Abstract
We investigated the predictive value of the corticobulbar tract (CBT) for dysphagia using diffusion tensor tractography in the early stage of intracerebral hemorrhage (ICH) for dysphagia. Forty-two patients with spontaneous ICH ± intraventricular hemorrhage (IVH) and 22 control subjects were recruited. The patients were classified into three groups: group A—could remove nasogastric tube (NGT) in the acute stage of ICH, group B—could remove NGT within 6 months after onset, and group C—could not remove NGT until 6 months after onset. The CBT were reconstructed, and fractional anisotropy (FA) and tract volume (TV) values were determined. The FA of the CBT in the affected hemisphere in group A was lower than in the control group (p < 0.05). The FA and TV of the CBT in the affected hemisphere in group B were lower than those in the control group (p < 0.05). In group C, the FA and TV in the affected hemisphere and unaffected hemispheres were lower than in the control group (p < 0.05). The TV of the CBT in the affected hemisphere in group B showed a moderate negative correlation with the length of time until NGT removal (r = 0.430, p < 0.05). We found that patients with CBT injuries in both hemispheres were not able to remove the NGT until 6 months after onset, whereas patients who were injured only in the affected hemisphere were able to remove NGT within 6 months of onset. The severity of injury to the CBT in the affected hemisphere appeared to be related to the length of time until NGT removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dysphagia is one of the most serious disabling sequelae of stroke [1]. Dysphagia has been reported to occur in approximately 30–50% of stroke patients: 50.9% of hospitalized patients in the early stage of stroke and 30.5% of discharged patients after the early stage of stroke have been reported to require a nasogastric tube (NGT) due to dysphagia [2, 3]. Dysphagia has a significant negative impact on stroke outcome because it is related to aspiration pneumonia, malnutrition, increased mortality, and prolonged hospital stay [4,5,6,7,8]. Furthermore, dysphasia is common in diseases which are common in general population such as hypertension, gastro-esophageal reflux disease, anxiety and depression [2, 9,10,11]. As a result, accurate prognosis prediction as well as rehabilitative management of dysphagia in the early stage of stroke is important because it could provide useful information for planning specific rehabilitation strategies and for estimating the duration of rehabilitation.
Prognostic prediction of dysphasia has been conducted using demographic factors, clinical characteristics, radiologic findings, videofluoroscopic findings, swallowing endoscopy findings, and water swallowing test: negative predictors- older age, cognitive impairment, impaired consciousness, lower functional state, stroke severity, lesion location (bilateral lesions, infratentorial lesion, and internal capsule, frontal or insular cortices), delayed oral transit and contrast penetration of laryngeal cavity [7, 10,11,12,13,14,15,16,17,18,19,20,21,22,23]. However, these techniques are focused on the clinical characteristics and swallowing function, therefore, they were limited in estimating injury to the corticobulbar tract (CBT), which is the main neural tract for swallowing [24].
The CBT innervates the nuclei for cranial nerves V, VII, XI, and XII, and also contributes to the cranial nerves IX and X. The muscles of the face, head, and neck are controlled by the CBT and injury of the CBT can accompany bulbar symptoms including dysphagia [25]. Therefore, elucidation of the state of the CBT would be useful for prognostic prediction of dysphagia (especially, removal of the NGT) in stroke patients. Advances in diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), have enabled three-dimensional reconstruction and estimation of the CBT [27,27,28,29,30,31]. Several studies using DTT have demonstrated injury of the CBT in several brain pathologies including stroke [24, 25, 27,28,29, 33,33,34,35,36]. However, no study on the prognostic prediction of dysphagia using DTT of the CBT has been reported so far.
In this study, we hypothesized that the state of the CBT in the early stage of intracerebral hemorrhage (ICH) would have predictive value for removal of the NGT. We therefore investigated the predictive value of the CBT state on DTT in the early stage of ICH for removal of the NGT.
Methods
Subjects
Forty-two consecutive patients (22 men, mean age; 58 ± 11 years, range 28–80 years) were recruited according to the following inclusion criteria: (1) first ever stroke, (2) a spontaneous supratentorial ICH ± intraventricular hemorrhage (IVH) that was confirmed by a neuroradiologist, (3) age at onset of ICH: 20–80 years, (4) DTI was performed at an early stage (less than 6 weeks after onset), (5) patients who had alert mentality and could obey one step commands, and (6) dysphagia at the onset of stroke requiring NGT insertion. The exclusion criteria were as follows: (1) previous history of psychiatric or neurological disease, (2) previous history of dysphagia or vocal palsy, (3) inability to obey a verbal command due to cognitive impairment. Twenty-two age- and sex-matched healthy control subjects without previous history of psychiatric or neurological disease (11 men, 11 women; mean age 51 ± 11, range 33–77 years) were recruited for this study.
