Abstract
Background
Interest for minimal invasive approach of esophagus resection is increasing. Today, a minimally invasive transhiatal esophagectomy is possible and is accepted widespread. Since cardiopulmonary changes during laparoscopic dissection of the mediastinum has not been studied yet we assessed the anesthesiological consequences of pneumothorax during laparoscopic mediastinal dissection.
Methods
In this case control study, 25 laparoscopically assisted transhiatal espohagus resections were compared with a control group consisting of 20 open transhiatal esophagus resections. Patient characteristics and intraoperative haemodynamic, respiratory, and ventilatory parameters were assessed.
Results
The laparoscopic assisted procedure was performed successfully in 12 of the 20 patients. The duration of the laparoscopic assisted procedure, compared to the open group was significantly longer (p<0.05). Intraoperative blood loss was significantly less in the laparoscopic group (p<0.05). Mediastinal dissection resulted in entry of the pleura in 84% of the open and 93% of the laparoscopic assisted procedure. Carbondioxide pneumothorax resulted in increased end-tidal CO2 and airway pressure levels and decreased lunng compliance. Airway pressure showed a significant difference between the groups (p<0.05). Hemodynamic parameters did not differ between groups significantly. There were no differences in postoperative cardiopulmonary complications.
Conclusions
Laparoscopic assisted transhiatal esophagectomy is a safe procedure and has no increased risk of postoperative cardiopulmonary complications compared to thr conventional approach. The anesthesiologist and the surgeon must be aware of the potential risk of pleural injury to manage cardiopulmonary compromises and minimize complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Because conventional esophageal resection for the management of esophageal cancer is associated with high perioperative morbidity and significant mortality, there is increasing interest in the minimally invasive approach to esophageal resection. Recently different approaches—including laparoscopic/thoracoscopic mediastinal dissection by means of a modified mediastinoscope and combined laparoscopic and right thoracoscopic esophagectomy have been described as alternatives to open surgery [1, 2, 7, 8]. Although there have been numerous reports touting the advantages of the minimally invasive approach, there is still some concern about its safety [15].
Transhiatal esophagectomy is one of the most frequently performed procedures for esophageal cancer. The avoidance of a thoracotomy and intrathoracic anastomosis is an important advantage that serves to reduce the morbidity and mortality associated with the thoracoabdominal approach [10]. Moreover, in a series of patients with distal esophageal cancer, the transhiatal approach compared satisfactorily with the en bloc thoracoabdominal approach and lymphadenectomy in a recent randomized study [3]. Minimally invasive transhiatal esophagectomy for cancer is feasible and compares favorably in some aspects with the conventional approach [13]. It has not yet been determined whether this laparoscopic transhiatal approach, which provides better visualization and enables the avoidance of blunt manual dissection of the mediastinum, minimizes cardiopulmonary changes. Moreover, the insufflation necessary for the laparoscopic approach can create unpredictable hazards in cases where the pleural barrier is entered during dissection.
The aim of this study was to address the following questions:
-
1.
Does minimally invasive transhiatal esophageal resection pose a greater risk than conventional surgery?
-
2.
Does iatrogenic pleural entry lead to significant respiratory changes? What are the outcomes?
-
3.
What are the differences in cardiovascular responses between the minimally invasive and conventional approaches?
-
4.
Do cardiovascular alterations create an increased need for inotropic agents?
Patients and methods
This case control study was carried out in a population of 45 patients who underwent transhiatal esophagectomy at the Vrije Universiteit Medical Center. All patients were operated on between January 1997 and March 2003. There were of two groups. The first 20 patients, the open surgery group, underwent conventional transhiatal esophagectomy before June 2000. The second group, the laparoscopic-assisted group, consisted of 25 patients who underwent laparoscopic-assisted transhiatal esophagectomy after June 2000.
The diagnosis of esophageal cancer was based on a complete preoperative evaluation that included abdominal and thoracic CT and upper gastrointestinal endoscopy, including biopsy and esophageal endosonography. Patients were grouped according to the American Society of Anesthesiologists (ASA) classification of physical status.
