Abstract
The aim of this study was to examine the effect of food thickener on the pharmacodynamics of mitiglinide (MGN), a drug belonging to a class of rapid-acting insulin secretagogues. First, MGN tablets were coated by immersion in a xanthan gum-based food-thickening agent. This treatment was shown to delay disintegration rates of MGN tablets in vitro. The pharmacodynamics of MGN after ingestion of a single oral dose of an MGN tablet, with or without food thickener immersion, were then examined in an open-label crossover study comprising 5 healthy participants. It was observed that after administration of 75 g of oral glucose, the area under the blood glucose concentration–time curve was larger for treatment with MGN tablets that had been immersed in the food thickener than for nonimmersed tablets. The maximum blood glucose level was also higher in treatments with MGN tablets that had been immersed in food thickener. The extended time of higher glucose levels associated with thickener-immersed MGN tablets given to human volunteers may be associated with the reduced disintegration rates of immersed MGN tablets as observed in the in vitro experiment. Overall, our study suggests that commercially available food thickeners influence the pharmacodynamics of MGN and that their use should therefore be carefully assessed and monitored in certain clinical situations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic patients are at increased risk of complications from pneumonia. They also have an increased mortality rate that may be related to coexistent medical conditions. Food-thickening agents based on xanthan gum are used for the prevention of aspiration, and as a result prevent the onset of aspiration pneumonia. It has also been reported that food thickeners are useful in helping certain patients eat food. These patients include elderly individuals and those with eating or swallowing disorders. Thickening agents are also of value to patients taking medication administered as tablets [1–5].
However, there have been reports of undissolved tablets being found in the stool of patients taking quick-dissolving tablets, such as magnesium oxide tablets, which have been immersed in a thickening agent [6]. In addition, it has been shown that, not only do thickening agents delay the disintegration time of voglibose orally disintegrating tablets, they also have an effect on the manifestation of a remedial effect on post-prandial hyperglycemia [7].
Mitiglinide is a member of a class of rapid-acting insulin secretagogues. It has been widely administered either alone or in combination with other oral hypoglycemic drugs such as voglibose. For these reasons, we selected mitiglinide as the target molecule for our study on the effects of food-thickening agent on drug pharmacodynamics. Specifically, we examined the effect of immersion in a thickening agent on the inhibitory effect of mitiglinide (MGN) tablets on the incremental elevation of blood glucose levels. The goal was to seek ways to improve post-prandial hyperglycemia in diabetic patients.
Materials and Methods
Dissolution Test
Samples
The Glufast® tablet 5 mg (M-tab) made by Takeda Pharmaceutical Co., Ltd. (Japan), manufacturing number: CHR2103, was used as the MGN tablet model drug. A xanthan gum, Tsururinko Quickly (Clinico Co., Ltd., Japan), was used as the thickening agent. The thickening agent used in this study is the most widely used product in Japan. This product was used as a representative example because it has strong influence on health care workers.
Disintegration Test
The xanthan gum-based thickening agent was prepared with purified water at 1.5% (w/v) (coefficient of viscosity: approximately 180 mPa s) [8] and 3.0% (w/v) (coefficient of viscosity: approximately 470 mPa s) [8] based on instructions in the package insert of the thickening agent. Six tablets of M-tab were immersed in each concentration of the thickening agent and then allowed to stand for 1 or 10 min. After removing the thickening agent as much as possible, immersed M-tab was applied to the disintegration test.
We then immediately performed the disintegration test (disintegration apparatus: NT-40H [Toyama Sangyo Co., Ltd., Japan], immersing fluid: purified water, thermostatic arrangement: 37 ± 2 °C, disk: not in use) described in the Japanese Pharmacopoeia, Sixteenth Edition (JP16). We defined disintegration time as the time required for the six sample tablets of M-tab to completely disintegrate. Six tablets of M-tab that had not been immersed in thickening agent were used as the control.
Oral Study
We selected 5 healthy Japanese volunteers (2 female and 3 male) with a mean of 39 years of age (range of 34–47 years) with a mean BMI of 22.0 ± 2.1 kg/m2 (range 19.1–24.4 kg/m2) for the oral study. These volunteers had no history of drug allergies, abnormal laboratory data, digestive system disease, or diabetes. Prior to initiating the study, we thoroughly explained the purpose and details of the study to the volunteers and obtained written consent from all participants. In addition, we obtained approval for the planning of this study from the Hospital Bando Clinical Research Ethics Committee.
