Abstract
Changes in morphometry of head and neck muscles have received little attention in research relative to limb muscles. While recent literature suggests that high-frequency ultrasound transducers can provide superior spatial resolution compared to that of magnetic resonance imaging (MRI), no studies have compared these imaging methods for investigating the submental muscle group. This preliminary study sought to compare ultrasound and MRI as a method of quantifying the cross-sectional area (CSA) of the submental muscle group. Measurements were taken from coronal ultrasound and MRI images in 11 healthy participants. Comparisons were limited to the anterior belly of the digastric muscle because of differences in imaging resolution. Ultrasound CSA measurements were smaller than MRI measurements (p = 0.01) by 10 % (95 % CI = −18 to −2). Correlations were significant and relatively high (left: r = 0.909, p < 0.001; right: r = 0.776, p = 0.005). Ultrasound imaging has the advantages of natural participant positioning, superior clarity of muscle borders of the submental muscles, requires less acquisition time, and is a less expensive method of imaging compared to MRI. This preliminary study has shown that ultrasound is a viable imaging modality for quantitative measurements of the anterior belly of the digastric muscle and has advantages over MRI beyond cost and accessibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Muscle morphometry has been assessed using a variety of imaging techniques, including magnetic resonance imaging (MRI) [1–6], computed tomography (CT) [7–10], and ultrasonography (US) [6, 11–19]. Exposure to ionizing radiation poses an ethical barrier to research when using radiographic imaging to investigate muscle size in nonpatient populations, thus encouraging the use of techniques that rely on noninvasive methods. While MRI eliminates this exposure, it is a costly method of investigation for researchers. Ultrasound is another way to investigate muscle size, eliminating many issues that surround the use of CT and MRI.
While two-dimensional calculations made with ultrasound are reportedly reproducible for swallowing muscles [20] and are valid for limb muscles [21], no studies have compared submental muscle cross-sectional area (CSA) measurements made from ultrasonographic and MRI images. While historically MRI was considered superior to ultrasound in regard to spatial resolution for limb muscles [22, 23], modern day high-frequency linear transducers used in musculoskeletal ultrasound imaging can achieve superior spatial resolution compared to that of MRI [24, 25].
This preliminary study aimed to compare the CSA of the submental muscles obtained from both ultrasound and MRI images to see whether superior muscle definition can be achieved with ultrasound imaging. By assessing the capability of ultrasound to demarcate these muscles, we wished to provide preliminary evidence that ultrasound is a viable method of documenting CSA of the submental muscles. If ultrasound provides superior definition compared to MRI, changes in oral musculature that result from factors such as exercise or atrophy secondary to disease or aging can be investigated with reduced cost and time compared with MRI.
Methods
Participants
Eleven healthy volunteers (2 males and 9 females, age range = 20–42 years) were recruited for one session. Participants had no history of surgery or disease affecting the head and neck musculature. Ethical approval was obtained from the appropriate regional health research ethics committee, and informed consent was obtained prior to commencement of data collection.
Procedure
In the absence of previous literature documenting optimal methods and parameters for MRI and ultrasound imaging of the submental muscles, various sequences (MRI) and settings (ultrasound) were tried until subjective consensus was reached between the researchers on which provided the clearest images for each method.
MRI
Three-dimensional coronal oblique T2-weighted images (TE/TR = 87.8/3,000 ms, TI = 0 ms, flip angle = 90°, acquisition matrix = 288 × 192 × 36, reconstruction matrix = 512 × 512 × 36, field of view [FOV] = 160 × 160 mm2, slice thickness = 3 mm, voxel size = 0.31 × 0.31 × 3.00 mm3) were acquired on a 3-T GE HDx scanner (GE Healthcare, Waukesha, WI, USA) with an 8-channel head coil. While T1-weighted images provide high-resolution anatomical images, T2-weighted images provide superior contrast. Because the CSA for each muscle was manually traced using electronic calipers, high-resolution T2-weighted images were selected to provide greater differentiation of the muscle boundaries. Participants were supine in the MRI scanner, with the head and neck placed within the head coil fixed to the scan table. Participants were asked to remain stationary and inhibit swallowing for the duration of the 3-min scan.