All demographic data and intraoperative respiratory and cardiovascular data were collected retrospectively. Besides data on patient characteristics, the following hemodynamic parameters were studied: heart rate (HR) (beats/min); blood pressure, mean arterial pressure (MAP), and central venous pressure (CVP) (mmHg); dysrhythmia; and inotropic requirement and perioperative fluid balance (ml).
The following respiratory and ventilatory parameters were also assessed: end-tidal carbon dioxide (etCO2) (%), ventilation pressure (Pairway) (mmHg), positive end-expiratory pressure (PEEP) (cm H2O), inspired oxygen fraction (FiO2), oxygen saturation by pulse oximetry (SaO2), tidal volume (TV), ventilatory frequency (frequency/min), lung compliance (C) (ml/cm H2O) calculated as \({\text{C = }}\frac{{{\text{TV}}}} {{{\text{P}}_{{\text{end inpiratory}}} - {\text{PEEP}}}}, \) minute ventilation (ml/kg/min) calculated as TV × ventilatory frequency, and blood gas analyses.
Along with these parameters, the cardiopulmonary complications were recorded.
Midazolam, temazepam, diazepam, or lorazepam was administered as premedication before induction. Induction of anesthesia was carried out using fentanyl or sufentanil; penthotal or propofol, followed by vecuronium, recuronium, or pancuronium, were used for initial muscular relaxation. All patients were ventilated using a semi-closed circle respirator with a fresh gas flow and carbon dioxide absorber (Dräger model Cicero EM®; GCX instrument mounting systems, Petaluma, CA).
Similar anesthetic techniques were used for both groups. After mask ventilation with 100% oxygen, a right-sided double lumen tube (Broncho-Cath, Mallinckrodt, Medical Ltd., Athlone, Ireland) was inserted in case a right thoracotomy was needed. Anesthesia was maintained with fentanyl or sufentanil; but propofol and isoflurane or sevoflurane were also used. In all patients an attempt was made to introduce a nasogastric tube. For urinary catheterization, a Foley or suprapubic catheter was used. Blood gas analyses were performed using a RapidLab 865 analyzer (Chiron Diagnostics, Emeryville, CA).
Esophageal resection and reconstruction were performed via the laparoscopic-assisted or conventional approach as described elsewhere [3, 9]. Carbon dioxide was used for insufflation in patients undergoing the minimally invasive procedure. Patients were positioned in the supine and anti-Trendelenburg position. The stomach was used as the esophageal substitute in all patients except one; this patient underwent a colonic interposition.
Data were reviewed from phases of the operative procedures, as follows:
-
Phase I: Laparotomy or laparoscopic insufflation
-
Phase II: Mediastinal dissection of the esophagus
-
Phase III: Gastrolysis and preparation of the gastric tube
-
Phase IV: Preparation of the cervical esophagus and the stripping procedure
-
Phase V: Placement of the gastric tube, performing a cervical esophagogastric anastomosis and feeding jejunostomy
All hemodynamic and respiratory changes were corrected by the anesthesiological team to maintain optimal oxygenation and blood pressure.
All patients underwent postoperative control mode ventilation in the intensive care unit (ICU). Pain was controlled with an epidural infusion of bupivacaine or intramuscular injections of morphine.
All results are expressed as means ± SD. Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, IL, USA). For significance of parametric data, t-tests were used. Comparison of perioperative results between groups was performed using the Kruskal-Wallis and Mann-Whitney U tests. Statistical significance was set at p < 0.05.
Results
The demographic data were comparable for the two groups (Table 1). Tumor characteristics—histological type, localization and stages—were similar. The laparoscopic-assisted procedure was completed successfully in 16 of the 25 patients. Nine cases (36%) were converted to an open procedure during different phases of the operation. The reasons for conversion are listed in Table 2. Laparoscopic mediastinal dissection was possible in 22 cases (88%).
