Abstract
We tested the effects of surface electrical stimulation on hyoid elevation during swallowing in healthy volunteers. Sixteen people were recruited and randomly divided into two groups. Electrical stimulation was applied to the skin above the infrahyoid muscle in the experimental group. The stimulation current was adjusted until muscle contraction occurred and the hyoid bone became depressed. Participants were asked to swallow forcefully so as to elevate the hyolaryngeal complex when the stimulation began. The same experiment was performed in the control group except the intensity of stimulation was adjusted to just above the sensory threshold. The two groups received ten 20-min treatments over 2 weeks. We recorded the myoelectrical activity of the submental muscles and the amount of hyoid bone movement at three time points (pretreatment, immediately post-treatment, and 2 weeks after treatment). In the experimental group, the amount of y-axis hyoid bone excursion was increased immediately post-treatment, but this effect faded within 2 weeks following the treatment. Myoelectrical activity was not affected by either treatment regimen. We concluded that effortful swallowing coupled with electrical stimulation increases the degree of hyoid elevation in healthy volunteers. It needs to be evaluated for its long-term effectiveness in increasing the elevation of hyolaryngeal complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Transcutaneous electrical stimulation is a novel treatment for dysphagia. The first study regarding this kind of treatment was published in 1997 and investigated the triggering of the swallowing reflex by oral electrical stimulation when a palatal prosthesis was applied [1]. Freed et al. [2] reported a new treatment for dysphagia consisting of transcutaneous electrical stimulation applied through electrodes placed on the neck. They compared the effectiveness of electrical stimulation treatment with that of thermal-tactile stimulation treatment and concluded that electrical stimulation was the more effective of the two. Another study showed that synchronized electrical stimulation improved the contraction of the thyrohyoid muscle and led to laryngeal elevation, an essential step in opening the cricopharyngeal sphincter [3].
There has been some controversy in recent studies over the efficacy of electrical therapy for dysphagia. Some reports provide positive outcomes for this therapy type within a certain population or individual, while others do not. Blumenfeld et al. [4] reported that patients who received 40 consecutive electrical stimulations showed a significant improvement in their swallowing function compared to those who had received traditional dysphagia therapy. Another retrospective analysis showed an improvement in swallowing function in 61% of patients who received transcutaneous electrical stimulation [5]. On the other hand, two different studies [6, 7] that compared the outcome of neuromuscular electrical stimulation (NMES) versus traditional swallowing treatments showed no additional improvement in the NMES treatment group.
Many researchers have attempted to determine the effects of transcutaneous electrical stimulation on the physiology of swallowing, but little is known in this regard. Suiter et al. [8] failed to produce evidence of an increase in submental muscle strength after NMES. Another study showed that surface electrical stimulation to the laryngeal regions caused significant hyoid and laryngeal descent at rest and reduced hyoid and laryngeal peak elevation during swallowing in healthy adults [9]. The authors suggested that in those patients who had the ability to raise their hyolaryngeal complex, hyoid depression with stimulation might serve as “resistance” during therapy. On the other hand, if a patient was unable to produce any hyolaryngeal elevation and therefore would not be able to resist the hyoid depression induced by stimulation, then stimulation might put such a patient at greater risk of aspiration as the hyolaryngeal complex would remain held down during swallowing [10]. Thus, the mechanism underlying electrical stimulation therapy is unclear.
Base on the findings of Ludlow et al. [10], we used electrical stimulation as a form of resistance training to increase hyoid elevation in healthy volunteers. This represents a different approach from the existing method of electrical stimulation. We thus tested the effect of a novel method of electrical stimulation therapy with the intention of providing an alternative treatment mechanism for dysphagia.
Materials and Methods
Subjects
Sixteen healthy volunteers between the ages of 21 and 30 years were recruited for this study from December 2006 to January 2007 and randomly assigned into two groups. They were all examined by a physician and a speech-language pathologist. Our volunteers were free from neurologic, phonologic, psychiatric, speech, and swallowing disorders. This study was approved by the Institution Review Board and informed consent was obtained from every volunteer. The average age of the study group was 26.3 ± 2.4 years (8 males and 8 females). The general characteristics of the participants are given in Table 1.
