Abstract
Background: Internal drainage with transhepatically or endoscopically placed endoprostheses has been used for many years as a temporary or definitive treatment for biliary tract obstruction. As a late complication, stent migration may occur. Methods: We reviewed our records to identify patients who were operated on for a migrated endoprosthesis that was causing complications. In all, five such patients were identified. Results: One patient had a large bowel perforation. Bowel penetration led to an interenteric fistula in one patient and to a biliocolic fistula formation in another. Small bowel distension was found in two patients. Surgical treatment consisted of local excision in three patients, segmental resection in one patient, and a bypass operation in the patient with biliocolic fistula. Postoperatively, four patients recovered without problems, but one patient died during a complicated postoperative course. Conclusion: If a stent becomes stuck in the gastrointestinal tract and is not accessible for endoscopic removal, early operative revision is mandatory to prevent further complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Interventional internal drainage of the biliary tract has become an established procedure for both the temporary and definitive treatment of biliary obstruction due to malignant or benign disease. The complication rate is reported to be low, so, if feasible, this technique is preferred over a surgical drainage procedure, especially in critically ill patients [1]. For benign stenosis, plastic stents remain the standard [4, 6, 12]. In malignant disease, metal stent implantation can be performed for palliative purposes [5].
Dislocation and migration is a late complication after endoscopic or transhepatic biliary endoprosthesis placement; it has been reported to occur in about 7% (0–40%) of cases [1, 4, 5, 6]. Proximal migration, the further advancing of the stent into the biliary duct, may lead to biliary obstruction [12]. Its correction is technically challenging, but it can usually be achieved endoscopically by forceps, snare, or balloon and rarely necessitates operative intervention [4, 11, 15, 25]. In the early stages of distal migration, the stent can be replaced endoscopically [12]. Endoscopic treatment is limited to early and endoscopically accessible cases [7, 23]. In most other cases, the stent passes naturally due to the contractions and distensibility of the intestinal wall. Under rare circumstances, excretion does not occur spontaneously [2, 14, 20, 23, 26]. Sometimes the stent gets stuck, leading to further complications. Complications due to distal migration other than the recurrence of biliary obstruction have only been reported anecdotally [2, 14, 19, 20, 22, 26]. Thus, close observation and clinical control, in combination with digestion-inducing means, is the primary therapy [24]. With distal migration, bowel obstruction or perforation may occur, requiring surgical intervention [2, 19].
We report five cases of stent migration, that necessitated surgical intervention.
Materials and methods
We reviewed all patients that had been operated on at our institution from 1995 to 2001 for a dislocated biliary stent. Data collected included patient demographics, diagnosis, indications for stent placement, type of stent, operative procedure, and intraoperative findings. Outcome criteria included survival data, need for further treatment, and postoperative outcome.
Results
In the reported period, we placed 987 plastic endoprostheses endoscopically; for those patients, we observed a migration rate of 3.7%. In most of these cases, endoscopic correction or exchange was performed or the stent passed naturally. In the same period, 20 biliary metal stents were placed endoscopically and ~180 were placed percutaneously for malignant biliary obstruction.
Five patients had to be operated on for a migrated biliary stent (see Table 1). The diagnosis was biliary obstruction in four of these cases; in the remaining case, a stent had been implanted prophylactically in a patient undergoing liver transplantation. The causes of the biliary stenosis were acute pancreatitis, stenosis of the papilla, pancreatic head carcinoma, and bile duct carcinoma, respectively. Patient histories are detailed below. The clinical signs that led to the diagnosis of stent dislocation were as follows: local pain that was provoked or that increased on palpation, with no signs of inflammation or bowel obstruction at the onset of symptoms. Conservative approaches for stent removal (high-fiber diet, bowel stimulation, colonoscopy, and enteroscopy) failed.
Case 1
A 27-year-old woman had experienced biliary necrotizing pancreatitis. After 6 weeks, she developed a pancreatic pseudocyst measuring 17 × 20 cm. The cyst was drained externally operatively but caused further biliary obstruction. A 12-F, 12-cm endoprosthesis was inserted endoscopically and had to be changed once due to occlusion. After the patient had recovered from pancreatitis, a cholecystectomy was performed. Endoprosthesis removal was planned for 6 weeks after cholecystectomy, but the patient did not show up. Nine months later, she presented with unspecific abdominal pain in the middle abdomen that increased on palpation. Endoscopic retrograde cholangiopancreatography (ERCP) showed a dissolution of the biliary obstruction but also revealed a dislocated stent. Further examination revealed a partial involution of the pseudocyst, which was now 10 × 13 cm in size. The endoprosthesis had moved into the small bowel. Because her pain was increasing, the patient underwent operation. The cyst was externally drained — its content was clear, with no signs of infection — and the stent was removed by jejunotomy. The jejunal loop was distended and fixed by adhesions. Postoperatively, the patient’s recovery was uneventful. The pancreatic drain was removed after 3 weeks.
