Abstract
Our previous study regarding the tongue-hold swallow (THS) demonstrated that the tongue-to-palate contact during THS could be influenced by the maximum tongue protrusion length (MTPL) of individual subjects, resulting in two different patterns of pressure generation. The present study further analyzed the influence of MTPL on the tongue pressure production along with submental surface electromyography (sEMG) during THS, in order to establish an index to better control THS effects. Tongue pressure using a sensor sheet system and concurrent submental sEMG activities were measured during swallowing tasks in 18 healthy young adults. Task conditions comprised THS at two different degrees of tongue protrusion and dry swallow. Tongue pressures and sEMG activities were compared among three task conditions, and correlations of MTPL with tongue pressure were also investigated. Additionally, a ROC curve was used to find a cut-off value for MTPL to predict changes (increases and decreases) in tongue pressure during THS. The duration and the amount of submental muscle activity increased concurrently during THS. Two trends were shown on the change in tongue pressure at the posterior-circumferential part of the hard palate during THS compared to dry swallow; the maximal magnitude and the integrated value of tongue pressure increased in some subjects, while these values decreased in others. Thirty-two millimeters was found to be the cut-off value of MTPL, which distinguishes increase/decrease pattern of tongue pressure with sensitivities of 60.0–85.7%. The present finding suggests that more reliable THS effects should be attainable using MTPL to set the tongue-hold position.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tongue-hold swallow (THS) proposed by Fujiu and Logemann [1] involves a dry swallow while holding the anterior portion of the tongue between the upper and lower front teeth. Contact between the base of the tongue and the posterior pharyngeal wall during the pharyngeal stage of swallowing generates swallowing pressure, which plays an important role in the passage of the bolus through the hypopharynx [2]. Use of this technique as an exercise by individuals with reduced contact between the base of the tongue and the pharyngeal wall strengthens the superior pharyngeal constrictor muscle and improves contact. According to Saigusa et al. [3], there is a ring-like connection between the transverse intrinsic lingual muscle and the superior pharyngeal constrictor muscle at the base of the tongue. These findings provide the anatomical basis for the therapeutic role of THS. During swallowing, the tongue moves posteriorly, bringing it in contact with the pharyngeal wall [2]. During THS, posterior movement of the tongue is restricted, thereby causing a compensatory increase in the range of movement of the pharyngeal wall. In other words, THS potentially increases the contraction capacity of the superior pharyngeal constrictor muscle, building muscle strength in this area. This is further supported by the study of Hammer et al. [4], which showed increased tongue and pharyngeal muscle activities during THS.
The number of studies which examined the mechanisms of THS to date is limited and they have mainly investigated pharyngeal contractile function using video fluoroscopy or manometry [5,6,7]. Fujiu-Kurachi et al. [8] investigated the contact between the tongue and the palate during THS from the perspective of the tongue resistance exercise and reported changes in the degree of contact in accordance with the extent of tongue protrusion. They found that these changes are affected by individual differences in tongue length, shown by the maximum tongue protrusion length (MTPL), indicating that the required degree of tongue protrusion should be determined using an objective, quantitative scale in performing THS.
The purpose of this study is to further investigate the relationship between MTPL and tongue pressure during THS by adding simultaneous recording of submental surface electromyography (sEMG), with the following hypotheses:
-
1.
There is a correlation between the tongue pressure production during THS and MTPL.
-
2.
It is possible to predict the changes in tongue pressure during THS based on the individual’s MTPL.
-
3.
The amount of the load can be adjusted by changing the degree of tongue protrusion.
Methods
Subjects and Setting
The subjects were 18 healthy young dentate individuals (8 women, 10 men; ages 26.8 ± 1.3 years) without any history of disease that causes dysphagia, removable prosthodontic treatment, temporomandibular disorder, or orthodontic treatment. All subjects had more than 28 natural teeth. Dataset of the present study was partially overlapped with that of our previous study [8]. We excluded five subjects (2 males and 3 females) without EMG data from the dataset of the previous study and added another five subjects (3 males and 2 females) with EMG data. Written, informed consent was obtained from each subject after explanation of the aim and methodology of this study. All study protocols were approved by the Ethics Committee of Osaka University Graduate School of Dentistry.