The duration of the laparoscopic-assisted procedure, was significantly longer than the open procedure (290 ± 37 vs 257 ± 34 min; p < 0.05). Intraoperative blood loss was significantly less in the laparoscopic group (600 ± 290 vs 900 ± 113 ml; p < 0.05). There was a significant difference between groups in terms of ICU stay, whereas parameters such as time of extubation and total hospital stay were not significant (Table 3).
Intraoperative fluid balance was 1,143 ± 1,557 ml in the laparoscopic group and 1,458 ± 1,162 ml in the open group (p > 0.05). The need for blood and fresh-frozen plasma transfusion was increased, but not significantly, in the laparoscopic-assisted group due to conversions (p > 0.05).
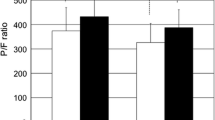
Mediastinal dissection resulted in entry of the pleura in 84% of the open and 93% of the laparoscopic-assisted procedures, including patients converted during phase III and IV. The risk of pleural perforation of both sides was documented in 41% of the patients in the open group and 85% in the laparoscopic group. The anesthesiologists were informed immediately of this problem in all cases. Hemodynamic parameters, such as mean CVP, mean HR, and mean systolic blood pressure, did not differ significantly between group, although mean CVP and mean HR showed a slight increase during phase II. The difference of mean diastolic blood pressure and MAP between groups during phase IV were statistically significant (p < 0.05). Systolic and diastolic blood pressures and MAP showed an insignificant decrease during phases I, II, and III. They remained constant after phase III (Fig. 1).
There was no difference between the two groups in need for inotropic agents. They were mostly applied before incision (43%) or during phase II (30%) in 95% of the open procedures and 85% of the laparoscopic-assisted procedures. Dopamine was the agent of choice in patients where an inotropic agent was indicated.
Although etCO2 remained stable during the open procedure, there was an increase in the laparoscopic-assisted group during phases I and II. There were also significant differences between the groups in phases I, II, IV, and V in terms of etCO2 (Fig. 2a). There was a significant difference in Pairway between the groups during phases I, II, and III. The increase during phases I and II in the laparoscopic-assisted group was remarkable. The Pairway of the open group remained constant (Fig. 2b). The PEEP changed remarkably during phase II in the laparoscopic-assisted group, but there were no significant differences between groups (Fig. 2c). A comparison of ventilatory frequency between groups showed an increase during phase II in both groups. The increase in the laparoscopic-assisted group was remarkable, resulting in significance during phases II, II, and V (Fig. 2d).
The SaO2 did not differ between the groups or among phases, and all patients had adequate saturation during the operation. Similar to SaO2, tidal volume showed no difference. The FiO2 increased from phase I to phase II in the laparoscopic-assisted group. There was a significant difference between groups during phases I, II, and IV. Tidal volume showed constant levels, with slight (nonsignificant) increases and decreases during surgery.
During phases I and II, significant decreases in lung compliance were seen inthe laparoscopic group (Fig. 3a). This group showed a large increase in minute ventilation in phase II, whereas the values for the open group remained constant (Fig. 3b). A significant increase in mean blood pCO2 levels was noted during the mediastinal dissection (phase II) for both groups. Moreover, blood pH levels decreased (but not significantly) between the initial and final phases of the surgical procedures.
There were five cases of postoperative cardiopulmonary complications—pneumonia in four patients and atrial fibrillation in another patient—but no difference between the groups in this parameter.
Discussion
One of the advantages of the transhiatal approach for esophageal resection is that pulmonary complications are obviated due to avoidance of the thoracotomy [9]. Nevertheless, there are still concerns about the conventional transhiatal approach. Notably, blind and frequent blunt manual dissection of the esophagus may result in cardiopulmonary complications [16].
The minimally invasive approach for esophagus dissection and resection enables a perfect view of the esophagus and the tumor up to the carina, and the dissection can be done in an avascular plane through anatomic layers under direct vision of the laparoscope [11]. A major disadvantage of this technique may be the pleura perforation, or the necessity to resect a part of the pleura as a part of the mediastinal resection of the tumor, which can be hazardous.