Ultrasound
A Philips (Philips Healthcare, Surrey, UK) IU22 ultrasonography instrument was used with a 12–5 MHz linear array transducer to acquire gray-scale images of the submental muscles in a coronal plane. Images of the submental muscles were acquired with the participant sitting upright. Participants were instructed to sit comfortably and relax their head in a neutral position. Once the participant achieved this posture, they were asked to maintain it while the transducer was placed under the chin and not to accommodate the transducer by flexing the neck. A generous amount of Aquasonic 100 ultrasound conductive gel was placed over the transducer, which was then placed perpendicular to the submental muscle group, with minimal pressure to ensure that transducer pressure did not distort muscle structure [15]. The transducer was placed in a coronal plane, approximately midway between the mentalis of the mandible and the superior palpable edge of the thyroid cartilage. Depth settings were tailored to accommodate individual anatomy, and gain settings were adjusted to allow optimal visualization of muscle borders. Images were recorded at between 30 and 34 frames/s, depending on the depth, focus points, and two-dimensional (2D) gain applied to the individual’s image.
Measurements were derived from both techniques offline.
Data Analysis
All images were imported into the DICOM viewing software Osirix™. The zoom function was used to enlarge the muscles of interest to approximately the same size on both the MRI and the ultrasound images. Although unable to ensure that the exact same section of muscle was being measured across the MRI and the ultrasound images for each person, the following steps were taken in an attempt to measure the same muscle section from each method. MRI images obtained coronal slices of the entire length of the submental muscles. The images were reviewed offline and the total number of slices which included any portion of the anterior belly of the digastric was counted. The midslice (in the case of an odd total number of slices) or the two midslices (in the case of an even total number of slices) of the scan were selected for measurement of the CSA. Coronal ultrasound images were taken at the midpoint between the mentalis of the mandible and the superior palpable edge of the thyroid cartilage in an attempt to image the middle of the muscle belly. Therefore, the midslice(s) on MRI was used in an attempt to measure the same muscle section imaged using ultrasound. Continuous trace callipers available in the Osirix™ software were used to outline the left and the right belly of the anterior belly of the digastric muscle, from which the software automatically generates a 2D quantity in cm2.
Statistical Analysis
Means and standard deviations were calculated for each belly of the anterior digastric muscle for both methods. A linear mixed-effects model [26, 27] was used to compare values obtained by ultrasound and MRI for the left and the right anterior belly of digastric muscle. Pearson correlation coefficients were used to determine the relationship between the measures obtained by the two methods.
Results
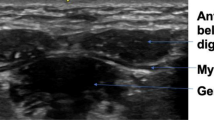
The aim of this study was to compare CSA values obtained by MRI and ultrasound for all submental muscles. However, the geniohyoid and mylohyoid muscle boundaries could not be visualized sufficiently to allow measurement of the CSA from MRI images (Fig. 1); hence, no comparison could be made for these muscles, although these measurements could be obtained from ultrasound images (Fig. 2). CSA measurements of the mylohyoid muscle were not possible using ultrasound images (Fig. 2), as the lateral borders of the muscle were not visible. Therefore, only calculation of one-dimensional (1D) thickness of the mylohyoid was possible from ultrasound images (Fig. 2). As the clarity of the mylohyoid muscle was not sufficient to measure 1D thickness using MRI images (Fig. 1), analysis was limited to comparison of the anterior belly of the digastric muscle.
The mean (±SD) CSAs (cm2) for each muscle belly derived from MRI were 0.96 ± 0.23 (left) and 0.97 ± 0.27 (right), and from ultrasound they were 0.87 ± 0.19 (left) and 0.86 ± 0.21 (right). A scatterplot of the values for the left and right anterior bellies is shown in Fig. 3. Ultrasound CSA measures were smaller than MRI measures by −0.10 ± 0.12 (95 % CI = −0.18 to −0.02, p = 0.01). There was a mean difference of 0.0 ± 0.025 cm2 between the left and right muscles (95 % CI = −0.08 to 0.08, p = 0.96). Pearson correlation coefficients for the left (r = 0.909) and the right (r = 0.776) measurements of CSA by MRI versus ultrasound were significant (p < 0.001 and p = 0.005, respectively).