Swallow Tasks
Experimental tasks included swallows with three different tongue positions, as in the previous study [8]: (1) dry swallow with no tongue protrusion (NP) (i.e., saliva swallow); (2) THS with slight tongue protrusion (SP) (i.e., tongue was protruded approximately 1 cm outside the upper incisors); and (3) THS with greater tongue protrusion (GP) (i.e., tongue was protruded at least 2 cm outside the upper incisors). The extent of tongue protrusion was adjusted using the line marking engraved on a disposable cotton swab (Kawamoto, Osaka, Japan) (Fig. 1). Each subject was instructed to hold his/her tongue by the upper and lower incisors for stabilization during THS, with gentle but reasonably sufficient biting force based on his/her own judgment. Subjects were provided with enough practice time to familiarize themselves with THS, but no specific instructions were given with respect to the manner of swallowing (e.g., how to move the tongue or how to push saliva) to look at the natural changes in swallow physiology with and without THS. After practicing, the subjects were allowed to rest for more than 15 min before starting the measurement. A total of 15 saliva swallows (five swallows for each of the three swallow tasks) were recorded from each subject in random order. Subjects were allowed to take a sip of water as needed between each recording to keep the oral cavity moist.
Experimental tasks with three different tongue protrusion tasks. a The cotton swab used for adjusting the extent of tongue protrusion before swallowing. b Detail of the cotton swab. c Dry swallow with no tongue protrusion (NP). d THS with slight tongue protrusion (SP). e THS with greater tongue protrusion (GP)
Maximum Tongue Protrusion Length (MTPL)
The subject protruded the tongue maximally, while the jaw was closed gently and naturally. The distance between the maximally protruded tongue tip and the upper incisors was measured as each subject’s maximum tongue protrusion length (MTPL) (Fig. 2).
Tongue Pressure Measurement
Tongue pressure was measured with the Swallow Scan System (Nitta Corporation, Osaka, Japan) (Fig. 3). The tongue pressure sensor sheet in this system forms a T shape that follows the curve of the palatal surface; it is extremely thin, with a thickness of approximately 0.1 mm, and thus poses no impediment to the measurement of physiological swallowing [9]. Depending on the size of the palate of each subject, a small, medium, or large sensor sheet was selected and attached individually to the palate with a sheet-type denture adhesive (Touch Correct II, Shionogi, Osaka, Japan). The tongue pressure sensor sheet incorporates pressure-sensitive points at five locations regarded as necessary for measuring tongue pressure during swallowing: the anterior-median part of the hard palate (Ch. 1), the mid-median part of the hard palate (Ch. 2), the posterior-median part of the hard palate (Ch. 3), the right posterior-circumferential part of the hard palate (Ch. R), and the left posterior-circumferential part of the hard palate (Ch. L). The wires from the sensor sheet were passed out of the mouth through the oral vestibule so as not to interfere with occlusion and connected to a personal computer.
The sensor sheet for measuring tongue pressure at five measuring points (Chs. 1–3, R, and L) attached to the palatal mucosa with a sheet-type denture adhesive and comparison of the patterns of pressure generation obtained during the three swallow tasks: a dry swallow with no tongue protrusion (NP), b THS with slight tongue protrusion (SP), and c THS with greater tongue protrusion (GP)
Before starting tongue pressure measurements, a vacuum pump was used to apply a specific negative pressure via the air duct provided at the exit of the tongue pressure sensor sheet for calibration of the pressure-sensitive points. During measurements, participants were seated in an upright position with their heads immobilized by a head-rest. The Frankfort plane was parallel to the floor, and participants’ feet were touching the floor. The sampling frequency was 100 Hz.
