Abstract
Background: The laparoscopic use of fluorescein and ultraviolet light may be a useful diagnostic tool that potentially could reduce the time until diagnosis and the subsequent mortality of mesenteric ischemia. Methods: Eight pigs were subjected to a pneumoperitoneum pressure of 7 mmHg, and another eight pigs were exposed to a pressure of 14 mmHg. A segment of small bowel was devascularized. Two filters were used to create ultraviolet light. Pigs from each pressure group were given various intravenous fluorescein doses. The ischemic segment of the small intestine and other structures were inspected laparoscopically with the filters attached. A videotape was evaluated by resident and attending surgeons. Results: Ischemic bowel was seen as a darkened silhouette against the viable fluorescent tissue. Overall, the results show that the use of ultraviolet light and fluorescence in the laparoscopic model is adequate for allowing the identification of ischemic bowel. Conclusions: The laparoscopic use of ultraviolet light combined with intravenous fluorescein dye is an effective diagnostic tool for evaluating mesenteric ischemia in pigs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite advances in diagnostic and therapeutic technologies, acute mesenteric ischemia continues to be a rare but devastating condition with a mortality rate of 70% to 90%. Delayed diagnosis is cited as an important factor contributing to poor outcome, yet early diagnosis of intestinal ischemia remains one of the most challenging aspects of the disease [1, 4, 8, 11, 13].
Several factors are implicated in the difficulty diagnosing acute mesenteric ischemia. The typical patient reports abdominal pain out of proportion to physical findings, but often chronic illnesses or altered mental status can obscure this classic presentation. Currently, the most accurate method for diagnosing acute bowel ischemia is mesenteric angiography. However, administration of intravenous contrast and transport of the patient to the radiology department are required for this procedure and pose potential risks to medically unstable patients [9, 11]. Computed tomography and ultrasound, frequently obtained for evaluation, contribute to the delay in treatment because they are nondiagnostic of mesenteric ischemia or infarction. Blood tests, radionucleotide scanning, peritoneal fluid analysis, and endoscopy have been explored as methods for improving diagnostic accuracy and time until diagnosis, yet delayed and inaccurate diagnoses continue to occur. Moreover, no diagnostic intervention so far has been shown to reduce the mortality rate [4, 11, 13].
Diagnostic laparoscopy is used with increasing frequency to evaluate the acute abdomen in the critically ill patient. First described in 1989 as a means to evaluate mesenteric ischemia at the bedside, this technique allowed for laparoscopic inspection of the intestine’s serosal surface [8]. Over the past decade, a growing number of studies have concluded that bedside laparoscopy is a safe and efficient means for assessing bowel viability [11, 15]. This technique avoids the problems of transport and nephrotoxic agents, potentially reducing the number of diagnostic tests, and hence, time until diagnosis.
The use of intravenous sodium fluorescein with ultraviolet light to produce fluorescence in tissue is an established technique to determine intraoperative intestinal viability [1, 2, 3, 5, 6, 7, 10, 11, 12, 14, 18]. First performed by Lange in 1942, this technique results in fluorescence of viable, perfused tissue when it is exposed to long wave ultraviolet light [12]. Conversely, tissues that are not perfused do not fluoresce and are deemed nonviable. Fluorescein dye is inexpensive, readily available, and safe [2, 5, 7, 11, 12, 18]. Gorey [6] reported a sensitivity of 96% and a specificity of 95% with the use of fluorescein fluorescence in open exploration for mesenteric ischemia in the rat model. Pearse et al. [17] found similar results in the early assessment of intestinal ischemia in the canine model with a sensitivity of 88%. In human trials, Paes et al. [16] found fluorescein to be highly effective in identifying mesenteric infarcts in 38 patients. Bulkley et al. [2] found fluorescein testing to have 100% sensitivity and specificity in predicting bowel ischemia in a prospective, controlled study of 28 consecutive patients.