Case 2
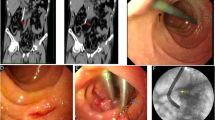
A 58-year-old man underwent a liver transplant for irresectable multifocal hepatocellular carcinoma. Intraoperatively, because the bile duct was rather small and an anastomotic stricture was expected, a 7-F, 10-cm straight endoprosthesis was inserted prophylactically. One month later, stent dislocation was observed, but there were no signs of biliary obstruction. As seen on control radiographs over 1 week, the endoprosthesis had advanced to the sigmoid colon, which was twisted and showing diverticula (Fig. 1). The stent had gotten stuck in a sigmoid diverticulum 50 cm above the dentate line. It was removed by colonoscopy. In the course of events, the patient developed a diverticular perforation and required an emergency laparotomy with sigmoid resection. The patient’s recovery was uneventful.
Case 3
A 60-year-old woman with coronary heart disease and a history of two myocardial infarctions had already undergone a sigmoid resection for diverticular disease and a right-sided nephrectomy for pyelonephritis. She then underwent cholecystectomy for biliary pancreatitis. Postoperatively, she was found to have recurrent bile duct stones and received an endoprosthesis (14-F) at another institution because of a papillary stenosis after bile duct clearance of the stones. After 2 months, she presented with recurrent abdominal pain that increased over time and was aggravated by food intake and local pressure. Stent dislocation was diagnosed radiographically. Conservative treatment esp. bowel stimulation led to an increase in abdominal pain. On radiologic controls, it was observed that the stent had become stuck in a jejunal loop. At the time of operation, extensive adhesiolysis was necessary. As a cause of the abdominal pain, we found that the endoprosthesis had penetrated the jejunal wall into the neighboring jejunal loop. Treatment consisted of local excision and closure of the penetrated regions. Postoperatively, the patient recovered without further complications.
Case 4
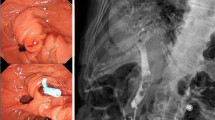
A 64-year-old man with a diagnosis of irresectable pancreatic head cystadenocarcinoma had had a pancreaticocysto-jejunostomy 5 years previously. He had received irradiation and chemotherapy for the tumor 4 years earlier. He then presented with obstructive jaundice. Endoscopy revealed a tumorous infiltration of the duodenum, so that the papilla of Vater could not be reached with the duodenoscope. Therefore, a Wallstent (Boston Scientific) (10-mm) was placed percutaneously transhepatically. After 6 months, he presented with duodenal obstruction and abdominal pain. The control CT scan showed a large tumor mass with duodenal infiltration (Fig. 2). The stent was dislocated, with its distal tip in the colon, thus creating a biliocolic fistula. Because he presented with a near total stop of food passage and signs of infection, operative revision was undertaken. The stent-carrying colonic segment was excluded from the colonic passage and connected to the jejeunal loop of the cystojejunostomy. The remaining colon was reanastomosed and a gastrojejunostomy performed. The postoperative course was complicated due to perfusion disturbance of the colon. This resulted in anastomotic insufficiency, leading to peritonitis, which necessitated operative revision twice. Finally, the patient developed pulmonary insufficiency and died.
Case 5
A 65-year-old man had undergone subtotal gastrectomy with Roux-en-Y-reconstruction for signet ring carcinoma 10 years previously. Eight years later, a biliodigestive anastomosis had been performed because of obstructive jaundice. During this operation, no definite diagnosis of the underlying disease could be made. He then presented with recurrent jaundice. Endoscopic retrograde cholangiography (ERC) was not possible due to the preceding gastric resection. CT scan revealed a tumorous lesion in the region of the pancreatic head and liver hilum. Percutaneous puncture of that process did not yield a clear histological diagnosis. An external percutaneous transhepatic cholangiodrainage was established. Later, explorative laparotomy was performed. It showed a diffuse infiltration of the hepatoduodenal ligament with a poorly differentiated cholangiocellular carcinoma and a subhepatic abscess. Due to the local extent of the disease, the tumor was not resectable. Histologic examination confirmed the diagnosis of a poorly differentiated cholangiocellular carcinoma. In the further course, the external drain was replaced with a 10-mm Wallstent. Unfortunately this metal stent dislocated into the jejunal loop of the biliodigestive anastomosis. Because of abdominal pain, operative revision was indicated. The stent was removed by local jejunotomy. The postoperative course was uneventful. The patient died 6 months later due to progression of his tumor.