Surface Electromyographic (sEMG) Measurements
After attaching the pressure sensor sheet on the hard palate, bipolar surface electrodes (DUO-TRODE; Myotronics, Kent, Washington, USA) were placed in the submental region along the anterior bellies of the digastric muscles. Then, sEMG signals were amplified using a bio-amplifier (BA1104 m; Nihonsanteku, Osaka, Japan) and recorded by a personal computer via a sensor interface (PCI-3133A; Nihonsanteku). The sampling frequency was 1000 Hz, the gain of the amplifier was 10,000 times, the time constant was 0.03, and the low-pass filter was set to 1000 Hz. A synchronization signal from the Swallow Scan System was entered to the sensor interface so that the measurements of both tongue pressure and sEMG were synchronized.
Data Analysis
-
1.
Tongue pressure during THS.
The tongue pressure data (Fig. 3) were analyzed to determine the duration, the maximal magnitude, and the integrated value of tongue pressure during swallowing at each pressure-sensitive location. The duration of tongue pressure was defined as the time from onset to offset of tongue pressure, the maximal magnitude of tongue pressure during swallowing was defined as the maximal value of tongue pressure that appeared during the time from onset to offset, and the integrated value of tongue pressure was defined as the integrated value that appeared during the time from onset to offset. The mean value of each parameter for every pressure-sensitive point was calculated for each subject. Repeated-measures analysis of variance was used to compare these parameters among different measurement tasks. If the difference was significant, multiple comparisons were then performed using Tukey’s post hoc test. Effect sizes were calculated using partial η 2, with the value of more than 0.14 considered large.
-
2.
sEMG activity during THS.
EMG signals were rectified and smoothed to analyze the duration and the integrated value. Onset of EMG was defined as the time when a processed wave exceeded the mean value plus the 2SD value for 1 s at rest, and offset was defined as the time when the wave felt below the value. The duration of EMG was defined as the time from onset to offset of EMG, and the integrated value of EMG was defined as the integrated value that appeared during the time from onset to offset. EMG data were normalized in each subject, using the duration and integrated value of NP as a baseline. The values of SP and GP were calculated as change rates with respect to that of NP. Friedman’s test was used to compare these parameters between different measurement tasks, and if the difference was significant, multiple comparisons were then performed using Wilcoxon signed rank-sum test with Bonferroni correction. Effect sizes were calculated using r, with the value of more than 0.5 considered large.
-
3.
Relationship between maximum tongue protrusion length and tongue pressure.
The influence of MTPL on tongue pressure generation during THS was analyzed at Chs. R and L because two different pressure patterns (i.e., increase and decrease) appeared in connection with MTPL at these pressure-sensitive points in the previous study [8]. In order to normalize the individual data, the maximal magnitude and the integrated values of tongue pressure during SP and GP were calculated relative to NP in each subject, and correlations of MTPL with the relative value were analyzed using Spearman’s rank correlation coefficient. Additionally, a receiver operating characteristic (ROC) curve [10] was used to find a cut-off value for MTPL to predict changes (increases or decreases) in the maximal magnitude and integrated value of tongue pressure at Chs. R and L during SP and GP. MTPL was set as the border value, and it was then assumed that the evaluation items increased if each subject’s MTPL was longer than the border value, and they decreased otherwise. The sensitivity and specificity were calculated as the border value was changed, and the ROC curve of each evaluation item was created.
PASW Statistics 18 (SPSS, Chicago, IL) was used for statistical analysis, and the level of significance was 5% in all cases.