Discussion
This is the first study to correlate the measurement of the CSA of the anterior belly of the digastric muscle acquired using ultrasound with that acquired by MRI. The results suggest that measurements of the CSA of this muscle acquired from ultrasound images are highly correlated with those obtained from gold standard MRI images. Furthermore, ultrasound was found to be superior in some aspects of measurement, thus calling into question the application of MRI for quantification of CSA of this specific muscle. Overall, the results from this study provide confidence in the use of ultrasound for the measurement of the anterior belly of the digastric muscle. Additionally, despite being unable to compare measurements of the geniohyoid and the mylohyoid muscles across imaging modalities, the CSA of the geniohyoid and the thickness measurements of the mylohyoid were possible with ultrasound but not with MRI, suggesting that there are advantages of ultrasound for measuring the entire submental muscle group.
These findings have implications for swallowing research and clinical management of dysphagia. Prior to the clinical use of ultrasound for assessing changes in these muscles associated with dysphagia treatments, further research is required to investigate its usefulness in identifying underlying mechanisms of pathophysiology and recovery. An increased scope for such research investigations (and eventual clinical application) is brought about by the decrease in cost and increase in portability associated with ultrasound compared with MRI. Quantifying decreases in submental muscle CSA may provide insight into the underlying mechanisms of dysphagia caused by sarcopenia (the degeneration of skeletal muscle associated with aging) or muscle weakness. Additionally, as researchers endeavour to understand the mechanisms that drive recovery from dysphagia, hypertrophy of these muscles following treatment can be explored more readily with ultrasound than with MRI.
Mean CSA values from MRI images exceeded those from ultrasound images by approximately 10 %. Factors that possibly contributed to this include positioning during image acquisition and image clarity. Inspection of Fig. 3 shows that the increase in CSA measured from MRI images is not systematic, with three instances having larger ultrasound values than MRI. One of these has a minimal difference of 0.03 cm2, suggesting that the CSAs obtained from both MRI and ultrasound are almost identical. Interestingly, the two remaining data points with larger values from ultrasound than from MRI were taken from the same participant (female). This suggests that something specific to this participant resulted in increased CSA measurements from the ultrasound images. This could be related to the composition of fatty tissue and muscle and/or interaction with either the transducer placement in the acquisition of the ultrasound image or positioning in the MRI scanner. As all other measurements made from MRI images were identical (1 data point) or larger (18 data points), exploration of factors possibly contributing to systematic increases in CSA for MRI measurements is worthy of consideration. Such factors may include variations of the plane from which the CSA is derived, which is related to participant positioning. Additionally, as our data set included mostly young females, inclusion of a more variable participant sample will likely reveal differences in the relationship between MRI and ultrasound measures.
The positioning of participants varied across methods in the present study. Participants were seated upright with their head in a neutral position for ultrasound, while they were supine for MRI. The head coil used for MRI dictated head position to some extent, possibly decreasing the length of the submental muscle group. Post hoc analysis of the MRI images showed that the CSA of the anterior belly increased posteriorly and decreased anteriorly from the midslice. This tendency for an increase in CSA toward the superior palpable edge of the thyroid cartilage, rather than a maximal CSA at the muscle belly, may suggest that positioning of participants substantially deformed the muscle during MRI scans. As ultrasound images were taken only at the midway point between the mentalis of the mandible and the superior palpable edge of the thyroid cartilage, this assumption cannot be confirmed but should be investigated in future studies. If future studies demonstrate that this difference results from positioning, this information should be considered when contemplating sources of variance seen in MRI data of the neck muscles.