Discussion
Interventional internal bilioenteric drainage using a stent is a minimally invasive procedure that maintains intact enterohepatic circulation. Endoscopic stenting procedures of the biliary system for malignant and benign lesions are well established and increasing in number. If stenting is performed for benign lesions, late complications should also be taken into consideration.
In addition to stent occlusion, the migration of a stent is a late complication that occurs with varying frequency and may cause further problems. Also, once the stent is removed from the bile duct, it may not be possible to retrieve it, leaving the stent to migrate spontaneously [23].
Especially in benign strictures, the dislocation of an endoprosthesis may not even be noticed because the biliary stenosis has resolved [9, 10, 12], as was the case in patients 1 and 3. In patients with intraabdominal adhesions, diverticular disease, or any other cause of fixation of the bowel, spontaneous stent passage may not be possible. The longer and more rigid the migrated stent is, the higher the risk of it becoming stuck [24]. Because the concrete symptoms result from the shifting of the stent and the organ thereby afflicted and do not relate to the underlying disease, the correct diagnosis may sometimes be delayed [18].
The observed frequency of stent migration varies greatly, from 0% to 40%; in average 5–10% for plastic endoprostheses [1, 5, 12, 21]. The migration of self-expandable metal mesh stents occurs less frequently (0–8%); the actual rate is probably <1% because the surrounding tissue grows through the struts, thus fixing them and sometimes even leading to occlusion [4, 5, 10, 21]. After the placement of self-expanding stents migration may occur, due to the shortening during their expansion as was observed in patient 5 [16].
In patient 4, the proximal end of the Wallstent impacted into the bile duct wall and its lack of flexibility, together with the sharp wire at its end, may have provided a base for the development of a duodenocolic fistula; thereafter, the scarring and contraction of the duodenal wall following radio chemotherapy completed it. Due to the stent’s rigidity, local erosions of neighboring vessels or the neighboring bowel wall may occur [4].
There is an increased risk of proximal stent migration with large-diameter or short stents [12]. Their migration can lead to symptoms of biliary obstruction if it is apparent at all [4, 12, 21]. It may be symptomatic with biliary obstruction or often not display any symptoms at all. Longer stents in the bile duct are less likely to migrate because a longer section is fixed in the common bile duct, thus limiting proximal movement [12].
The only risk factors for distal migration that have been identified are papillary stenosis [12] and — in a smaller number of cases — the omission of sphincterotomy [17]. Stenting is complicated relatively more frequently by distal migration in benign strictures, than in malignant ones [4, 12]. One possible explanation for this phenomenon is that the benign stenoses are not as tight, possibly due to regression of the inflammatory component. In malignant strictures, tumor growth may help to anchor the stent, thus preventing its migration.
Migration may lead to impaction in the distal gut. The common denominator of an impacted stent is the extrinsic fixation of the bowel wall, impeding the passage of the stent through the bowel lumen [14, 19, 23]. On very rare occasions, a perforation occurs without any obvious associated bowel pathology [20].
Complications after stent migration can be classified into penetration, perforation, and obstruction of the intestine. Rarely, other organs—such as the pleura or pancreas—can be affected [11, 15]. Penetration requires adherence between the perforated organ and another organ. It does not lead to diffuse peritonitis or intraabdominal contamination, but eventually causes the development of a fistula (e.g., duodenocolic) [22]. Penetration of the intestinal wall can lead to an abscess, which may resolve after stent extraction and conservative treatment [23]. Cases of stent migration leading to complications that have been published in the last 2 years are summarized in Table 2.
Intestinal wall lesions due to a migrated stent are observed relatively frequently in the duodenum [2, 7, 22]. In these cases, the stent is too rigid and the duodenal wall is fixed in the retroperitoneal space. Perforation can occur in diverticular sacs of the colon because the walls of the diverticula are extremely thin [13, 23]. Because stents move naturally along the lumen of the intestine, the therapeutic approach to stent migration is primarily conservative. If they become impacted and they are endoscopically accessible, endoscopic removal is the therapy of choice. Endoscopy may even be sufficient in cases of local wall penetration [7, 23]; however, an operation is mandatory in cases of perforation and peritonitis [2, 13, 24].
A review of the literature shows that the overwhelming majority of complications with bowel perforation were caused by a straight stent [2, 13, 15, 18, 19, 20].
