Abstract
The stylopharyngeus plays a critical role in the clearance of the piriform recess. We dissected 78 sides of the pharynx from 55 donated cadavers and observed histology of another seven sides of the pharynx from seven cadavers. The stylopharyngeus consistently comprised (1) a descending muscle bundle surrounding the piriform recess and (2) an additional short sheet inserting into the tonsillar bed. Histologically, the former bundle connected to a thick fascia providing the lateral glossoepiglottic fold, extending along the submucosa of the piriform recess, and covering the thyroid cartilage, whereas the latter sheet intermingled with other pharyngeal wall muscles at and near the tonsillar bed. Notably, in 44.4% of female specimens, the additional sheet occupied a greater proportion in cross section than the descending muscle bundle. Given the different directions, the additional sheet seemed to check clearance function of the descending bundle for the piriform recess. Thus, particularly in women, interindividual differences in pharyngeal clearance were likely to depend on whether the additional sheet is strong or weak. Chin down in combination with tilting and rotating the head may represent effective exercises of the stylopharyngeus that could compensate for the disadvantages of additional insertion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Silent aspiration or postdeglutitive overflow aspiration represents a major mechanism for dysphagia in elderly patients [1–4]. Longitudinal pharyngeal muscles, namely, the stylopharyngeus, palatopharyngeus, and salpingopharyngeus, are responsible for pharyngeal shortening and, thus, play a critical role in pharyngeal clearance to avoid postdeglutitive overflow aspiration [1, 2, 5]. In particular, the stylopharyngeus exhibits a specific topographical relationship with the piriform recess, with muscle insertions surrounding the piriform recess according to the figure in Gray’s Anatomy (Figs. 11–26 in [6]). A lateral margin of the piriform recess, i.e., the lateral glossoepiglottic fold [6] or pharyngoepiglottic fold [7], is composed of the stylopharyngeus with the palatopharyngeus. The piriform recess is well known as the largest pocket that provides postdeglutitive retention, in addition to being the major route for pharyngeal swallowing. Of the three longitudinal pharyngeal muscles, stylopharyngeus contraction seems to be the most effective for pharyngeal clearance.
Taken together, we hypothesized that interindividual anatomic variations in stylopharyngeal insertion, if present, are committed to pharyngeal clearance function. However, little information is available on interindividual anatomic variations of the stylopharyngeus, particularly, in elderly individuals. The present study, therefore, aimed to (1) describe anatomic variations in the stylopharyngeal insertion and (2) discuss treatment of patients depending on anatomic limitations to pharyngeal clearance.
Materials and Methods
For macroscopic observations we dissected specimens of the pharynx in 78 sides of 55 donated cadavers (38 left sides and 40 right sides; 42 sides from 30 male cadavers and 36 sides from 25 female cadavers). Bilateral observations were made in 23 of the 55 cadavers. These cadavers had been fixed with 10% formalin and the mean age at death was 76.4 years (range = 52–98 years). The cadavers included three that were less than 69 years old (52- and 66-year-old females and 64-year-old male). These specimens included the pharyngeal opening of the auditory canal, soft palate, and the extrapharyngeal part of the stylopharyngeus superiorly, and the epiglottis, thyroid cartilage, and piriform recess inferiorly. After bisection of the pharynx along the midsagittal plane and removal of the pharyngeal mucosa, the palatopharyngeus and salpingopharyngeus were observed first. We then opened a slit between the superior and the middle constrictors of the pharynx and made sure there was a continuation of the extra- and intrapharyngeal portions of the stylopharyngeus.
In addition to the aforementioned 55 cadavers, seven specimens that included the stylopharyngeus, tonsillar bed, and piriform recess were processed for routine paraffin-embedded histology (seven sides of seven cadavers, four males and three females; mean age = 75.8 years). For easy identification in histology, the stylopharyngeus was labeled with carbon particles at the entrance to the pharyngeal wall. These specimens for histology were immersed in 5% nitric acid for 1 week to decalcify the hyoid bone. Semiserial sections with 0.5- or 1.0-mm intervals were prepared and stained using hematoxylin and eosin.
The protocol for the present research project did not include any specific issue that needed to be approved by the Ethics Committees of our institutions. The present work conformed to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000).