CSA measurements of the geniohyoid muscle and thickness measurements of the mylohyoid muscle were not possible on MRI images due to poor border delineation (Figs. 1 and 2). These muscles could be differentiated and measured from ultrasonographic images, suggesting that there is a difference in the sensitivity of the two methods for demarcating these muscles. The acquisition of MRI images in this study required a longer duration of movement inhibition (3 min) than ultrasound image acquisition (0.3 s). As the submental muscles are activated during swallowing at least once every minute [28], it is likely that image clarity was compromised to a greater degree for MRI than for ultrasound. CSA measurements were taken from the muscle’s belly in both ultrasound and MRI images. As most movement occurs at the muscle’s belly, controlling movement is essential for CSA measurements from this location, which suggests that the advantage of rapid acquisition time of ultrasound is especially applicable in this case. The difference in signal generation between the two methods also may contribute to the superior image quality documented for ultrasound in this study. Because ultrasound uses the change in reflective properties of adjacent tissues, it may be more sensitive to the muscle borders in this confined space than MRI, which generates a signal based on proton density. In addition, because of scanning schedules, image acquisition duration, and limited funding for the acquisition of the MRI images, it was possible to scrutinize and repeat image acquisition if movement artifact was detected during only the ultrasound procedure.
As these preliminary results suggest, ultrasound imaging can provide a viable method of accurately measuring the CSA of the anterior digastric muscle, but the repeatability of measurements needs further attention if ultrasound is to be used to document changes in muscle morphometry across sessions. The investigation by Emshoff et al. [20] into the reliability of ultrasound measurements of the anterior belly of the digastric muscle included a time lapse of more than 5 min between image acquisitions. Further research is required to look at the reliability and also the precision of ultrasound measurements in these muscles, obtained with a longer period of time between sessions. Documenting the variance associated with a longer time lapse between sessions will help determine the magnitude of the effects required to override such variance. To validate the sensitivity of ultrasound in detecting such changes, longitudinal research with an expected outcome of modified muscle morphometry is also required. Comparison of results obtained over repeated measures from both ultrasound and MRI is necessary to further clarify sources of variation specific to each method.
Once methodological confounds such as reliability, precision, and validity are addressed, normative submental muscle CSA values may be desired. This study included a small participant sample to explore the potential of ultrasound images in providing superior measures of submental CSA compared with MRI. Because a within-participant design was used for this comparison, normative values of submental CSA are not provided. Future studies aimed at quantifying normative values of the submental muscles must account for differences in the CSA associated with age and gender by incorporating a larger participant sample with more variable participant characteristics. The variance estimates in the current data set suggest that most variability occurs across subjects. This will undoubtedly increase with the inclusion of a more variable participant sample. Future studies attempting to provide normative values of these muscles using a more variable sample must consider anthropometric measurements such as height and weight to account for these differences. The within-participant variance for each method was very small (SD = 0.03 cm2), suggesting that studies using within-participant designs will be most sensitive to measuring changes in these muscles. As this study was unable to complete comparisons for the mylohyoid and geniohyoid muscles due to poor muscle boundary definition in the MRI images, future research investigating these muscles with ultrasound should include these two muscles.
MRI and ultrasound measurements of the CSA of the digastric muscle are highly correlated, although MRI images have a larger group mean. Ultrasound imaging has the advantages of natural participant positioning, superior clarity of muscle borders of all submental muscles, requires less acquisition time, and is a much less expensive method of examination than MRI. In summary, this preliminary study has shown that ultrasound provides a viable imaging modality for quantitative measures of the anterior belly of the digastric muscle, with promise for the entire submental muscle group, and has advantages over MRI beyond cost and accessibility.
References
Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–23.
Kubo K, Ohgo K, Takeishi R, Yoshinaga K, Tsunoda N, Kanehisa H, Fukunaga T. Effects of isometric training at different knee angles on the muscle-tendon complex in vivo. Scand J Med Sci Sports. 2006;16:159–67.
Narici MV, Landoni L, Minetti AE. Assessment of human knee extensor muscles stress from in vivo physiological cross-sectional area and strength measurements. Eur J Appl Physiol Occup Physiol. 1992;65:438–44.
Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53:1483–9.
Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88:150–8.
Stonecipher MR, Jorizzo JL, Monu J, Walker F, Sutej PG. Dermatomyositis with normal muscle enzyme concentrations. A single-blind study of the diagnostic value of magnetic resonance imaging and ultrasound. Arch Dermatol. 1994;130:1294–9.