Procedures
This study was designed as a single-blind, randomized, controlled study and was scheduled for a total of 4 weeks. Before stimulation therapy, we obtained baseline data for submental muscle electrical activity during swallowing by using surface electromyography (sEMG). Hyoid bone excursion was also measured by videofluoroscopy (VFS). Electrical stimulation therapy was then applied to the participants over 2 weeks, which was followed by sEMG and VFS to evaluate the therapeutic effects of electrical stimulation. During the final 2 weeks of the study the participants received no treatment after which the last set of sEMG and VFS data was gathered to determine the long-term effects of electrical therapy.
Electrical Stimulation
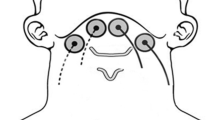
Electrical stimulation was performed using the Microstim® (Medel GmbH, Hamburg, Germany) which is a two-channel functional electrical stimulation device for neuromuscular rehabilitation, especially where activation of paralyzed muscles after stroke is concerned. Two sets of electrodes were placed in the infrahyoid area targeting the sternohyoid muscles (Fig. 1). A pulse rate of 35 Hz for 600 μs was used. The “on” and “off” stimulation cycle ratio was 6:4 s. In the control group, the intensity of stimulation was adjusted until the participants felt a tingling sensation in the neck. In the experimental group, the intensity of stimulation was increased until muscle contraction was visible. All participants received one 20-min treatment each weekday for a total of ten 20-min treatments over 2 weeks. Participants were asked to forcefully swallow 2 ml of water every 10 s during the “on” stimulation so as to elevate the hyolaryngeal complex. Between two 10-min treatments, a 2-min rest period was allowed for all participants so as to avoid muscle fatigue.
Surface Electromyography
The surface EMG signal was obtained using a MedelecSynergy instrument (Oxford Instruments, Surrey, UK). The active electrodes were placed on the anterior bellies of the digastric muscle with the reference electrodes on the mentis. The peak amplitude and area of the EMG signal were measured three times during forceful swallowing of 2 ml of water (sweep speed = 3 s; amplitude sensitivity = 100 μV). To facilitate consistent electrode placement for the three recordings, we marked the points of stimulation on the submental area.
Videofluoroscopy
A videofluoroscopic swallowing study was performed for analysis of hyoid bone movement. Participants sat upright and their heads were stabilized and immobilized with the examiner’s hands. A 1.5-m distance from the X-ray tube to the head was maintained. Participants were asked to forcefully swallow 2 ml of 35% barium (w/v) three times. Videofluoscopic images of a lateral view were directly captured by using a digital Picture Archiving and Communication System (PACS). The capture rate was 30 frames/s and the frame size was 1,021 × 1,021 pixels.
Hyoid Bone Movement Analysis
We used a PiView STAR program (Infinitt Co., Ltd., Seoul, Korea) for analysis of hyoid bone movement. When we identified a point with the cursor, the program showed its coordinate using a pixel location (Fig. 2). We determined the anterior inferior corner of the forth vertebra as the origin and measured the x and y coordinates of the anterior tip of the hyoid bone twice (when the hyoid bone reached the superior and inferior positions during swallowing). We calculated the difference between the x and y coordinates of the superior and inferior positions. The distance between pixels was 0.184 mm.
Statistical Analysis
The following measurements were obtained for comparison pretreatment, immediately post-treatment, and 2 weeks after treatment cessation: sEMG amplitude, sEMG area, x-axis values, and y-axis values of the hyoid bone. For paired nonparametric variables analysis, the Wilcoxon signed rank test was used (α = 0.05), and was performed with SPSS v12.0 (SPSS, Inc., Chicago, IL, USA).