In this study, we assessed the anesthesiological consequences of pneumothorax during laparoscopic mediastinal dissection. It has not yet been determined whether there are significant changes after pleural entry in minimally invasive esophageal cancer surgery. But there are studies attesting to the cardiopulmonary changes after intraoperative pleural entry during laparoscopic antireflux surgery [5, 6].
In this series, mediastinal dissection resulted in pneumothorax in 93% of the patients undergoing the laparoscopic-assisted procedure. Carbon dioxide pneumothorax resulted in increased etCO2 and airway pressure levels and decreased lung compliance. As expected, these parameters did not change in the conventional surgery group.
Pneumoperitoneum may decrease the functional residual capacity and thus decrease SaO2. To counteract this conditions, it may be necessary to increase the PEEP level. Using a pig model, Sandbu et al. created a similar situation of communication with carbon dioxide between the insufflated abdomen and the thoracic cavity [12]. They observed that this pneumoperitoneum had adverse effects on blood gases: hypercarbia, respiratory acidosis, and hypoxemia were early manifestations that occurred even in the presence of hemodynamic stability. They also observed that the increase of PEEP equal to or higher than the carbon dioxide pressure improved blood gases; in particular, the hypoxemia could be avoided. In the series presented here, after constant correction, it is remarkable that the SaO2 level did not change in both groups. In our experience with a insufflation pressure of 10 mmHg (after entry of the pleural cavity), the increased PEEP never surpassed the level of 8 cm H2O in order to maintain optimal oxygenation.
The anesthesiologist and the surgeon must be aware of the potential risk of pleural injury. Intraoperative findings such as increased etCO2 elevated airway pressure, and decreased lung compliance should alert the anesthesia team that pleural entry may have occurred. Hyperventilation, increasing minute volume, use of PEEP, and decreasing insufflation pressure can be helpful [14]. We believe that there is no need to discontinue the procedure so long as the patient remains stable. All pneumothoraces can be treated easily and safely by inserting chest tubes through the trocar holes on the side of pleural tear at the end of the surgical procedure. Suturing of the pleurotomy is not needed.
The nonsignificant decrease in blood pH levels and significant increase in PaCO2 levels can be accounted for by carbon dioxide absorbtion through the peritoneum, leading to hypercapnia and respiratory acidosis. In this situation, an increase in minute ventilation is required to prevent hypercarbia.
It is known that carbon dioxide pneumoperitoneum induces hemodynamic changes as metabolic and ventilatory changes [4]. The carbon dioxide insufflation and pneumothorax had no significant cardiac effect on the patients in either group and was hemodynamically well tolerated. Meanwhile, HR CVP showed a slight increase during mediastinal dissection, while MAP decreased during the first three phases and remained stable after phase III.
The results of this study show that laparascopic-assisted transhiatal esophagectomy is safe and entails no increased risk of postoperative cardiopulmonary complications, as compared to the conventional approach. Close patient monitoring and good communication between the surgeon and anesthesiologist are essential to manage cardiopulmonary hazards and minimize complications.