Results
Macroscopic Observations
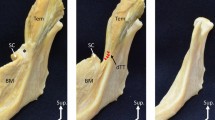
During and immediately after passing through a slitlike space between the superior and the middle pharyngeal constrictors, the stylopharyngeus consistently divided into (1) a major muscle bundle descending toward the piriform recess and (2) a short muscle sheet extending into the dorsoinferior part of the submucosal layer at the tonsillar bed (Fig. 1A). Because the latter insertion into the tonsillar bed had not yet been described, this was termed the “additional insertion” (Figs. 1–3). This insertion comprised superficial (mucosal side) and superior components of the extrapharyngeal stylopharyngeus muscle fibers. The additional insertion into the tonsillar bed was sometimes very thin (Figs. 1B, 2B; 15 of 78 sides and 12 of 55 cadavers). In the tonsillar bed, the insertion was located in the superficial or mucosal side of the styloglossus and constrictor pharyngis superior. The styloglossus was sometimes located in a wedged position between the additional insertion and descending bundle of the stylopharyngeus. The major descending muscle bundle usually formed the medial or ventral aspect of the lateral glossoepiglottic fold in combination with the palatopharyngeus for the lateral aspect (Fig. 2). In the lateral glossoepiglottic fold, the stylopharyngeus was thicker than the palatopharyngeus in almost two thirds of the 78 specimens.
Variations of the stylopharyngeus, viewed from the medial side of the right half of the pharynx. A A strong additional insertion to the tonsillar bed in an 82-year-old male. Almost half of the entire muscle fiber of the stylopharyngeus (SP) contributes to the additional insertion. The descending bundle runs along the lateral glossoepiglottic fold (SP to fold). B In contrast, a 78-year-old male exhibits a relatively strong descending bundle (SP to cartilage) lining the piriform recess (PR) and running inferiorly toward the thyroid cartilage. The palatopharyngeus (PP) was divided to show the SP. CC, cricoid cartilage; CPS, constrictor pharyngis superior; PT, palatine tonsil; TONG, tongue (cut); UV, uvula
Left/right differences in the stylopharyngeus. All four panels display the same specimen of a 93-year-old female. A From the dorsal side of the pharynx. EPI, epiglottis. B The left side of the pharynx exhibits a strong descending bundle of the stylopharyngeus going inferiorly toward the thyroid cartilage (SP to cartilage). C, D The right side of the pharynx shows a relatively strong additional insertion and a weak descending bundle (SP to fold) running along the lateral glossoepiglottic fold (LGEF). The mucosal membrane was absolutely removed in D. CC, cricoid cartilage; CPS, constrictor pharyngis superior; PT, palatine tonsil; TONG, tongue (cut); UV, uvula
Schematic representation of the well-developed descending bundle of the stylopharyngeus with the additional insertion to the tonsillar bed. Right side of the pharynx viewed from the medial side after bisection in the midsagittal plane. The lateral glossoepiglottic fold comprises the stylopharyngeus (SP) and palatopharyngeus (PP). The PP is also divided into two bundles. The salpingopharyngeus (SA) occupies a large area in the dorsal side of the piriform recess. CC, cricoid cartilage; CPS, constrictor pharyngis superior; PT, palatine tonsil; UV, uvula
In addition to forming the fold (see above), dorsal or lateral parts of the descending muscle bundle, when developed well (26 of 78 sides of the pharynx), ran inferiorly along the lateral margin and/or bottom of the piriform recess toward the thyroid cartilage (Fig. 1B). The salpingopharyngeus was adjacent to the lateral or dorsal side of the well-developed descending bundle along the recess. Figure 3 shows an example of a well-developed stylopharyngeus.
In the cut surface of an extrapharyngeal portion of the stylopharyngeus outside the pharyngeal wall, 19 of 78 specimens of the pharynx (24.4%) displayed the additional insertion that occupied a greater area than the descending muscle bundle (Fig. 1A; Table 1). In another eight specimens of the pharynx, the tonsillar bed insertion shared equally with the descending bundle. Conversely, the descending muscle bundle was evident in the remaining 51 of 78 sides of the pharynx. Notably, in 16 of 36 sides of the pharynx obtained from female cadavers, the additional insertion displayed a greater area than the descending bundle (44.4%), in contrast to 3 of 42 sides of the male pharynx (7.1%).
We performed statistical analysis using the Mann-Whitney U test on the size distribution of the additional insertion between the male and the female groups and derived significant differences with respect to gender (p < 0.01, Table 1).