Results
Table 2 gives the change in sEMG peak amplitude over 4 weeks. Following forceful swallowing training, the peak amplitude of the sEMG immediately post-treatment increased compared with the baseline data in six of eight subjects in the experimental group, but these responses were not statistically significant (p = 0.123). The sEMG signals 2 weeks after treatment cessation showed a similar outcome. In the control group, there was no significant difference between the peak amplitudes of the sEMG signals regardless of when the data were gathered. Also, there was no significant difference between pre- and post-treatment sEMG signals in either group, indicating that myoelectrical activity was not affected by the treatment.
In the case of hyoid movement, increased elevation just after 2 weeks of treatment was observed only in the experimental group. This elevation involved no forward hyoid movement. However, the effects faded within 2 weeks following treatment. Figure 3 shows the degree of x- and y-axis movement of the hyoid bone. There was no significant difference in the degree of x-axis movement in either group pretreatment, immediately post-treatment, and 2 weeks after treatment. However, in the experimental group, the amount of y-axis hyoid bone excursion was increased immediately post-treatment. This movement, however, could not be maintained for the 2 weeks following treatment. There was no significant difference in the control group.
Discussion
Although surface electrical stimulation for the treatment of dysphagia is gaining in popularity, the mechanism whereby it affects swallowing remains unclear. The findings of the present study contribute to our understanding of how electrical stimulation affects swallowing physiology.
Upward and superior movement of the larynx is an important aspect of swallowing. It is very closely related to airway protection and decreased laryngeal elevation causes aspiration [11]. Opening of the upper esophageal sphincter also results from the traction generated by the anterior movement of the larynx [12].
The premise of this study was based on the findings of Ludlow et al. [10], i.e., electrical stimulation applied to the anterior neck results in decreased hyolaryngeal excursion. Evidence in the research literature indicates that resistance exercises are effective for strength training [13]. Individuals with decreased hyolaryngeal excursion during swallowing presumably have weak muscles. Therefore, we used electrical stimulation to provide resistance against hyolaryngeal excursion hypothesizing that it would lead to an increase in hyoid movement and a strengthening of the muscles involved in hyoid movement.
Participants in the experimental group trained their submental muscles by elevating their hyoid against the downward movement induced by electrical stimulation (the current intensity was also raised to induce downward movement). In the first 2 weeks of training, hyoid movement was enhanced but this enhancement did not last an additional 2 weeks without exercise. It usually takes at least 4 weeks for muscle hypertrophy to occur and so the strength gained during the early training period might be explained by the neural factor theory [14]. Two weeks of muscle unloading results in the loss of strength via the mechanism of neural adaptation [15]. These theories may offer a possible explanation for our results.
However, our sEMG signal findings did not support the neural factor theory. In this study, six participants in the experimental group actually increased their sEMG amplitudes just after treatment, although the increases were not statistically supported. One possible explanation for our findings could lie in our method of effortful swallow. According to Huckabee et al. [16], how one performs the effortful swallow affects pharyngeal pressure and submental sEMG. If tongue-to-palate is not emphasized during execution of effortful swallowing, the amplitude of the submental sEMG cannot be increased. This factor could have affected our results. Another possible reason is that we could not measure the activity of all the muscles involved in hyoid elevation. The muscles that elevate the hyoid bone consist of the mylohyoid, geniohyoid, and anterior bellies of the digastric muscle. However, we placed the recording electrodes on only the anterior bellies of the digastrics, which are superficially located, and could record signals only from those. So, it is possible that our data did not reflect the effects of training.
There are conflicting views about the physiologic effect of effortful swallowing as a means of altering pharyngeal pressure in bolus swallowing. Hind et al. [17] documented that effortful swallowing of a thin liquid bolus resulted in increased oral pressure and increased duration of maximal anterior hyoid excursion, laryngeal vestibule closure, and extent of superior hyoid movement, with a trend toward oral bolus clearance. However, Bülow et al. [18] documented a decreased hyomandibular distance before swallowing and reduced hyoid movement during swallowing in nonimpaired subjects. In our study, effortful swallowing training without motoric electrical stimulation did not increase either sEMG activity or hyoid excursion compared to the baseline data.