Engstrom CM, Loeb GE, Reid JG, Forrest WJ, Avruch L. Morphometry of the human thigh muscles. A comparison between anatomical sections and computer tomographic and magnetic resonance images. J Anat. 1991;176:139–56.
Hudash G, Albright JP, McAuley E, Martin RK, Fulton M. Cross-sectional thigh components: computerized tomographic assessment. Med Sci Sports Exerc. 1985;17:417–21.
Sipila S, Suominen H. Muscle ultrasonography and computed tomography in elderly trained and untrained women. Muscle Nerve. 1993;16:294–300.
Sipila S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol. 1995;78:334–40.
Hodges PW, Gandevia SC. Pitfalls of intramuscular electromyographic recordings from the human costal diaphragm. Clin Neurophysiol. 2000;111:1420–4.
Maganaris CN, Baltzopoulos V, Sargeant AJ. Repeated contractions alter the geometry of human skeletal muscle. J Appl Physiol. 2002;93:2089–94.
Reimers CD, Harder T, Saxe H. Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. J Neurol Sci. 1998;159:60–6.
Reimers CD, Schlotter B, Eicke BM, Witt TN. Calf enlargement in neuromuscular diseases: a quantitative ultrasound study in 350 patients and review of the literature. J Neurol Sci. 1996;143:46–56.
Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve. 2003;27:693–8.
Sipila S, Suominen H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve. 1991;14:527–33.
Sipila S, Suominen H. Quantitative ultrasonography of muscle: detection of adaptations to training in elderly women. Arch Phys Med Rehabil. 1996;77:1173–8.
Watkin KL, Diouf I, Gallagher TM, Logemann JA, Rademaker AW, Ettema SL. Ultrasonic quantification of geniohyoid cross-sectional area and tissue composition: a preliminary study of age and radiation effects. Head Neck. 2001;23:467–74.
Weiss LW, Clark FC, Howard DG. Effects of heavy-resistance triceps surae muscle training on strength and muscularity of men and women. Phys Ther. 1988;68:208–13.
Emshoff R, Bertram S, Strobl H. Ultrasonographic cross-sectional characteristics of muscles of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:93–106.
Miyatani M, Kanehisa H, Ito M, Kawakami Y, Fukunaga T. The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur J Appl Physiol. 2004;91:264–72.
Juul-Kristensen B, Bojsen-Møller F, Holst E, Ekdahl C. Comparison of muscle sizes and moment arms of two rotator cuff muscles measured by ultrasonography and magnetic resonance imaging. Eur J Ultrasound. 2000;11:161–73.
Schedel H, Reimers CD, Nagele M, Witt TN, Pongratz DE, Vogl T. Imaging techniques in myotonic dystrophy. A comparative study of ultrasound, computed tomography and magnetic resonance imaging of skeletal muscles. Eur J Radiol. 1992;15:230–8.
Ahmed R, Nazarian LN. Overview of musculoskeletal sonography. Ultrasound Q. 2010;26:27–35.
Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol. 2009;193:619–27.
Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press; 2007.
Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000.
Rudney JD, Ji Z, Larson CJ. The prediction of saliva swallowing frequency in humans from estimates of salivary flow-rate and the volume of saliva swallowed. Arch Oral Biol. 1995;40:507–12.
Acknowledgments
This research was conducted during the tenure of a Postgraduate Scholarship of the New Zealand Neurological Foundation. The manuscript was completed with the support of a Ngā Pae o te Māramatanga Doctoral Bridging Grant. The authors thank the Christchurch Radiology Group for the generous use of their ultrasound equipment, and Gareth Leeper and Simon Felton for their input regarding the MRI sequences. The authors also thank Professor Thomas Marquardt for the generous donation that made the MRI scans possible.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macrae, P.R., Jones, R.D., Myall, D.J. et al. Cross-Sectional Area of the Anterior Belly of the Digastric Muscle: Comparison of MRI and Ultrasound Measures. Dysphagia 28, 375–380 (2013). https://doi.org/10.1007/s00455-012-9443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-012-9443-8