The present population included three cadavers that were younger than 69 years old; these three cadavers were examined unilaterally after bisection along the midsagittal plane, as the other halves were dissected by students. One of these three specimens displayed a strong tonsillar bed insertion, while the other two showed a strong descending bundle.
We bilaterally examined 23 cadavers, most (19) of which displayed no laterality in stylopharyngeus insertions. However, in the remaining four cadavers, a significant left/right difference was found in proportion to additional insertion and descending bundle. The additional insertion was thicker than the descending bundle in the left sides of two cadavers (Fig. 2), and vice versa in the right sides. In two cadavers the descending bundle was thicker unilaterally and almost equal on the other side of the pharynx.
Histologic Observations
The tonsillar bed insertion or additional insertion was composed of superior parts of the stylopharyngeus and displayed a short recurrent course to intermingle with the constrictor pharyngis superior and palatopharyngeus (Fig. 4). At the entrance of the stylopharyngeus into the pharyngeal wall, fascia existed between the additional insertion and the constrictor pharyngis superior, but no fascia was present between the stylopharyngeus and the palatopharyngeus (Fig. 4D). The additional insertion was thin in two of seven specimens for histologic examination (Fig. 4) and was thick in five of seven specimens (Fig. 5).
Histology of the pharyngeal wall and stylopharyngeus. A-C A longitudinal section of the pharynx. D A higher-magnification view rotated 90° clockwise of the square outlined in C. Panel A (along the lateral glossoepiglottic fold) is the most medial, while panel C (along the piriform recess) is the most lateral or dorsal. Additional insertion is weak (D). Note a thick fascia (arrow, panels A-C) lining the stylopharyngeus (SP). CPS, CPM, and CPI, constrictor pharyngis superior, medial, and inferior; HB, hyoid bone; M, mucosal membrane; PP, palatopharyngeus; TC, thyroid cartilage
Histology of the stylopharyngeus and additional insertion. A longitudinal section along the piriform recess. Arrows indicate the strong additional insertion. The space labeled by asterisks represents an artifact made during dehydration process. SLA, superior laryngeal artery. CPS, CPM, and CPI, constrictor pharyngis superior, medial, and inferior; HB, hyoid bone; M, mucosal membrane; PP, palatopharyngeus; TC, thyroid cartilage
At the lateral glossoepiglottic fold, the palatopharyngeus was located on the superficial or mucosal side of the stylopharyngeus, although no definite fascia existed between these muscles. However, in the inferior part of the fold, both muscle fibers intermingled mutually to provide a single conjoined muscle plate in the submucosal tissue. In the superficial and deep sides of the plate at the piriform recess, clear thick fasciae were evident. In particular, a definite fascia lined the deep side of the muscle plate. The fascia clearly separated the conjoined muscle plate from the constrictor pharyngis medius. These fasciae continued inferiorly and loosely covered the thyroid cartilage. Pharyngeal mucosal glands were seldom embedded in the stylopharyngeus in contrast to abundant glands inserted deeply into the palatopharyngeus (not shown).
Discussion
According to present histology, a thick fascia was evident between the constrictor pharyngis medius and the descending bundle of the stylopharyngeus. This observation indicated that stylopharyngeal function differed substantially from the function of the constrictor pharyngis, and that contraction of the descending bundle of the stylopharyngeus effectively flattened the piriform recess. The descending bundle thus seems effective for clearance of the piriform recess. Pharyngeal mucosal glands were seldom embedded in the stylopharyngeus, in contrast to abundant glands inserted deep into the palatopharyngeus. This morphology also seemed to suggest much greater contraction for the stylopharyngeal muscle than for the palatopharyngeal muscle. In addition, a thick fascia underlying the conjoined muscle plate of the stylopharyngeus and the palatopharyngeus continued inferiorly and covered loosely the thyroid cartilage. The classical description “the stylopharyngeus inserts the cartilage [6, 7]” thus appears to be imprecise, as the muscle fibers themselves did not connect directly to the cartilage.