We defined M-tab after 10-min immersion in 3% (w/v) thickening agent as the study drug (M-tabims). Nonimmersed M-tab served as the control drug. We administered M-tabims (study drug) and M-tab (control drug) using a crossover study design. The dosage interval was set to 7 days or more [9]. For the medical dosage, participants abstained from eating for 10 h or more before administration of the drug. We administered either the study or control drug with 150 mL water directly after collecting a blood sample to measure the blood glucose level after the 10 h of fasting. Five healthy volunteers took a tablet immersed in a thickening agent. M-tabims was coated by immersion in a xanthan gum-based food-thickening agent. In healthy volunteers, after removing the thickening agent as much as possible, M-tabims was administered orally. Then, following the literature protocol described by Kurihara et al. [9], we immediately administered 75 g of oral glucose (225 mL of 33.3% glucose solution, Trelan®-G75, AY Pharmaceuticals Co., Ltd., Japan).
We collected blood samples at 0, 15, 30, 45, 60, 75, 90, 105, and 120 min after administering the 75 g of oral glucose. Blood glucose levels were measured in these samples. The blood was sampled by the participants themselves using a puncturing device (OneTouch Pen, Johnson & Johnson K.K., Japan), and a single-use automatic lancet (OneTouch Pen Lancets, Johnson & Johnson K.K., Japan). For glucose measurements, the participants used a self-examination glucose-measuring device (OneTouch UltraVue®, Johnson & Johnson K.K., Japan) equipped with a glucose kit (LFS Quick Sensor®, Johnson & Johnson K.K., Japan).
We defined the maximum blood glucose level after taking 75 g oral glucose as C max. We defined the time it took to reach C max as T max. In addition, we calculated the area under the blood glucose concentration–time curve (AUC) using the trapezoid formula.
Statistical Analysis
Data were analyzed by two-way ANOVA followed by the Tukey–Kramer test and paired t test. All analyses were performed using Microsoft Excel 2013.
Results
Disintegration Test
The disintegration time (median value) for the M-tab samples that were not immersed in thickening agent was 92.2 s. The disintegration time for M-tab samples that had been immersed in the 1.5% (w/v) thickening agent for 1 min was 101.0 s (p = 0.188, compared with M-Tab samples; Table 1), whereas the disintegration time for M-tab samples that had been immersed for 10 min was 285.0 s (p = 0.001, compared with M-Tab samples; Table 1). The disintegration time for M-tab samples that had been immersed in the 3.0% (w/v) thickening agent for 1 min was 140.5 s (p = 0.009, compared with M-Tab samples; Table 1), whereas the disintegration time for M-tab samples that had been immersed for 10 min was 294.5 s (p = 0.001, compared with M-Tab samples; Table 1).
Oral Study
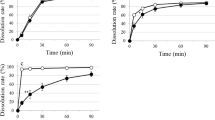
As shown in Fig. 1, a shift in blood glucose level was apparent when M-tab and M-tabims were administered. There was no difference between the M-tab and M-tabims treatments in mean T max, (the time it took to reach maximum blood glucose level, C max) after ingestion of 75 g glucose. Mean T max for M-tab or M-tabims administration was 30 min. The mean C max for M-tab administration was 120 mg/dL, while that for M-tabims administration was 156 mg/dL. In other words, the mean C max for M-tabims administration was 1.3 times higher than that for M-tab administration.
Blood glucose levels for M-tabims administration after ingestion of 75 g glucose remained higher than for M-tab administration for up to 90 min. There were significant differences between the M-tabims and M-tab administration as follows; 129 versus 110 mg/dL at 15 min; 156 versus 120 mg/dL at 30 min; 142 versus 98 mg/dL at 45 min; 123 versus 82 mg/dL at 60 min; and 106 versus 88 mg/dL at 75 min, p < 0.01). Blood glucose levels for M-tab administration gradually decreased between 30 and 60 min after taking 75 g glucose, and the lowest value was recorded 60 min after glucose ingestion. Blood glucose levels for M-tabims administration drastically decreased between 30 and 90 min after taking 75 g glucose, and then levels remained constant. The difference between M-tabims and M-tab was greatest (1.5 times higher for M-tabims) at 60 min. The AUC for M-tabims administration up to 120 min after taking glucose solution increased more than for M-tab administration (Table 2).
During the testing period, none of the participants showed any adverse symptoms such as, for example digestive abnormalities, after glucose administration.
Discussion
Commercially available instant thickening agents are becoming more popular in medicinal applications. In particular, a new generation of xanthan gum-based thickening agents are now being used in many medical institutions and nursing homes. For this reason, a xanthan gum agent was used as the thickening agent in this study.
We set the food thickener concentrations to 1.5 and 3.0% [8]. In our previous report, interviews conducted on clinical practice revealed that up to 10 min is needed to coat voglibose orally disintegrating tablets in a thickening agent before administration to diabetic patients. Since similar interview results were obtained in the case of M-tab also. For this reason, in our study, we let the mitiglinide M-tab stand for up to 10 min in the thickening agent.