Results
Tongue Pressure During THS
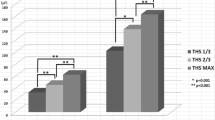
The duration of tongue pressure during GP was longer at the posterior-circumferential parts of the hard palate (Chs. R and L) than during NP, and these differences were statistically significant (Ch.R: p < 0.05, ηp 2 = 0.212, 95% CI [1.03 1.20]. Ch.L: p < 0.05, ηp 2 = 0.298, 95% CI [1.06 1.23]) (Fig. 4a; Table 1). As in the previous study [8], the maximal magnitude and integrated value of tongue pressure at the median parts of the palate (Chs. 1, 2, and 3) decreased during THS, while two types of changes were identified for the posterior-circumferential parts (Chs. R and L): eleven subjects demonstrated an increase in the maximal magnitude with an increase in the extent of tongue protrusion (increase group, Fig. 4b; Table 2), and seven subjects exhibited a decrease as the extent of protrusion became greater (decrease group, Fig. 4c; Table 2).
a Comparison of the duration of tongue pressure during the three swallow tasks measured at five different points. NP dry swallow with no tongue protrusion. SP THS with slight tongue protrusion. GP THS with greater tongue protrusion. b Two types of changes were identified for the maximal magnitude of tongue pressure at the posterior-circumferential parts (Chs. R and L) during THS. a A subject in increase group. This subject’s MTPL was 33 mm. b A subject in decrease group. This subject’s MTPL was 28 mm
sEMG Activity During THS
The durations of sEMG activity during SP and GP tasks were both longer than that during the NP task, and these differences were significant (NP–SP: p < 0.05, r = 0.73. NP–GP: p < 0.05, r = 0.80) (Fig. 5a; Table 3). The integrated value of sEMG activity was higher during GP than during NP and SP, and these differences were significant (NP–GP: p < 0.05, r = 0.75. NP–GP: p < 0.05, r = 0.75) (Fig. 5b; Table 3). Next, the same analysis was performed after dividing all subjects into increase and decrease groups. Increasing tendency in relation to the extent of tongue protrusion was shown in both groups (Fig. 5a, b). The duration and integrated value of sEMG activity were higher during GP than during NP and SP in the decrease group (The duration NP–GP: p < 0.05, r = 0.81. The duration SP–GP: p < 0.05, r = 0.73. The integrated value NP–GP: p < 0.05, r = 0.78. The integrated value SP–GP: p < 0.05, r = 0.81.), but these differences did not reach statistical significance in the increase group (Fig. 5a, b; Table 3).
a Comparison of the duration of sEMG activity during the three swallow tasks. The values of SP and GP were normalized as change rates with respect to that of NP. The results of analysis for all subjects, increase group, and decrease group are shown. The values of SP and GP were normalized as change rates with respect to that of NP. b Comparison of the integrated value of sEMG activity during the three swallow tasks
Relationship Between Maximum Tongue Protrusion Length and Tongue Pressure
The minimum MTPL value in the present subjects was 24 mm, and the maximum value was 48 mm. The mean value of MTPL was 32.7 ± 6.5 mm. MTPL was positively correlated with the change rate of the maximal tongue pressure at Chs. R and L during SP and GP (p < 0.05), and strongly correlated with that of the integrated value at Ch. L during SP and GP (p < 0.01), suggesting some right and left differences in the relationship between MTPL and tongue pressure generation (Table 4).
With respect to the area under the curve (AUC) of the ROC curve (Fig. 6; Table 5), the values exceeded 0.7 (indicating moderate accuracy) except for the maximal magnitude at Ch. L and the integrated value at Ch. R during SP. Each cut-off value for MTPL to predict the changes that maximized both sensitivity and specificity was 32.0 mm, except for the integrated value at Ch. R during SP and GP. The other cut-off values for these two items were both 30.5 mm (Tables 6, 7). At an MTPL cut-off value of 32.0 mm, prediction of whether tongue pressure would increase or decrease showed a sensitivity of 60.0–85.7% and a specificity of 60.0–81.8% (Table 8).