The application of fluorescein and long-wave ultraviolet light to laparoscopy would be a powerful adjunct to the current bedside procedures for determining mesenteric ischemia. The procedure would allow for direct, bedside assessment of tissue perfusion, thus permitting identification of ischemic and viable tissue. Potentially, the accuracy in identifying ischemic bowel would be enhanced, thereby reducing the time to diagnosis and mortality.
The current study describes the use of two light filters attached to standard videolaparoscopic equipment to achieve effective laparoscopic fluorescence of viable tissues and visualization of ischemic bowel in the porcine model. We propose that the laparoscopic use of ultraviolet light and fluorescein provides the surgeon with the ability to discern between viable and ischemic tissues on a consistent basis. Furthermore, the fluorescent visualization of different ischemic segments as a function of pneumoperitoneum pressures and fluorescein dye dosages is examined.
Materials and methods
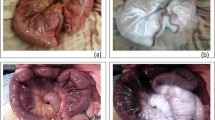
The Institutional Animal Care and Use Committee at Allegheny General Hospital approved the study. Sixteen 25-lb pigs were anesthetized with ketamine 15 mg/kg intramuscularly (IM), xylozine 2 mg/kg IM, and isoflurane 4% via an endotracheal tube, then maintained on ventilators. Arterial catheters were placed to monitor hemodynamics. Abdominal access was achieved with a Veress needle, and pneumoperitoneum was established with carbon dioxide gas. Eight of the pigs were subjected to a pneumoperitoneum pressure of 7 mmHg, whereas the other eight were exposed to a pressure of 14 mmHg. After laparoscopic evaluation of the abdominal cavity, a 10- to 15-cm segment of small bowel was devascularized with surgical clips, and the bowel became visibly ischemic (Fig. 1). Two filters (Karl Storz Endoscopy, Tuttlingen, Germany) were used. One filter was placed between the light source and the light cable to filter all but long-wave (380–440 nm) ultraviolet light. The second filter was inserted between the laparoscope and the camera to filter all but fluorescent frequency.
Two pigs from each pneumoperitoneum pressure group (7 mmHg and 14 mmHg) were given an intravenous sodium fluorescein dose of 5 mg/kg, 10 mg/kg, 15 mg/kg, or 20 mg/kg. The ischemic segment of the small intestine, surrounding bowel, and other structures were inspected laparoscopically with the filters attached. Videotape and digital still images recorded the laparoscopic findings from the 16 pigs. The pigs were then euthanized with intracardiac potassium chloride.
Eight representative segments of the video documentation and digital images after the fluorescein injection were presented in randomized order to 15 surgical residents and attending surgeons. Each segment contained the laparoscopic findings from both pressure groups (7 mmHg and 14 mmHg) and all four fluorescein dose groups. These evaluators, who had a wide range of laparoscopic experience, were blinded to the fluorescein dosage and pneumoperitoneum pressure and were not affiliated with this study. On the basis of the premise that green fluorescence was perfused tissue and darkened tissue was nonperfused tissue, the evaluators were asked first if an ischemic segment could be identified. Then they rated their ability to discern ischemic bowel from viable tissue and their ability to visualize surrounding structures. The rating occurred on a scale of 1 to 5. A rating of 1 denoted complete inability to define an area of ischemia or to see any surrounding bowel or organs. A rating of 2, 3, or 4 represented ability to discern ischemia or visualize structures as fair, good, or very good, respectively. A rating of 5 reflected excellent distinction between an ischemic and perfused segment of intestine and excellent visualization of surrounding structures.
Results
All of the pigs remained hemodynamically stable during the procedure. The investigators noted that in all 16 pigs, viable tissue and intestine were visualized as fluorescent within 30 s after fluorescein injection. Additionally, the ischemic segment of intestine was noted to be a darkened silhouette against the fluorescent tissue. In all cases, sufficient fluorescence was achieved to visualize surrounding organs and intestine, such that the investigators were able laparoscopically to inspect the bowel from proximal to distal end (Fig. 2).