In a tablet disintegration test, we observed that the median time to complete disintegration of M-tab immersed in thickening agent was up to 3.2 times slower than that for disintegration of uncoated M-tab. We observed a trend where the disintegration time increased with longer immersion times. In this study, we allowed the M-Tab to stand for between 1 and 10 min in a thickening agent. The slower disintegration of the M-tab immersed in thickening agent may explain results from our experiment where immersed M-tabs (M-tabims) and nonimmersed M-tabs were administered to human volunteers. We consider the Tmax at the time of M-tabims administration that is slower than that at the time of M-Tab administration to be the result of delayed disintegration time caused by attenuation of water permeability. As noted in the results section, blood glucose levels for M-tabims administration after ingestion of 75 g glucose remained higher than for M-tab administration for up to 90 min. At 60 min, the difference was as much as 1.5-fold. The slower time to complete disintegration of M-tabims may be associated with the extended time of higher glucose levels associated with M-tabims given to human volunteers. The mechanism of oral disintegration of tablet was assumed involving a three-step disintegration process. Saliva permeates the surface of tablet at first. And then the permeated region of tablet is softened. The softened portion is finally destroyed. In general, tablet disintegration requires penetration of water into the interior of the tablet. Although the mechanism of extension of tablet disintegration time due to the thickening agent is unclear, we believe that simply coating the outer surface of the tablet increases attenuation of water permeation through the tablet surface.
The extended increase in blood glucose levels associated with M-tabims as noted above and as described in detail in the results section is a key finding of our study. Our results clearly suggest that the commercially available xanthan gum-based thickening agents are likely to be associated with changes in the pharmacodynamics profile of a clinically important oral hypoglycemic drug such as mitiglinide. In our previous report (same as current report), we had shown that thickening agents do not only delay the disintegration time of voglibose oral-disintegrating tablet, but also have an effect on the manifestation of remedial effect on post-prandial hyperglycemia. We believe it is possible that thickening agents will affect the pharmacodynamics profile of other clinically important oral hypoglycemic drugs.
References
Deto A, Yamagata Y, Kayashita J. Effects of temperature on the physical properties of various commercial thickening gents for dysphagia. Bull Fac Hum C Sci Pref Univ. Hiroshima. 2007;2:39–47.
Nakamura M, Yoshida S, Nishioka Y, Hayashi S, Suzuki Y. Categorization of commercial thickeners on the basis of their effects on the physical properties of foods. Jpn J Nutr Diet. 2012;70:59–70.
Tomita T, Goto H, Yoshimura Y, Tsubouchi Y, Nakanishi R, Kojima C, Yoneshima M, Yoshida T, Tanaka K, Sumiya K, Kohda Y. Effect of food thickener on disintegration and dissolution of magnesium oxide tablets. Yakugaku Zasshi. 2015;135:835–40.
Tomita T, Goto H, Sumiya K, Nakanishi R, Kojima C, Yoneshima M, Yoshida T, Tanaka K, Kohda Y. Effect of food thickener on disintegration of donepezil hydrochloride orally disintegrating tablets. Igaku no Ayumi. 2015;253:677–8.
Goto H, Tomita T, Sumiya K, Nakanishi R, Kojima C, Yoneshima M, Yoshida T, Tanaka K, Kohda Y. Effect of food thickener on tablet disintegration of rapid-disintegration over-the-counter drugs. J Community Pharm Pharm Sci. 2016;8:88–91.
Yamaguchi H, Ochiai A, Takiya H. Abstracts of papers, the 24th annual meeting of Japanese society of pharmaceutical health care and sciences, Nagoya. 2014, p. 371.
Tomita T, Goto H, Sumiya K, Yoshida T, Tanaka K, Kohda Y. The effect of food thickener on the inhibitory effect of voglibose oral disintegrating tablet on post-prandial elevation of blood sugar levels. Yakugaku Zasshi. 2016;136:1171–6.
Fujitani J, Uyama R, Okoshi H, Kayashita J, Koshiro A, Takahashi K, Maeda H, Fujishima I, Ueda K. Japanese society of dysphagia rehabilitation: classification of dysphagia modified food. Jpn J Dysphagia Rehabil. 2013;17:255–67.
Kurihara A. Effects of combination therapy with mitiglinide and sitagliptin on insulin and glucagon secretion. Prog Med. 2011;31:2737–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tomita, T., Goto, H., Sumiya, K. et al. Effect of Food Thickener on the Inhibitory Effect of Mitiglinide Tablets on Post-prandial Elevation of Blood Glucose Levels. Dysphagia 32, 449–453 (2017). https://doi.org/10.1007/s00455-017-9787-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-017-9787-1