Bedside screening test for dysphagia was performed for all patients who had been admitted or transferred to the rehabilitation department using the Gugging Swallowing Screen (GUSS) [37]. When the GUSS score was more than 16 points, the confirmatory videofluoroscopic swallowing study (VFSS) was performed. Penetration-aspiration scale and the vallecular and pyriform sinus residue scale after swallowing on the VFSS image (grade 0, no residue; grade 1, < 10% of all width of vallecular or pyriform sinuses; grade 2, 10–50% of width; grade 3, ≥ 50% of width) were used to determine whether NGT could be removed and the patients could start oral feeding [38, 39]. NGT was removed when (1) the penetration-aspiration scale is less than score 2 and (2) vallecular and pyriform sinus residue scale result is grade 1 or 2.
All patients who had failed to start oral feeding based on the initial evaluation of GUSS or VFSS, dysphagia rehabilitation therapy was performed by occupational therapists (60 min/day, five times/week). Dysphagia rehabilitation therapy includes direct and indirect methods to facilitate the sensory and motor function of orofacial and laryngopharyngeal muscles. The compensatory strategies of swallowing were also provided; these methods include Mendelsohn’s maneuver, head and neck positioning, supraglottic swallowing. Neuromuscular electrical stimulation (NMES) was applied to the extrinsic laryngeal muscles, especially suprahyoid muscles, for 30 min per session (5 five times/week). When patients showed clinical improvement during the dysphagia rehabilitation therapy, the GUSS and VFSS were performed again. When VFSS findings meet the aforementioned criteria, NGT was removed and oral feeding was started.
The patients were classified into three groups: (1) patient group A—patients who could remove the NGT in the acute stage of ICH (within 2 days after onset); (2) patient group B—patients who could not remove the NGT in the acute stage of ICH (within 2 days after onset) but were able to remove it within 6 months; (3) patient group C—patients for whom the NGT could not be removed until 6 months after onset. Ten patients [6 males; 4 female; mean age 51 ± 15 years; range 28–80 years; 5 with concurrent IVH (50.0%)] belonged to patient group A, 27 patients belonged to patient group B [13 males; 14 females; mean age 60 ± 8.9 years; range 47–79 years; 13 with concurrent IVH (48.1%)] and 5 patients belonged to patient group C [3 males; 2 females; mean age 59 ± 10 years; range 47–79 years; 3 with concurrent IVH (60.0%)]. The patients in group B were able to remove the NGT at an average of 36.44 ± 16.21 days after onset.
The volume of hematoma and total lesion on T2-weighted brain magnetic resonance images (21.39 ± 9.38 days) were calculated by using the following formula: [A (cm) × B (cm) × C (cm)]/2 (A the length, B the width of the largest cross-sectional area, C the total height from the bottom to the top slice of the hematoma) [40]. The peri-hematomal edema volume which the region of high signal on T2-weighted images around the hematoma, was calculated by subtracting the hematoma volume from the total lesion volume [41, 42]. The volume of the IVH was estimated using the modified Graeb Scale (mGS), which show separate ventricular compartments to IVH volume and regional accumulation [43]. The mGS consists of the eight parts of the ventricle which are the fourth, third, right and left lateral ventricles (maximum score 4 for each), right and left occipital horns (maximum score 2 for each), and the right and left temporal horns (maximum score 2 for each). The volumes were calculated by a single blinded expert. This study was conducted retrospectively, and the Institutional Review Board of a university hospital approved the study protocol.
Diffusion Tensor Imaging and Tractography
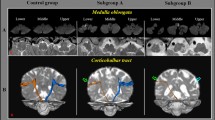
DTI scanning was performed once at an average of 21.39 ± 9.38 days after onset using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips Healthcare, Best, The Netherlands) with single-shot, spin echo-planar imaging. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; TR = 10,398 ms; TE = 72 ms; parallel imaging reduction factor (SENSE factor) = 2; EPI factor = 59; b = 1000 s/mm2; and a slice thickness of 2.5 mm. The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library was used to analyze DTI data. Eddy current correction was applied to correct the head motion effect and image distortion [44]. FMRIB Diffusion Software with routines option (0.5 mm step lengths, 5000 streamline samples, curvature thresholds = 0.2) was used for fiber tracking [44,45,46]. For analysis of the CBT, the seed region of interest (ROI) was placed on the lower pons (anterior blue portion on the color map), and the target ROI was placed on the lower portion of the precentral gyrus which was in the section of the top of the lateral ventricles [24,25,26, 30, 31]. The fractional anisotropy (FA) and tract volume (TV) of the CBT in both hemispheres were measured. DTT of all patients was analyzed by a single blinded expert (3 year’s experience of DTT analysis) (Fig. 1).