All 15 surgical residents and staff evaluating the selected video documentation identified the ischemic segment of the small bowel from all 16 pigs. In rating their ability to identify an ischemic intestinal segment or visualize surrounding structures, the evaluators did not register any one particular fluorescein dosage or pneumoperitoneum pressure as superior. The average scores for each group are listed in Tables 1 and 2.
These data were analyzed with the Friedman test, a nonparametric comparison of the distributions of several related variables. The average rating for the ability to identify an ischemic segment of bowel was significantly worse (p < 0.05) in the pigs exposed to 14 mmHg and 15 mg/kg of fluorescein than in the remaining groups. The mean rating in this group for identifying ischemia and visualizing viable bowel was 3.1 and 3.7, respectively. The ratings ranged from 2 to 5 (fair to excellent), with 25% of the ratings documented as excellent or very good, 58% as good, and 17% as fair for discerning ischemia. With regard to visualizing surrounding viable structures, 66% of the ratings were documented as excellent or very good, 25% as good and 8% as fair.
Overall, the results show that the use of ultraviolet light and fluorescence in the laparoscopic model is adequate for allowing the identification of ischemic bowel. Specifically, the ability to determine ischemic bowel using fluorescein dosages of 5, 10, and 20 mg/kg was classified as very good or excellent in 83% to 91% of all evaluator ratings. Visualization of surrounding structures was rated as very good to excellent in 75% to 100% of all evaluator ratings for these same dosages.
Discussion
The preliminary data from this study demonstrate that perfused and ischemic bowel can be identified laparoscopically in the porcine model using intravenous sodium fluorescein dye and two ultraviolet light filters on standard laparoscopic equipment. Additionally, varying the dose of fluorescein or amount of pneumoperitoneum does not interfere with accurate assessment of bowel viability.
The dosage of fluorescein for optimal fluorescence has not been established, with most studies reporting 2 to 15 mg/kg [2]. We were interested in investigating differences in fluorescence over a range of dosages. We hypothesized that lower doses of fluorescein might not provide enough fluorescence to permit inspection of the small intestine from proximal to distal points, and to see surrounding structures. The visualization of surrounding viable tissue is dependent on the fluorescence created by the perfusion of fluorescein through the interstitial fluid of the tissue, which is excited by long-wave ultraviolet light. Conversely, we hypothesized that higher doses of fluorescein might alter the ability to determine ischemic tissue through microvascular encroachment of fluorescein from viable segments into ischemic segments or staining from peritoneal fluid that contains fluorescein.
This study was conducted using a standard pneumoperitoneum pressure of 14 mmHg and a lower pressure of 7 mmHg. The rationale for investigating the lower pneumoperitoneum pressure was based on previous studies concluding that a pneumoperitoneum pressure higher than 12 to 15 mmHg is associated with decreased capillary blood flow [19]. As such, we were interested in seeing whether a lower pneumoperitoneum pressure would allow for increased capillary blood flow, thereby improving perfusion of the tissue with fluorescein. The amount of fluorescence generated by the ultraviolet light then would be increased, and visualization of viable structures would be better than for those tissues subjected to a higher pneumoperitoneum pressure. Our results failed to demonstrate this.
The ability to discern ischemia was comparable in all pressure groups, excluding the group of pigs receiving a pneumoperitoneum pressure of 14 mmHg and a fluorescein dose of 15 mg/kg. Similar results were achieved when the evaluators judged their visualization of viable structures. The significance of this lower rating is unknown because better ratings were seen in categories with lower and higher fluorescein doses and lower pressures. In addition, the hemodynamics of the pig and the technique used to create ischemia were the same as with other models. Possible mechanisms that could have caused this finding include inadequate clip placement and staining of the serosa with fluorescein from surrounding perfused organs.
Although acute mesenteric ischemia is a rare disorder, its persistently high mortality rate continues to challenge clinicians. Intestinal necrosis can occur within hours, making prompt diagnosis and treatment critical to a successful outcome. Improvement in this outcome is hampered by late presentation of patients, delayed or incorrect diagnosis, and delay in treatment.