The present study demonstrated an additional muscle sheet of the stylopharyngeus inserted into the tonsillar bed. In 19 of 78 sides of the pharynx (24.4%), the additional insertion was stronger than the descending bundle running in and around the piriform recess. Notably, the significantly high incidence of strong additional insertion was observed in females (44.4%) compared with that in males (7.1%). The descending bundle seems to play a critical role in pharyngeal clearance, but the additional insertion seems unlikely to contribute to this function because this is likely to pull the tonsillar bed dorsosuperiorly. Moreover, the tonsillar bed might be fixed at the deep side by the stylohyoid ligament. Indeed, the strong additional insertion does not seem to always correlate with the reduced clearance function of the stylopharyngeus, because the strong descending bundle is able to compensate.
The additional insertion was likely to cause left/right differences in pharyngeal clearance (Fig. 2). In fact, nonpathologic left/right differences in pharyngeal swallowing [8] and in the size of the piriform recess [9] have often been reported. According to the present results, almost 20% of elderly patients seemed to unilaterally have reduced function of the stylopharyngeus due to the relatively strong additional insertion. Moreover, tonsillectomy, which is now performed even in elderly patients for control of some immunologic disorders [10], might induce scarring that involves the additional insertion and pulls up on the stylopharyngeal descending bundle. However, the incidence of muscle variation seems likely to differ between human populations as seen in pharyngeal constrictors [11]. Consequently, the additional insertion of the stylopharyngeus seems to represent an “unfavorable variation” against pharyngeal clearance, particularly in elderly women in whom muscle function is reduced.
Why did previous studies not mention this additional insertion to the tonsillar bed? Identification seemed to be difficult in gross anatomy because, according to the present histology, muscle fibers of the additional insertion were short in course and intermingled with the palatopharyngeus and constrictor pharyngis superior. Although muscular lamination of the tonsillar bed has been described in different ways [12–15], the stylopharyngeus was excluded from those components. Ohtsuka et al. [16] described the parts of the muscle that took part in formation of the tonsillar bed; this corresponded to the major descending bundle in the present study. We speculated that nobody paid attention to the additional insertion because in younger specimens it should be present but much smaller than the descending bundle. Thus, the relatively strong insertion to the tonsillar bed, as often seen in females (44.4%), was likely to be a result of aging, with degeneration of the descending muscle bundle. Conversely, the additional insertion, even when replaced by collagenous fibers with degeneration, could change the direction of the longitudinal pharyngeal muscle. Unfortunately, however, we were unable to retrieve specimens from younger subjects to confirm this hypothesis.
Of the three longitudinal muscles of the pharynx, the stylopharyngeus is the only muscle with an origin outside the pharyngeal wall (i.e., the styloid bony process). Thus, because the topographical relationship between the stylopharyngeus origin and the point of insertion changes depending on the posture of the head, stylopharyngeus contraction or pharyngeal clearance function are likely to be easily influenced by posture of the head. Flexion of the head enhances closure of the laryngeal vestibule [17]. In the face-forward position, the opening diameter of the upper esophageal sphincter is increased and pharyngeal retention in the piriform recess is decreased [18, 19]. When the chin is tucked or in a chin-down position, posterior shift of the anterior pharyngeal structures occurs in combination with narrowing of the distance from the epiglottis to the pharyngeal wall [20–26]. This distance nearly corresponds to the working distance for the descending bundle of stylopharyngeus along the lateral glossoepiglottic fold. Thus, a chin-down position does not seem good for pharyngeal clearance by the stylopharyngeal descending bundle. In fact, according to Logemann et al. [18], in contrast to head rotation, the face-forward position decreases pharyngeal retention in the piriform recess. In the present study we hypothesized that interindividual and left/right differences in pharyngeal clearance ability are likely to depend on whether the additional insertion of the stylopharyngeus is strong or weak. Conversely, exercise for the stylopharyngeus should be effective for the descending bundle, not for the additional insertion. Overall, we would like to try a chin-down position in combination with lateral tilt and rotation of the head for acceleration of pharyngeal clearance and exercise of the stylopharyngeus.
References
Dejaeger E, Pelemans W, Ponette E, Joosten E. Mechanisms involved in postdeglutition retention in the elderly. Dysphagia 1997;12:63–67.
Olson R, Castell J, Johnston B, Ekberg O, Castell DO. Combined videomanometric identification of abnormalities related to pharyngeal retention. Acad Radiol 1997;4:349–354.
Smith CH, Logemann JA, Colangelo LA, Rademaker AW, Pauloski BR. Incidence and patient characteristics associated with silent aspiration in the acute care setting. Dysphagia 1999;14:1–7.