One example of the ROC curve. The sensitivity and specificity were calculated as the border value (MTPL) was changed, and the ROC curve of each evaluation item was created. The area under the curve (AUC) gives an indication of prediction accuracy and the border value which makes the shortest distance from the point (0, 1) on the graph become the cut-off value with the highest sensitivity and specificity
Discussion
In the present study, similar changes were observed in each tongue pressure parameter during THS compared with those during NP, as reported in a previous study [8]. Specifically, the duration of tongue pressure was significantly longer at Chs. R and L during GP. Conversely, the maximal magnitude and the integrated value of tongue pressure were significantly lower at Chs. 1, 2, and 3 during SP and GP. These findings may be explained by increased compensatory posterior tongue activity during THS resulting from holding and thus restricting the movement of the anterior tongue. However, it remains a matter of speculation because tongue movement was not recorded in the present study. In a subject from the increase and decrease groups, respectively, the magnitude of tongue pressure declined dramatically in SP, suggesting that the restriction of tongue tip in SP was more effective for declining tongue activity than the restriction of tongue dorsum in GP. The results of the present study also demonstrated that tongue pressure production in the posterior-circumferential part of the hard palate during THS was dependent on the individual’s maximal tongue protrusion length (MTPL). At an MTPL cut-off value of 32.0 mm, it is possible to predict whether changes in tongue pressure will increase or decrease.
In the present analysis of the relationship between MTPL and tongue pressure, all the items showed positive correlation at Ch.L; however, no correlation was found at Ch.R except for positive correlation between MTPL and maximum tongue pressure with SP and GP. Ono et al. [11] and Tamine et al. [12] reported that there were no right and left differences in maximum magnitude, duration, and the sequential order of tongue pressure at the posterior-circumferential parts on the hard palate. From these reports, it is supposed that the tongue moves bisymmetrically during swallowing. As for the reason why the right and left difference was found in the present study, it might be because that THS is not a physiological tongue action. In voluntarily initiated swallow, tongue–palate contact has fixed sequential pattern where the initial contact between tongue tip and anterior hard palate is followed by the contact between tongue blade and posterior palate [13,14,15]. Restriction of the initial tongue–palate contact in THS makes the following peristaltic tongue wave difficult. Therefore, it was speculated that some irregular compensative tongue action for bolus transfer might cause the right and left difference in the tongue–palate contact pattern. Our results suggested the incidence of rotational tongue action, which facilitated the tongue contact against the left side of hard palate and resulted in a higher correlation between tongue pressure items and MTPL. However, these speculations should be confirmed in the future experiments using tongue motion tracking system [16, 17].
As discussed previously, THS strengthens the superior pharyngeal constrictor muscle by restricting posterior movement of the tongue during THS. The condition of tongue pressure can be predicted from one’s MTPL. Standard THS stipulates a fixed degree of tongue protrusion in all patients. Therefore, the present findings show that tongue pressure during THS is influenced by MTPL, suggesting that the degree of tongue protrusion should be determined for each individual patient based on that patient’s MTPL in order to achieve consistent THS effects. Since a fixed degree of tongue protrusion was stipulated for all subjects in the present study, it is difficult to further clarify the adequate degree of tongue protrusion determined on the basis of MTPL. At the beginning of the present study, there was no idea to divide subjects into “Increase group” and “Decrease group,” because it was hypothesized that the task burden on tongue and supra-hyoid muscles became heavier according to the amount of tongue protrusion in THS. Additionally, there was no standard for tongue protrusion in THS, so the amount of tongue protrusion was set by absolute value such as 1 cm, 2 cm, and so on. However, task with the relative degree of tongue protrusion considering MTPL in each subject should be conducted in the future studies.