Because stent migration can entail concomitant risks — especially in patients with adhesions, diverticular disease, or a hernia — the insertion of a straight plastic stent should be undertaken cautiously. Close follow-up is mandatory in this group.
For liver transplantation, we no longer use prophylactic stenting. The use of stents can even result in a higher incidence of stenosis, not to mention further possible complications from T-tubes or endoprostheses [3].
For the treatment of biliary obstruction, short-term stenting is a simple and useful procedure that obviates the need for surgical intervention. However, in patients requiring long-term therapy, stent migration has to be considered as a relevant complication that may eventually lead to a life-threatening situation. Therefore, the retrieval of migrated stents should be attempted in all cases. A migrated stent that has become symptomatic should be removed within 24 h. If conservative and endoscopic approaches fail, early operation is mandatory.
References
H Ahlström LE Lörelius G Jacobson (1986) ArticleTitleInoperable biliary obstruction treated with percutaneously placed endoprosthesis. Acta Chir Scand 152 301–303 Occurrence Handle2426890
A Basile A Macri S Lamberto S Caloggero A Versaci C Famulari (2003) ArticleTitleDuodenoscrotal fistula secondary to retroperitoneal migration of an endoscopically placed plastic biliary stent. Gastrointest Endosc 57 136–137
SM Bawa A Mathew H Krishnan E Minford D Talbot DF Mirza MG Thick P Gibbs D Manas (1998) ArticleTitleBiliary reconstruction with or without an internal biliary stent in orthotopic liver transplantation: a prospective randomised study. Transpl Int 11 S245–247 Occurrence Handle10.1007/s001470050470 Occurrence Handle9664988
WC Culp TC McCowas RP Lieberman TC Goertzen RF LeVeen TG Heffron (1996) ArticleTitleBiliary strictures in liver transplant recipients: treatment with metal stents. Radiology 199 339–346 Occurrence Handle1:STN:280:BymB3c3ns1I%3D Occurrence Handle8668775
PHP Davids AK Groen EAJ Rauws GNJ Tytgat K Huibregtse (1992) ArticleTitleRandomised trial of self expanding metal stents versus polyethylene stents for malignant biliary obstruction. Lancet 340 1488–1492 Occurrence Handle1:STN:280:ByyC3cfptFY%3D Occurrence Handle1281903
GD De Palma C Cantanzano (1999) ArticleTitleSenting or surgery for treatment of irretrievable common bile duct calculi in elderly patients? Am J Surg 178 390–393 Occurrence Handle1:STN:280:DC%2BD3c%2FnslOrsw%3D%3D
M Distefano G Bonnano A Russo (2001) ArticleTitleBiliocutaneous fistula following biliary stent migration. Endoscopy 33 97 Occurrence Handle10.1055/s-2001-17404 Occurrence Handle1:STN:280:DC%2BD3M3jt1Crug%3D%3D Occurrence Handle11204999
RG Figueiras MO Echart AG Figueiras GP Gonzalez (2001) ArticleTitleColocutaneous fistula relating to the migration of a biliary stent. Eur J Gastroenterol Hepatol 13 1251–1253 Occurrence Handle10.1097/00042737-200110000-00021 Occurrence Handle1:STN:280:DC%2BD3MnmsVamug%3D%3D Occurrence Handle11711785
E Fiori G Mazzoni G Galati SE Lutzu A De Cesare M Bononi A Bolognese A Tocchi (2002) ArticleTitleUnusual breakage of a plastic biliary endoprosthesis causing an enterocutaneous fistula. Surg Endosc 16 870 Occurrence Handle10.1007/s004640042021 Occurrence Handle1:STN:280:DC%2BD383lvVagug%3D%3D
DA Howell SF Nezhad RM Dy (1999) ArticleTitleEndoscopically placed Gianturco endoprostheses in the treatment of malignant and benign biliary obstruction. Gastrointest Endosc Clin North Am 9 479–490 Occurrence Handle1:STN:280:DyaK1MzhvFSnsA%3D%3D
MB Jendresen LB Svendsen (2001) ArticleTitleProximal displacement of biliary stent with distal perforation and impaction in the pancreas. Endoscopy 33 195 Occurrence Handle10.1055/s-2001-12808 Occurrence Handle1:STN:280:DC%2BD3M7ntF2itQ%3D%3D Occurrence Handle11272227