T2-weighted brain magnetic resonance images (a) and results of diffusion tensor tractography for the corticobulbar tract (CBT) (b). Control group: a representative subject (42-year-old female), group A: a representative patient (62-year-old female), group B: a representative patient (64-year-old male) shows narrowing (yellow arrow) of the CBT in the affected (left) hemisphere, and group C: a representative patient (77-year-old female) shows narrowing (yellow arrow) of the CBT in the unaffected (right) hemisphere and non-reconstruction (red arrow) of the CBT in the affected (left) hemisphere
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (IBM Corp. Released 2014, IBM SPSS Statistics for Windows, Version 23.0., Armonk, NY: IBM Corp.) Differences in baseline values between the four groups for independent variables were tested with the Kruskal–Wallis (one-way ANOVA) test and the Mann–Whitney test. Using Spearman correlation, the values of FA and TV of the CBT were used in determination of correlation with the length of time to NGT removal. A correlation coefficient of more than 0.60 indicated a strong correlation, between 0.40 and 0.59 indicated a moderate correlation, between 0.20 and 0.39 indicated a weak correlation, and less than 0.20 indicated a very weak correlation [47]. The significance level of the p value was set at 0.05.
Results
The baseline demographic and clinical data of the patient and control groups are summarized in Table 1. No significant differences were observed in terms of age, sex, Mini-Mental State Examination, mean duration to DTI scanning between the three patient and control groups (p > 0.05). However, in the lesion location, the thalamic lesion was significantly higher in the patient groups A and C than the patient group B (p < 0.05).
The comparison of the volumes of hematoma, peri-hematomal, total lesion and IVH between the patient groups are summarized in Table 2. The hematoma volume was significantly higher in the patient group C than the patient group B (p < 0.05). The volumes of the peri-hematomal edema and total lesion were significantly higher in the patient group C than the patient groups A and B (p < 0.05). The comparison of IVH scale did not show significant difference between the patient groups (p > 0.05).
The comparison of DTT parameters of the CBT between the patient and control groups is summarized in Table 3. The CBT in the affected hemisphere was not reconstructed in any of the five patients in patient group C. The FA value of the CBT in the affected hemisphere in patient group A was significantly lower than that of the control group (p < 0.05). However, the TV value of the CBT in the affected hemisphere and the values of FA and TV of the CBT in the unaffected hemisphere in patient group A were not significantly different from those of the control group (p > 0.05). The values of FA and TV of the CBT in the affected hemisphere and the FA value in the unaffected hemisphere in patient group B were significantly lower than those of the control group (p < 0.05) without significant differences in the TV value in the unaffected hemisphere. By contrast, in patient group C, the values of FA and TV in the affected and unaffected hemispheres were significantly lower than those of the control group (p < 0.05).
Table 4 shows a summary of correlations between the length of time to NGT removal in patient group B and DTT parameters of the CBT in both hemispheres. The TV value of the CBT in the affected hemisphere showed a moderate negative correlation with the time to NGT removal (r = 0.430, p < 0.05) [47].
Discussion
In the current study, by using DTT, we investigated the predictive value of the CBT state on DTT for dysphagia in the early stage of ICH and found the following results: (1) in patient group A, the FA value of the CBT in the affected hemisphere was lower than that of the control group; (2) in patient group B, the values of FA and TV of the CBT in the affected hemisphere were lower than those of the control group; (3) in patient group C, the values of FA and TV in the affected and unaffected hemispheres were lower than those of the control group; (4) the TV value of the CBT in the affected hemisphere showed a moderate negative correlation with the time to NGT removal; and (5) the volumes of the peri-hematomal edema and total lesion were larger in the patient group C than the patient groups A and B.