Diagnostic laparoscopy for the evaluation of abdominal vascular catastrophes is not yet an established method, but is being used increasingly to assess the acute abdomen at the bedside [8, 15, 20]. However, the visual assessment of ischemic intestine under white light is unreliable in both open and laparoscopic conditions [3, 7, 11]. The adjuvant use of fluorescein dye has been shown to increase the sensitivity and specificity of clinical judgement alone in open laparotomies for intestinal ischemia, and it is more accurate than other operative means of diagnosis, such as Doppler flowmetry [1, 2, 6, 7, 10, 14].
Two studies have examined the use of fluorescence and endoscopic technology to determine bowel ischemia [5, 10]. However, both studies used an argon laser light transmitted through a fiberoptic strand inserted through a working port in the endoscope. Visualization of ischemic bowel was adequate but most standard laparoscopes do not have a working port. In addition, many institutions do not have access to an argon laser, particularly at the bedside.
The laparoscopic use of ultraviolet light created by a long-wave ultraviolet light filtering system combined with intravenous fluorescein dye is an effective diagnostic tool in the evaluation of mesenteric ischemia in the porcine model. This pilot study investigated the effects of fluorescein dosage and pneumoperitoneum pressure in the detection of mesenteric ischemia. The data presented in this study serve as preliminary findings for future investigation of the same technique in a larger sample and perhaps with human subjects. These results should be interpreted cautiously. A greater number of cases are needed to determine an optimal dosage of fluorescein.
The purpose of this study was to present a new technique that, if proved efficacious, may have clinical implications on humans. Its use at the bedside of humans for early diagnosis of mesenteric ischemia should be investigated. This technique has the potential to reduce the mortality associated with this condition by reducing the time to diagnosis through elimination of the need for unnecessary or potentially harmful tests. The application of the light-filtering system to standard laparoscopic equipment is simple and can be provided universally to all hospitals with standard laparoscopic capabilities.
References
J Ballard W Stone J Hallett P Pairolero K Cherry (1993) ArticleTitleA critical analysis of adjuvant techniques used to assess bowel viability in acute mesenteric ischemia. Am Surg 59 309–311 Occurrence Handle1:STN:280:ByyB2MnjslA%3D Occurrence Handle8489100
G Bulkley G Zuidema S Hamilton C O’Mara P Klacsmann S Horn (1981) ArticleTitleIntraoperative determination of small intestinal viability following ischemic injury. Ann Surg 193 628–637
D Dyess B Bruner C Donnell J Ferrara R Powell (1991) ArticleTitleIntraoperative evaluation of intestinal ischemia: a comparison of methods. South Med J 84 966–974 Occurrence Handle1:STN:280:By6A2M3msVE%3D Occurrence Handle1882273
J Eldrup-Jorgensen R Hawkins C Brendenberg (1997) ArticleTitleAbdominal vascular catastrophes. Surg Clin North Am 77 1305–1320 Occurrence Handle1:STN:280:DyaK1c%2Fptleluw%3D%3D Occurrence Handle9431341
S Galandiuk V Fazio R Petras (1988) ArticleTitleFluorescein endoscopy. Dis Colon Rectum. 31 848–853 Occurrence Handle1:STN:280:BiaD3s%2FhtFM%3D Occurrence Handle3180956
T Gorey (1980) ArticleTitleThe recovery of intestine after ischemic injury. Br J Surg 67 699–702 Occurrence Handle1:STN:280:Bi6D2c%2Fms1w%3D Occurrence Handle7427023