Surface electromyography is widely used to evaluate the muscle activities involved in swallowing [17], and the submental sEMG reflects the activity of the submental muscles (the mylohyoid, geniohyoid, and digastric muscles) [18]. Furthermore, a correlation with superficial lingual muscle activity during tongue pressure generation has also been reported [19]. Therefore, the submental sEMG enables the observation of not only the activity of the submental muscles involved in hyoid bone and laryngeal elevation during THS, but also physiological changes in the muscle groups involved in tongue pressure production. In the present study, the duration and the integrated value of sEMG activity during THS showed notable changes when compared with those during NP. Specifically, compared to NP, the durations of submental muscle activity during SP and GP were significantly longer, and the integrated value of sEMG was significantly higher during GP than during NP and SP. These results mostly agree with the previous study about EMG recording during THS [4].
Compared to dry swallow without tongue protrusion, THS makes hyoid bone and laryngeal elevation much harder to achieve, which requires more time and effort for one to swallow. This can explain the increases in the duration of submental muscle activity and the integrated value of sEMG. The integrated value of the sEMG was significantly higher during GP than during SP, demonstrating that the amount of load during the THS maneuver can be modified by the amount of tongue protrusion. This suggests that the principle of progressive overload may be satisfied. Furthermore, since the activities of the submental muscles and the superficial lingual muscle are correlated [20], if THS activates the intrinsic lingual muscle along with the submental muscles, it may be considered an effective exercise technique to strengthen the intrinsic lingual muscles.
In the present study, a correlation was observed between MTPL and changes in tongue pressure production at the posterior-circumferential part of the hard palate (Chs. R and L) during THS. The large MTPL presumably resulted in greater tongue pressure production at that part of the hard palate even when the anterior portion of the tongue was held. ROC curve analysis enabled changes in tongue pressure production at the posterior-circumferential part of the hard palate during THS to be predicted based on MTPL with moderate accuracy. Trends in increases and decreases in tongue pressure changed based on an MTPL of around 32.0 mm. With the current experimental conditions, an MTPL of 32.0 mm marked the boundary between increases and decreases in tongue pressure. In this research, the ROC analysis with an absolute value of MTPL was conducted in order to obtain the clear cut-off value.
The increases in tongue pressure production and submental muscle activity in subjects with a large MTPL observed in the present study are similar to those reported by Yeates et al. [21], who found that tongue pressure production and submental muscle activity increased during an effortful swallow. Since THS involves swallowing in conditions unlike a normal physiological state, it is predictable that the muscle activities of the various organs involved in swallowing will increase compared to normal, and that similar biomechanical effects will be exerted on the oral and pharyngeal organs as in the effortful swallow, in which the tongue is more strongly pressed than normal. One effect of effortful swallowing is the increase in posterior movement of the base of the tongue during the pharyngeal stage of swallowing [5, 21]. A similar mechanism is likely to be responsible for the increase in tongue pressure production in the posterior-circumferential part of the hard palate during THS in subjects with a large MTPL.
However, this effect is counterproductive to the goal of THS, which is to activate the superior pharyngeal constrictor muscle by suppressing posterior movement of the base of the tongue [1]. In order to reliably build the strength of the superior pharyngeal constrictor muscle, posterior movement of the tongue must be sufficiently suppressed. The present findings suggest that tongue pressure production patterns in the posterior-circumferential part of the hard palate during THS depend on MTPL. Therefore, by using MTPL to set the tongue-hold position at a point that decreases tongue pressure production at the posterior-circumferential part, in other words, at a point that can sufficiently suppress posterior movement of the tongue, more reliable THS effects should be attainable. From this perspective, using MTPL to predict posterior tongue pressure production during THS is fundamental to further consolidating the effects of THS.
Limitation
The results of the present study were provided by the task of THS with an absolute value of SP (10 mm) and GP (20 mm). Therefore, it is impossible to suggest what percent of patient’s MTPL is to be set for effective amount of tongue protrusion. As the next step for investigating the mechanism of THS, another study with a relative value of tongue protrusion is in progress.
References
Fujiu M, Logemann JA. Effect of a tongue-holding maneuver on posterior pharyngeal wall movement during deglutition. Am J Speech Lang Pathol. 1996;5:23–30.
Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103:128–36.
Saigusa H, Yamashita K, Tanuma K, Saigusa M, Niimi S. Morphological studies for retrusive movement of the human adult tongue. Clin Anat. 2004;17:93–8.
Hammer Michael J, Jones Corrine A, Mielens Jason D, Kim Chloe H, McCulloch Timothy M. Evaluating the tongue-hold maneuver using high-resolution manometry and electromyography. Dysphagia. 2014;29:564–70.
Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002;54:171–6.
Doeltgen SH, Witte U, Gumbley F, Huckabee ML. Evaluation of manometric measures during tongue-hold swallows. Am J Speech Lang Pathol. 2008;18:65–73.
Umeki H, Takasaki K, Enatsu K, Tanaka F, Kumagami H, Takahashi H. Effects of a tongue-holding maneuver during swallowing evaluated by high-resolution manometry. Otolaryngol Head Neck Surg. 2009;141:119–22.
Fujiu-Kurachi M, Fujiwara S, Tamine K, Kondo J, Minagi Y, Maeda Y, Hori K, Ono T. Tongue pressure generation during tongue-hold swallows in young healthy adults measured with different tongue positions. Dysphagia. 2014;29:17–24.
Hori K, Ono T, Tamine K, Kondo J, Hamanaka S, Maeda Y, Dong J, Hatuda M. Newly developed sensor sheet for measuring tongue pressure during swallowing. J Prosthodont Res. 2009;5:28–32.
Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7.
Ono T, Hori K, Nokubi T. Pattern of tongue pressure on hard palate during swallowing. Dysphagia. 2004;19:259–64.
Tamine K, Ono T, Hori K, Kondoh J, Hamanaka S, Maeda Y. Age-related changes in tongue pressure during swallowing. J Dent Res. 2010;89:1097–101.
Dodds WJ, Taylor AJ, Stewart ET, Kern MK, Logemann JA, Cook IJ. Tipper and dipper types of oral swallow. Am J Roentgenol. 1989;153:1197–9.
Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phase of swallowing. Am J Roentgenol. 1990;154:953–63.
Palmer JB, Hiiemae KM, Liu J. Tongue-jaw linkages in human feeding. Arch Oral Biol. 1997;6:429–41.
Steele CM, Van Lieshout PH. Use of electromagnetic midsagittal articulography in the study of swallowing. J Speech Lang Hear Res. 2004;47:342–52.
Steele CM, Van Lieshout P. Tongue movements during water swallowing in healthy young and older adults. J Speech Lang Hear Res. 2009;52:1255–67.
Stepp Cara E. Surface electromyography for speech and swallowing systems: measurement, analysis, and interpretation. J Speech Lang Hear Res. 2012;55:1232–46.
Palmer PM, Luschei ES, Jaffe D, McCulloch TM. Contributions of individual muscles to the submental surface electromyogram during swallowing. J Speech Lang Hear Res. 1999;42:1378–91.
Palmer PM, Jaffe DM, McCulloch TM, Finnegan EM, Van Daele DJ, Luschei ES. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51:828–35.
Yeates EM, Steele CM, Pelletier CA. Tongue pressure and submental surface electromyography measures during noneffortful and effortful saliva swallows in healthy women. Am J Speech Lang Pathol. 2010;19:274–81.
Acknowledgements
This study was supported by the Global COE Program “In Silico Medicine” at Osaka University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have financial or other relationships that would influence the assessment of the data or that would constitute a conflict of interest.
Rights and permissions
About this article
Cite this article
Fujiwara, S., Fujiu-Kurachi, M., Hori, K. et al. Tongue Pressure Production and Submental Surface Electromyogram Activities During Tongue-Hold Swallow with Different Holding Positions and Tongue Length. Dysphagia 33, 403–413 (2018). https://doi.org/10.1007/s00455-017-9865-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-017-9865-4