Eisenhuber E, Schima W, Schober E, Pokieser P, Stadler A, Scharitzer M, Oschatz E. Videofluoroscopic assessment of patients with dysphagia: pharyngeal retention is a predictive factor for aspiration. AJR Am J Roentgenol 2002;178:393–398.
Kahrilas PJ, Logemann JA. Pharyngeal structure and function. Dysphagia 1993;8:303–307.
Bannister L, Respiratory System. In: Williams PL, Editor. Gray’s Anatomy, 39th ed. London: Churchill & Livingstone, 1995, pp 1627–1682.
Netter FH. Atlas of Human Anatomy, 4th ed. Oxford: Saunders, 2006, p 63.
Seta H, Inada H, Abo M, Sugimoto A, Miyano S. Upper esophageal imaging patterns by videofluoroscopic examination in the anterior-posterior planes: laterality of pharyngoesophageal segment flow. Jpn J Rehabil Med 2004;5:307–312.
Ohkuma R, Fujishima I, Takehara I, Ishii M, Miyono S. Transmucosal surface electromyography of cricopharyngeal muscle using a catheter electrode. Jpn J Rehabil Med 1999;6:410–414.
Masuda Y. Clinical and immunological study of IgA nephropathy before and after tonsillectomy. Acta Otolaryngol (Stockh) Suppl 1988;45:248–255.
Shimada L, Gasser RF. Variations of the pharyngeal raphe. Clin Anat 1988;1:285–294.
Todd TW, Fowler RH. The muscular relationships of the tonsil. Am J Anat 1927;40:355–371.
Moore KL. Clinical Oriented Anatomy, 2nd ed. Baltimore: Williams & Wilkins, 1980, pp 1036–1047.
Hollinshead WH. Anatomy for Surgeons, vol 1, 3rd ed. Philadelphia: Harper & Row, 1982, pp 394–400.
Beasley P. Anatomy of the pharynx and oesophagus. In: Wright D, Editor. Scott-Brown’s Otolaryngology, vol 1, 5th ed. London: Butterworths, 1987, pp 252–271.
Ohtsuka K, Tomita H, Murakami G. Anatomy of the tonsillar bed: topographical relationship between the palatine tonsil and the lingual branch of the glossopharyngeal nerve. Acta Otolaryngol Suppl 2002;546:99–109.
Ekberg O. Posture of the head and pharyngeal swallowing. Acta Radiol Diagn (Stockh) 1986;6:691–696.
Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil 1989;70:767–771.
Logemann JA. The role of exercise programs for dysphagia patients. Dysphagia 2005;20:139–140.
Welch MV, Logemann JA, Rademaker AW, Kahrilas PJ. Changes in pharyngeal dimensions effected by chin tuck. Arch Phys Med Rehabil 1993;74:178–181.
Shanahan TK, Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Chin-down posture effect on aspiration in dysphagic patients. Arch Phys Med Rehabil 1993;74:736–739.
Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffmann RG. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol 1997;272:G1518–G1522.
Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, Aydogdu I. The effect of head and neck positions on oropharyngeal swallowing: A clinical and electrophysiologic study. Arch Phys Med Rehabil 2001;82:1255–1260.
Lewin JS, Hebert TM, Putnam JB Jr, DuBrow RA. Experience with the chin tuck maneuver in postesophagectomy aspirators. Dysphagia 2001;16:216–219.
Büllow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia 2001;16:190–195.
Easterling C, Grande B, Kern M, Sears K, Shaker R. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia 2005;20:133–138.
Acknowledgments
The authors are grateful to Professor Toshihiko Yajima, Department of Oral Anatomy, Health Science University of Hokkaido; Professor Shigemitsu Yoshida, Department of Oral Anatomy, Hokkaido University School of Dentistry; and Professor Shigetaka Yoshida, Department of Anatomy, Asahikawa Medical University School of Medicine, for their permission to use their materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was performed at the Department of Anatomy, Sapporo Medical University, School of Medicine, Sapporo, Japan.
Rights and permissions
About this article
Cite this article
Meng, H., Murakami, G., Suzuki, D. et al. Anatomical Variations in Stylopharyngeus Muscle Insertions Suggest Interindividual and Left/Right Differences in Pharyngeal Clearance Function of Elderly Patients: A Cadaveric Study. Dysphagia 23, 251–257 (2008). https://doi.org/10.1007/s00455-007-9131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-007-9131-2