Among the DTT parameters, the FA and TV have most commonly been used in evaluating the state of neural tracts in patients with brain injury [48,49,50,51]. The FA value represents white matter organization by indicating the degree of directionality and the integrity of white matter microstructures such as axons, myelin, and microtubules, and a low FA value suggests a loss of white matter integrity [52]. The TV is determined by the number of voxels within a neural tract, thereby reflecting the total number of fibers in the tract [53]. Therefore, lower values of FA or TV of the CBT indicate an injury to the CBT [52, 53]. In detail, the CBT of the affected hemispheres in patient groups A and B and the CBTs in both hemispheres in patient group C appeared to be injured. However, the CBT of the affected hemispheres in patient group A showed relatively milder injury because only the FA value was decreased, whereas the CBT of the affected hemispheres in patient group C presented with more severe injury because none of the CBTs were reconstructed compared with the other patient groups. CBT injury in the unaffected hemisphere in patient group C might be ascribed to periventricular white matter injury by IVH or a neural tract injury in the unaffected hemisphere by large hematoma in patients with ICH, which were demonstrated in previous studies [54, 55]. As a result, our results can be summarized as follows: among patients who were obliged to feed using a NGT after the acute stage of ICH, patients who were injured the CBT only in the affected hemisphere were able to remove the NGT within 6 months after onset. However, patients with CBT injuries in both hemispheres were not able to remove the NGT until 6 months after onset. On the other hand, the total lesion volume was larger in the patient group C than the patient group A and B appears to be coincided with the results of the previous studies which dysphasia was related with lesion volume in stroke patients [13, 14].
Regarding the correlation between the time to NGT removal and DTT parameters of the CBT in patient group B, a moderate negative correlation was observed between the time to NGT removal and the TV value of the CBT in the affected hemisphere. Because the TV value indicates the total number of fibers in the CBT, the remaining fiber numbers in the CBT in the affected hemisphere appeared to be negatively related to the length of time until NGT removal. In other words, the severity of injury to the CBT in the affected hemisphere appeared to be related to the time to NGT removal in patients who could not remove the NGT due to dysphagia in the acute stage of ICH.
The fact that a patient could remove the NGT means the recovery of dysphasia in that patient. Regarding the recovery mechanisms of dysphasia, a few studies have reported [32, 56]. In 1998, Hamdy et al. reported that the pharyngeal motor cortex reorganizes after acute unilateral stroke, and an increase in cortical excitability in the unaffected hemisphere was associated the recovery of swallowing function using transcranial magnetic stimulation study [56]. In 2016, Jang et al. reported a patient who showed reorganization of a CBT at the subcortical white matter following cerebral infarct [32]. Therefore, further studies on the recovery mechanisms of dysphasia centered on the CBT should be warranted.
Since the introduction of DTI, a few studies have demonstrated dysphagia due to the CBT injury in stroke and traumatic brain injury [32, 35, 57]. However, to the best of our knowledge, this is the first study to demonstrate the predictive value of the CBT state on DTT in the early stage of ICH for dysphagia. However, the limitations of this study should be considered. First, the DTT analysis is operator-dependent, and regions of fiber complexity and crossing can prevent full reflection of the underlying fiber architecture by DTI [58]. Second, the presence of IVH in patients with supratentorial ICH is associated with a lack of homogeneity. Third, this study recruited a relatively small number of subjects. In addition, inequality of the number of patients for each patient group was another limitation of this study. Although it was inevitable because this study recruited the patients consecutively, further prospective studies including larger number of subjects should be encouraged to overcome the above limitations.
Conclusion
In conclusion, we found that patients with CBT injuries in both hemispheres could not remove the NGT until 6 months after onset, whereas patients who were injured only in the affected hemisphere were able to remove the NGT within 6 months after onset. Furthermore, the injury severity of the CBT in the affected hemisphere appeared to be related with the length of time to NGT removal. Our results suggest that the evaluation of the CBT state using DTT would be helpful for prognosis prediction of the removal of the NGT in the early stage of ICH.
Abbreviations
- CBT:
-
Corticobulbar tract
- DTT:
-
Diffusion tensor tractography
- DTI:
-
Diffusion tensor imaging
- ROI:
-
Regions of interest
- ICH:
-
Intracerebral hemorrhage
- IVH:
-
Intraventricular hemorrhage
- NGT:
-
Nasogastric tube
- FA:
-
Fractional anisotropy
- TV:
-
Tract volume
References
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63.
O’Neill PA, Smithard DG, Morris J. Complications and outcome following acute stroke: revised table. Stroke. 1998;29:1480–1.
Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000;10:380–6.
Guyomard V, Fulcher RA, Redmayne O, Metcalf AK, Potter JF, Myint PK. Effect of dysphasia and dysphagia on inpatient mortality and hospital length of stay: a database study. J Am Geriatr Soc. 2009;57:2101–6.
Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis. 2012;10:61–7.
Lin WC, Huang CY, Lee LF, Chen YW, Ho CH, Sun YT. Initial National Institute of Health Stroke Scale to early predict the improvement of swallowing in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:104297.
Schmidt J, Holas M, Halvorson K, Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994;9:7–11.
Kalra L, Smith DH, Crome P. Stroke in patients aged over 75 years: outcome and predictors. Postgrad Med J. 1993;69:33–6.
Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life—a population-based study. Aliment Pharmacol Ther. 2008;27:971–9.
Ickenstein GW, Stein J, Ambrosi D, Goldstein R, Horn M, Bogdahn U. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252:1510–6.
Ickenstein GW, Kelly PJ, Furie KL, Ambrosi D, Rallis N, Goldstein R, et al. Predictors of feeding gastrostomy tube removal in stroke patients with dysphagia. J Stroke Cerebrovasc Dis. 2003;12:169–74.
Broadley S, Croser D, Cottrell J, Creevy M, Teo E, Yiu D, et al. Predictors of prolonged dysphagia following acute stroke. J Clin Neurosci. 2003;10:300–5.
Kumar S, Doughty C, Doros G, Selim M, Lahoti S, Gokhale S, Schlaug G. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. J Stroke Cerebrovasc Dis. 2014;23:56–62.
Maeshima S, Okazaki H, Okamoto S, Mizuno S, Asano N, Tsunoda T, et al. Dysphagia following putaminal hemorrhage at a rehabilitation hospital. J Stroke Cerebrovasc Dis. 2016;25:389–96.
Maeshima S, Osawa A, Yamane F, Ishihara S, Tanahashi N. Dysphagia following acute thalamic haemorrhage: clinical correlates and outcomes. Eur Neurol. 2014;71:165–72.
Flowers HL, AlHarbi MA, Mikulis D, Silver FL, Rochon E, Streiner D, Martino R. MRI-based neuroanatomical predictors of dysphagia, dysarthria, and aphasia in patients with first acute ischemic stroke. Cerebrovasc Dis Extra. 2017;7:21–34.
Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–8.
Ween JE, Alexander MP, D’Esposito M, Roberts M. Incontinence after stroke in a rehabilitation setting: outcome associations and predictive factors. Neurology. 1996;47:659–63.
Daniels SK, McAdam CP, Brailey K, Foundas AL. Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6:17–24.
Horner J, Massey EW, Riski JE, Lathrop DL, Chase KN. Aspiration following stroke: clinical correlates and outcome. Neurology. 1988;38:1359–62.
Broadley S, Croser D, Cottrell J, Creevy M, Teo E, Yiu D, Pathi R, Taylor J, Thompson PD. Predictors of prolonged dysphagia following acute stroke. J Clin Neurosci. 2003;10:300–5.
Okubo PC, Fabio SR, Domenis DR, Takayanagui OM. Using the national institute of health stroke scale to predict dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2012;33:501–7.
Joundi RA, Martino R, Saposnik G, Giannakeas V, Fang J, Kapral MK. Predictors and outcomes of dysphagia screening after acute ischemic stroke. Stroke. 2017;48:900–6.
Kwon HG, Lee J, Jang SH. Injury of the corticobulbar tract in patients with dysarthria following cerebral infarct: diffusion tensor tractography study. Int J Neurosci. 2016;126:361–5.
Jenabi M, Peck KK, Young RJ, Brennan N, Holodny AI. Identification of the corticobulbar tracts of the tongue and face using deterministic and probabilistic DTI fiber tracking in patients with brain tumor. Am J Neuroradiol. 2015;36:2036–41.
Jang SH, Seo JP. The anatomical location of the corticobulbar tract at the corona radiata in the human brain: diffusion tensor tractography study. Neurosci Lett. 2015;590:80–3.
Jang SH, Kim SH, Kwon YH. Extensive traumatic axonal injury of brain due to violence: a case report. Medicine (Baltim). 2018;97:e13315.
Jang SH, Lee HD. Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study. Neural Regen Res. 2018;13:936.
Jang SH, Seo YS. Dysarthria due to injury of the corticobulbar tract in a patient with mild traumatic brain injury. Am J Phys Med Rehabil. 2016;95:e187–8.