References
G Buess J Kaiser K Manncke DH Walter JR Bessell HD Becker (1997) ArticleTitleEndoscopic microsurgical dissection of the esophagus (EMDE) Int Surg 82 109–112 Occurrence Handle1:STN:280:ByiH2s3oslA%3D Occurrence Handle9331833
AL DePaula K Hashiba EA Ferreira RA dePaula E Grecoo (1995) ArticleTitleLaparoscopic transhiatal esophagectomy with esophagogastroplasty Surg Laparosc Endosc 5 1–5 Occurrence Handle1:STN:280:ByqB2MzkvVA%3D Occurrence Handle7735533
JB Hulscher JW Sandick Particlevan AG de Boer BP Wijnhoven JG Tijssen P Fockens PF Stalmeier (2002) ArticleTitleExtended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus N Engl J Med 347 1662–1669 Occurrence Handle10.1056/NEJMoa022343 Occurrence Handle12444180
JL Joris (1994) Anesthetic management of laparoscopy RD Miller (Eds) Anesthesia Churchill Livingstone New York 2011–2029
JL Joris JD Chiche ML Lamy (1995) ArticleTitlePneumothorax during laparoscopic fundoplication: diagnosis and treatment with positive end-expiratory pressure Anesth Analg 81 993–1000 Occurrence Handle10.1097/00000539-199511000-00017 Occurrence Handle1:STN:280:BymD28fpslY%3D Occurrence Handle7486090
D Mangar GT Kirchhoff JJ Leal R Laborde E Fu (1994) ArticleTitlePneumothorax during laparoscopic Nissen fundoplication Can J Anaesth 41 854–856 Occurrence Handle1:STN:280:ByqD2c3ps1w%3D Occurrence Handle7955003
OJ McAnena J Rogers NS Williams (1994) ArticleTitleRight thoracoscopically assisted esophagectomy for cancer Br J Surg 81 236–238 Occurrence Handle1:STN:280:ByuB3cjnsVU%3D Occurrence Handle8156345
NT Nguyen PR Schauer JD Luketich (1999) ArticleTitleCombined laparoscopic and thoracoscopic aproach to esophagectomy J Am Coll Surg 188 328–332 Occurrence Handle10.1016/S1072-7515(98)00304-4 Occurrence Handle1:STN:280:DyaK1M7mtlOjtQ%3D%3D Occurrence Handle10065824
MB Orringer H Sloan (1978) ArticleTitleEsophagectomy without thoracotomy J Thorac Cardiovasc Surg 76 643–654 Occurrence Handle1:STN:280:CSaD3s%2FpvVQ%3D Occurrence Handle703369
MB Orringer B Marshall MD lannettoni (2001) ArticleTitleTranshiatal esophagectomy for treatment of benign and malignant esophageal disease World J Surg 25 196–203 Occurrence Handle10.1007/s002680020019 Occurrence Handle1:STN:280:DC%2BD3MrhsFCmug%3D%3D Occurrence Handle11338022
N Sadanaga H Kuwano M Watanabe M Ikebe M Mori S Maekawa M Hashizume et al. (1994) ArticleTitleLaparoscopy-assisted surgery: a new technique for transhiatal esophageal dissection Am J Surg 168 355–357 Occurrence Handle1:STN:280:ByqD3s%2FhvVA%3D Occurrence Handle7943595
R Sandbu B Birgisdottir D Arvidsson U Sjostrand S Rubertsson (2001) ArticleTitleOptimal positive end-expiratory pressure (PEEP) settings in differential lung ventilation during simultaneous unilateral pneumothorax and laparoscopy: an experimental study in pigs Surg Endosc 5 1478–1483
WT Broek ParticleVan den Ö Makay FJ Berends JZ Yuan APJ Houdijk S Meijer MA Cuesta (2004) ArticleTitleLaparoscopic-assisted transhiatal resection for distal malignancies of the esophagus Surg Endosc . .
RWM Wahba MJ Tessler SJ Kleiman (1996) ArticleTitleAcute ventilatory complications during upper abdominal surgery Can J Anaesth 43 77–83 Occurrence Handle1:STN:280:BymB2c%2FpvFM%3D Occurrence Handle8665641
SA Wajed JH Peters (2001) ArticleTitleLaparoscopic and endoscopic surgery in esophageal malignancy SurgOncol Clin N Am 10 493–510 Occurrence Handle1:STN:280:DC%2BD3MnhtFWktw%3D%3D
K Yakoubian B Bougeois J Marty JP Marmuse JM Desmonts (1990) ArticleTitleCardiovascular responses to manual dissection associated with transhiatal esophageal resection Cardiothorac Anesth 4 458–461 Occurrence Handle10.1016/0888-6296(90)90291-M Occurrence Handle1:STN:280:By2D2srptlY%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makay, Ö., den Broek, W.T.v., Yuan, J.Z. et al. Anesthesiological hazards during laparoscopic transhiatal esophageal resection: a case control study of the laparoscopic-assisted vs the conventional approach. Surg Endosc 18, 1263–1267 (2004). https://doi.org/10.1007/s00464-003-9176-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-003-9176-8