosc Endosc 6 35–37 Occurrence Handle1:STN:280:BymH3cvivVQ%3D Occurrence Handle8808558
MJ Wright K Bel’eed BF Johnson DW Eadington L Sellars MJ Farr (1999) ArticleTitleRandomized prospective comparison of laparoscopic and open peritoneal dialysis catheter insertion Perit Dial Int 19 372–375 Occurrence Handle1:STN:280:DyaK1MvjsFKktQ%3D%3D Occurrence Handle10507820
EJ Yun MV Meng TV Brennan JW McAninch RA Santucci SJ Rogers (2003) ArticleTitleNovel microlaparoscopic technique for peritoneal dialysis catheter placement Urology 61 1026–1028 Occurrence Handle12736031
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comert, M., Borazan, A., Kulah, E. et al. A new laparoscopic technique for the placement of a permanent peritoneal dialysis catheter: the preperitoneal tunneling method. Surg Endosc 19, 245–248 (2005). https://doi.org/10.1007/s00464-003-9302-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-003-9302-7