Pan C, Peck KK, Young RJ, Holodny AI. Somatotopic organization of motor pathways in the internal capsule: a probabilistic diffusion tractography study. Am J Neuroradiol. 2012;33:1274–80.
Yim SH, Kim JH, Han ZA, Jeon S, Cho JH, Kim GS, Choi SA, Lee JH. Distribution of the corticobulbar tract in the internal capsule. J Neurol Sci. 2013;334:63–8.
Jang SH, Lee J, Kwon HG. Reorganization of the corticobulbar tract in a patient with bilateral middle cerebral artery territory infarct. Am J Phys Med Rehabil. 2016;95:e58–9.
Kim HK, Han M, Lee HJ. Corticobulbar tract involvement in neuropsychiatric systemic lupus erythematosus: a case report. Iran J Radiol. 2016;13:e32927.
Liégeois F, Tournier J-D, Pigdon L, Connelly A, Morgan AT. Corticobulbar tract changes as predictors of dysarthria in childhood brain injury. Neurology. 2013. https://doi.org/10.1212/WNL.1210b1013e3182840c3182846d.
Jang SH, Kim SH, Seo JP. Image of the month: dysphasia due to injury of the corticobulbar tract following traumatic brain injury. Clin Med (Lond). 2017;17:584–5.
Moon HI, Kim GS, Lee E. Is the location of white matter lesions important in the swallowing function of older patients with mild stroke? Dysphagia. 2018;34(3):407–14.
Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, Brainin M. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38:2948–52.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001;82:677–82.
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Staykov D, Wagner I, Volbers B, Hauer EM, Doerfler A, Schwab S, Bardutzky J. Natural course of perihemorrhagic edema after intracerebral hemorrhage. Stroke. 2011;42:2625–9.
Lee SH, Park HK, Ryu WS, Lee JS, Bae HJ, Han MK, Lee YS, Kwon HM, Kim CK, Park ES, Chung JW, Jung KH, Roh JK. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicenter randomized controlled trial. Eur J Neurol. 2013;20:1161–9.
Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK, et al. The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 2013;44:635–41.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19.
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–55.
Behrens TE, Johansen-Berg H, Woolrich M, Smith S, Wheeler-Kingshott C, Boulby P, Barker G, Sillery E, Sheehan K, Ciccarelli O. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750.
Wuensch KL. Straightforward statistic for the behavioral sciences. J Am Stat Assoc. 1996;91:1750.
Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–74.
Filippi M, Cercignani M, Inglese M, Horsfield M, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–11.
Mori S, Frederiksen K, Van Zijl PC, Stieltjes B, Kraut MA, Solaiyappan M, Pomper MG. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002;51:377–80.
Jang SH, Park JS, Shin DG, Kim SH, Kim MS. Relationship between consciousness and injury of ascending reticular activating system in patients with hypoxic ischaemic brain injury. J Neurol Neurosurg Psychiatry. 2018. https://doi.org/10.1136/jnnp-2018-318366.
Mori S, van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15:468–80.
Seo JP, Jang SH. Different characteristics of the corticospinal tract according to the cerebral origin: DTI study. Am J Neuroradiol. 2013;34:1359–63.
Kwon HG, Choi BY, Kim SH, Chang CH, Jung YJ, Lee HD, Jang SH. Injury of the cingulum in patients with putaminal hemorrhage: a diffusion tensor tractography study. Front Hum Neurosci. 2014;8:366.
Yeo SS, Choi BY, Chang CH, Jung YJ, Ahn SH, Son SM, Byun WM, Jang SH. Periventricular white matter injury by primary intraventricular hemorrhage: a diffusion tensor imaging study. Eur Neurol. 2011;66:235–41.
Barritt AW, Smithard DG. Role of cerebral cortex plasticity in the recovery of swallowing function following dysphagic stroke. Dysphagia. 2009;24:83.
Jang SH, Shin SM. The usefulness of diffusion tensor tractography for estimating the state of corticobulbar tract in stroke patients. Clin Neurophysiol. 2016;127:2708–9.
Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009;8:165–74.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2018R1A2B6000996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jang, S.H., Kwak, S.Y., Chang, C.H. et al. Prognostic Prediction of Dysphagia by Analyzing the Corticobulbar Tract in the Early Stage of Intracerebral Hemorrhage. Dysphagia 35, 985–992 (2020). https://doi.org/10.1007/s00455-020-10093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10093-3