P Horgan T Gorey (1992) ArticleTitleOperative assessment of intestinal viability. Surg Clin North Am 72 143–154 Occurrence Handle1:STN:280:By2C3Mnis1M%3D Occurrence Handle1731381
T Iberti B Salky D Onofrey (1989) ArticleTitleUse of bedside laparoscopy to identify intestinal ischemia in postoperative cases of aortic reconstruction. Surgery 105 686–688 Occurrence Handle1:STN:280:BiaB3czntFY%3D Occurrence Handle2523091
R Kaleya S Boley (1992) ArticleTitleAcute mesenteric ischemia: an aggressive diagnostic and therapeutic approach. 1991 Roussel lecture. Can J Surg 35 613–623 Occurrence Handle1:STN:280:ByyD1M3gtlc%3D Occurrence Handle1458387
D Kam D Scheeres (1993) ArticleTitleFluorescein-assisted laparoscopy in the identification of arterial mesenteric ishemia. Surg Endosc 7 75–78 Occurrence Handle1:STN:280:ByyB3cnitVc%3D Occurrence Handle8456372
B Kurland L Brandt H Delany (1992) ArticleTitleDiagnostic tests for intestinal ischemia. Surg Clin North Am 72 85–101 Occurrence Handle1:STN:280:By2C3MnislM%3D Occurrence Handle1731391
K Lange L Boyd (1942) ArticleTitleThe use of fluorescein to determine the adequacy of the circulation. Med Clin North Am 26 943–952
N Mamode I Pickford P Leiberman (1999) ArticleTitleFailure to improve outcome in acute mesenteric ischemia: seven-year review. Eur J Surg 165 203–208 Occurrence Handle10.1080/110241599750007054 Occurrence Handle1:STN:280:DyaK1M3kvVCgtg%3D%3D Occurrence Handle10231652
A Mann V Fazio F Lucas (1982) ArticleTitleA comparative study of the use of fluorescein and the Doppler device in the determination of intestinal viability. Surg Gynecol Obstet 154 53–55 Occurrence Handle1:STN:280:Bi2D1crgslI%3D Occurrence Handle6976009
R Orlando K Crowell (1997) ArticleTitleLaparoscopy in the critically ill. Surg Endosc 11 1072–1074 Occurrence Handle10.1007/s004649900532 Occurrence Handle9348376
E Paes J Vollmar S Hutschenreiter M Schoenberg R Kubel E Scholzel (1988) ArticleTitleMesenteric infarct: new aspects of diagnosis and therapy. Chirurg 59 828–835 Occurrence Handle1:STN:280:BiaC28bptVE%3D Occurrence Handle3234093
W Pearce D Jones G Warren E Bartle T Whitehill R Rutherford (1987) ArticleTitleThe use of infrared photoplethysmography in identifying early intestinal ischemia. Arch Surg 122 308–310 Occurrence Handle1:STN:280:BiiC2MrpsVA%3D Occurrence Handle3827570
C Stolar J Randolph (1978) ArticleTitleEvaluation of ischemic bowel viability with a fluorescent technique. J Pediatr Surg 13 221–225 Occurrence Handle1:STN:280:CSeB38%2FhtFQ%3D Occurrence Handle671186
J Windsor M Bonham M Rumball (1997) ArticleTitleSplanchnic mucosal ischemia: an unrecognized consequence of routine pneumoperitoneum. Surg Laparosc Endosc 7 480–482 Occurrence Handle10.1097/00019509-199712000-00010 Occurrence Handle1:STN:280:DyaK1c%2FpvFCluw%3D%3D Occurrence Handle9438631
G Zamir P Reissmann (1998) ArticleTitleDiagnostic laparoscopy in mesenteric ischemia. Surg Endosc 12 390–393 Occurrence Handle10.1007/s004649900688 Occurrence Handle1:STN:280:DyaK1c3ktVKitw%3D%3D Occurrence Handle9569355
Acknowledgements
We thank Sandra Bell McGinty, PhD, for her editorial help, statistical expertise, and endless support, Laurel Omert, MD, for her editing assistance, and Ms. Susan Gardner for her assistance in preparing the text.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McGinty, J., Hogle, N. & Fowler, D. Laparoscopic evaluation of intestinal ischemia using fluorescein and ultraviolet light in a porcine model . Surg Endosc 17, 1140–1143 (2003). https://doi.org/10.1007/s00464-001-8255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-001-8255-y