JF Johanson MJ Schmalz JE Geenen (1992) ArticleTitleIncidence and risk factors for biliary and pancreatic stent migration. Gastrointest Endosc 38 341–346 Occurrence Handle1:STN:280:By2B1MbitVc%3D Occurrence Handle1607087
U Klein F Weiss O Wittkugel (2001) ArticleTitle[Migration of a biliary Tannenbaum stent with perforation of sigmoid diverticulum.] RoFo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 173 1057 Occurrence Handle10.1055/s-2001-18306 Occurrence Handle1:STN:280:DC%2BD3Mnlt1OrsQ%3D%3D Occurrence Handle11704920
JM Levey (2002) ArticleTitleIntestinal perforation in a parastomal hernia by a migrated plastic biliary stent. Surg Endosc 16 1636–1637 Occurrence Handle10.1007/s00464-002-4506-9 Occurrence Handle1:STN:280:DC%2BD38notlGhtQ%3D%3D
L Liebich-Bartholain U Kleinau H Elsbernd R Büchsel (2001) ArticleTitleBiliary pneumonitis after proximal stent migration. Gastrointest Endosc 2001 382–384 Occurrence Handle10.1067/mge.2001.113646
EJ Loveday (1997) ArticleTitleA migrating biliary wallstent: an unusual complication. Clin Radiol 52 246 Occurrence Handle1:STN:280:ByiB38nivFA%3D
C Margulies ES Siqueira WB Silverman XS Lin JA Martin M Rabinovitz A Slivka (1999) ArticleTitleThe effect of endoscopic sphincterotomy on acute and chronic complications of biliary endoprostheses. Gastrointest Endosc 49 716–719 Occurrence Handle1:STN:280:DyaK1M3ns1Gisg%3D%3D Occurrence Handle10343215
JW Marsman HP Hoedemaker (1996) ArticleTitleNecrotizing fasciitis: fatal complication of migrated biliary stent. Australas Radiol 40 80–83 Occurrence Handle1:STN:280:BymH3sngtFU%3D Occurrence Handle8838897
BM Mistry MA Memon R Silverman FR Burton CR Vanna H Solomon PJ Garvin (2001) ArticleTitleSmall bowel perforation from a migrated biliary stent. Surg Endosc 15 1043 Occurrence Handle1:STN:280:DC%2BD3MrlvVCmuw%3D%3D
R Mofidi K Ahmed A Mofidi WP Joyce Z Khan (2000) ArticleTitlePerforation of the ileum: an unusual complication of distal biliary stent migration. Endoscopy 32 S67 Occurrence Handle1:STN:280:DC%2BD3M7ptlKiuw%3D%3D Occurrence Handle11085487
T Nakamura R Hirai M Kitagawa Y Takehira M Yamada K Tamekoshi Y Kobayashi H Nakamura M Kanamori (2002) ArticleTitleTreatment of common bile duct obstruction by pancreatic cancer using various stents: single centre experience. Cardiovasc Intervent Radiol 25 373–380 Occurrence Handle10.1007/s00270-002-0426-2 Occurrence Handle12016522
AK Pathak LJ de Souza (2001) ArticleTitleDuodenocolic fistula: an unusual sequela of stent migration. Endoscopy 33 731 Occurrence Handle10.1055/s-2001-16220 Occurrence Handle1:STN:280:DC%2BD3MvktlCgug%3D%3D Occurrence Handle11490393
TA Ruffolo GA Lehman S Sherman R Aycock A Hayes (1992) ArticleTitleBiliary stent migration with colonic diverticular impaction. Gastrointest Endosc 38 81–83 Occurrence Handle1:STN:280:By2A3cjjslU%3D Occurrence Handle1612389
V Selivanov GF Sheldon JP Cello RA Crass (1984) ArticleTitleManagement of foreign body ingestion. Ann Surg 199 187–191 Occurrence Handle1:STN:280:BiuC38jgsFI%3D Occurrence Handle6696536
PR Tarnasky PB Cotton J Baillie MS Branch J Affronti P Jowell S Guarisco RE England JWC Leung (1995) ArticleTitleProximal migration of biliary stents: attempted endoscopic retrieval in forty-one patients. Gastrointest Endosc 42 513–519 Occurrence Handle1:STN:280:BymB3srot1M%3D Occurrence Handle8674920
A Wilhelm C Langer G Zoeller R Nustede H Becker (2003) ArticleTitleComplex colovesicular fistula: a severe complication caused by biliary stent migration. Gastrointest Endosc 51 124–126
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diller, R., Senninger, N., Kautz, G. et al. Stent migration necessitating surgical intervention. Surg Endosc 17, 1803–1807 (2003). https://doi.org/10.1007/s00464-002-9163-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-002-9163-5