The present study has several shortcomings. First, the sample size was too small. We randomly assigned the participants and used statistical analysis for nonparametric valuables, but the limitation still remained. Second, because we did not measure laryngeal movement, we could report on the elevation of the hyoid rather than the hyolaryngeal complex. We did not measure the cross-sectional area of the submental muscles that would have reflected muscle hypertrophy, and we did not measure real strength using a force transducer. Therefore, we could not claim that submental muscle strength increased. These limitations should be addressed in a subsequent study. Third, this treatment protocol was applied to young healthy people rather than patients with dysphagia. As a result it is difficult to extrapolate our findings to account for such patients. Therefore, clinical studies with patients suffering from dysphagia are required.
In conclusion, effortful swallowing coupled with electrical stimulation increases the degree of hyoid elevation in healthy volunteers. However, it needs to be evaluated for its short-term and long-term effectiveness in increasing hyoid and laryngeal elevation and improving swallowing safety in patients with dysphagia.
References
Park CL, O’Neill PA, Martine DF. A pilot exploratory study of oral electrical stimulation on swallow function following stroke: an innovative technique. Dysphagia. 1997;12:161–6. doi:10.1007/PL00009531.
Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46:466–74.
Leelamanit V, Limsakul C, Geater A. Synchronized electrical stimulation in treating pharyngeal dysphagia. Laryngoscope. 2002;112:2204–10. doi:10.1097/00005537-200212000-00015.
Blumenfeld L, Hahn Y, LePage A, Leonard R, Belafsky PC. Transcutaneous electrical stimulation versus traditional dysphagia therapy: a nonconcurrent cohort study. Otolaryngol Head Neck Surg. 2006;135:754–7. doi:10.1016/j.otohns.2006.04.016.
Shaw GY, Sechtem PR, Searl J, Keller K, Rawi TA, Dowdy E. Transcutaneous neuromuscular electrical stimulation (VitalStim) curative therapy for severe dysphagia: myth or reality? Ann Otol Rhinol Laryngol. 2007;116:36–44.
Kiger M, Brown CS, Watkins L. Dysphagia management: An analysis of patient outcomes using VitalStim therapy compared to traditional swallow therapy. Dysphagia. 2006;21:243–53. doi:10.1007/s00455-006-9056-1.
Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23:302–9. doi:10.1007/s00455-007-9145-9.
Suiter DM, Leder SB, Ruark JL. Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia. 2006;21:56–60. doi:10.1007/s00455-005-9010-7.
Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Crujido L, Wright-Harp W, et al. The effect of surface electrical stimulation on hyo-laryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol. 2006;101:1657–63. doi:10.1152/japplphysiol.00348.2006.
Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22:1–10. doi:10.1007/s00455-006-9029-4.
Lundy DA, Smith C, Colangelo L, Sullivan PA, Logemann JA, Lazarus CL, et al. Aspiration: cause and implications. Otolaryngol Head Neck Surg. 1999;120:474–8. doi:10.1053/hn.1999.v120.a91765.
Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748–59.
de Lateur BJ. Therapeutic exercise. In: Braddom RL, editor. Physical medicine and rehabilitation. 2nd ed. Philadelphia: W.B. Saunders; 2000. p. 399–401.
Moritani T, de Vries HK. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115–30.
Deschenes MR, Giles JA, McCoy RW, Volek JS, Gomez AL, Kraemer WJ. Neural factors account for strength decrements observed after short-term muscle unloading. Am J Physiol Regul Integr Comp Physiol. 2002;282:R578–83.
Huckabee ML, Steele CM. An analysis of lingual contribution to submental surface electromyographic measures and pharyngeal pressure during effortful swallow. Arch Phys Med Rehabil. 2006;87:1067–72. doi:10.1016/j.apmr.2006.04.019.
Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82:1661–5. doi:10.1053/apmr.2001.28006.
Bülow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14:67–72. doi:10.1007/PL00009589.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, JW., Oh, JC., Lee, H.J. et al. Effortful Swallowing Training Coupled with Electrical Stimulation Leads to an Increase in Hyoid Elevation During Swallowing. Dysphagia 24, 296–301 (2009). https://doi.org/10.1007/s00455-008-9205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-008-9205-9