Abstract
Children with CHARGE syndrome commonly experience feeding and swallowing problems. Difficulties may be associated with congenital structural anomalies, motor impairment, and/or oral sensory impairment. For many children with CHARGE syndrome, the introduction of functional oral feeding is delayed and there are often long-term feeding complications. Oral aversion or defensiveness is a frequent serious issue; however, it is uncertain whether this is a primary sensory disorder or secondary to delayed and/or negative oral sensory and feeding experiences. This article examines in detail the early oral sensory and feeding experiences of five children with CHARGE syndrome, through a review of medical records and caregiver questionnaires. Findings indicate variable early oral sensory experiences in this group of children, with all of the children having some difficulty or delay in the development of oral feeding and swallowing. The nature of these difficulties and the potential contributory factors are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
CHARGE syndrome is a diagnosis given to a nonrandom pattern of congenital anomalies [1,2]. The diagnosis is based on the presence of four major features, or three major and three minor features [3]. Major features include coloboma, choanal atresia, cranial nerve dysfunction, and characteristic ear anomalies, while minor features include cardiovascular malformations, growth deficiency, genital hypoplasia, orofacial clefting, tracheoesophageal fistula, developmental delay, and distinctive face. The reported incidence of CHARGE syndrome in Canada ranges from 1/8500 in the Atlantic Provinces to a national average of 3.5/100,000 live births [4]. This latter number is likely an underestimate due to the difficulty in properly diagnosing and reporting the condition nationally [5].
Feeding and swallowing disorders are highly prevalent in the CHARGE population [3,6]. For many children with CHARGE syndrome, feeding problems are identified in the neonatal period and may persist into and beyond preadolescence [7]. Prolonged gavage or gastrostomy tube feeding is common [6], and children with CHARGE syndrome require aggressive medical management and may need a fundoplication procedure due to chronic gastroesophageal reflux [3,8]. It has been reported that most of these children require involvement of a multidisciplinary feeding team to address medical, developmental, and behavioral feeding issues [3].
Feeding difficulties may be associated with cardiovascular malformations, with structural anomalies in the oral or nasal cavity, larynx, and/or pharynx, and with cranial nerve dysfunction. In general, the presence of congenital cardiovascular malformations may impede the successful introduction of oral feeding in infants [9,10], but it may be only one of many contributory factors in CHARGE syndrome. A common feature of CHARGE syndrome is unilateral or bilateral choanal atresia or stenosis [11,12], which may affect respiration during feeding and may coincide with reduced olfaction [13]. Feeding and swallowing may also be affected by the presence of a cleft palate, laryngeal malformations (laryngomalacia, laryngeal clefts, and short aryepiglottic folds), and/or esophageal deformities (esophageal atresia, tracheoesophageal fistula) [12,14].
Cranial nerve (CN) involvement in CHARGE syndrome is frequent; with CN V, VII, VIII, IX, and X most typically affected to varying degrees within the population [15]. CN II anomalies are also common [15], while CN I anomalies are probably underreported as an “occasional finding” [3]. Motor and sensory impairments are common sequelae of CN involvement with CHARGE syndrome and may explain many of the feeding difficulties experienced by these children. The cranial nerves that most significantly affect feeding in this population include CN V, VII, IX, and X [16]. All are involved in the pharyngeal phase of swallowing, while CN V, VII, and IX are involved in the oral phase of swallowing. CN X plays a predominant role in the esophageal phase of swallowing. Impairment of the trigeminal nerve (CN V) has been underreported [15] and may result in reduced sensory awareness in the mouth, palate, and throat and/or in impaired coordination of jaw, tongue, and palate movements involved in chewing and swallowing. This may contribute to nasal regurgitation, gagging, and/or choking. Impairment of the facial nerve (CN VII) may be associated with reduced or altered sensation of touch (lips) and taste and with impaired function of facial, pharyngeal, and laryngeal muscles [17], resulting in risk of gagging, choking, and/or aspiration. Furthermore, because the facial nerve controls the opening of the upper esophageal sphincter [17], CN VII involvement may include impaired esophageal motility. Disruption of glossopharyngeal nerve (CN IX) function may result in altered sensation and taste in the mouth and in poor coordination of velopharyngeal and laryngeal muscles during swallowing [17]. This may lead to nasal regurgitation and increased risk of aspiration. Problems resulting from vagus nerve (CN X) dysfunction may include altered sensory input to the pharynx, larynx, and base of tongue, as well as impaired motor control of the palate, pharynx, larynx, and esophagus [17,18]. Consequences include risk of nasal regurgitation, aspiration, and/or gastroesophageal reflux [12]. Cranial nerve I (olfactory) involvement may result in anosmia or reduced sense of smell [3,5] which may have a role in the development of appetite, mediated through hunger [19].
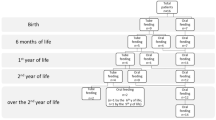
Swallowing is thought to depend on a “central patterned program that is modulated or reinforced by feedback from sensory input” [17]. Particularly with respect to the oral phase of swallowing, there appears to be a gradual transition from primitive/reflexive patterns to more complex voluntary patterns [16]. Infant reflexes, which are typically present at birth and facilitate early development of feeding and swallowing, maybe absent or impaired in CHARGE syndrome due to cranial nerve damage. Those most likely to be affected include the gag (CN IX, X), phasic bite (CN V), and rooting (CN V, VII) reflexes [17]. Oral sensorimotor development in children with CHARGE syndrome may be impeded as a consequence of sensory or motor impairments, medical intervention, and/or limited or negative feeding experiences. Sensory defensiveness may result from difficulty integrating oral sensory information from different channels [20]. This could result in limited tactile oral exploration, which is felt to increase tolerance for a variety of oral sensations and facilitate successful introduction and progression of oral feeding [18]. Many children with CHARGE syndrome demonstrate oral tactile sensitivity and it has been reported that more than 90% have difficulty swallowing textured foods [3]. Gastroesophageal reflux, frequently a long-term issue for children with CHARGE syndrome [3], may likely contribute to both oral and tactile hypersensitivity and persistent food refusal [21,22]. The early feeding difficulties experienced by many children with CHARGE syndrome often result in medical intervention such as frequent or prolonged orogastric or nasogastric tube feeding. These interventions may disrupt or prevent the development of oral feeding by exacerbating oral hypersensitivity and oral defensiveness, resulting in food aversion [23,24]. In addition, negative c feeding experiences associated with previous incidents of gagging, choking, coughing, and/or aspiration may lead to food selectivity or refusal [25,26]. Parents of children with significant feeding difficulties may give up attempts to feed their child or may delay introduction of soft or solid foods. Although controversial, there is thought to be a critical period for introduction of solid foods, when the child is developmentally ready to chew [27]. Infants who are introduced to lumpy solids after 10 months of age may be more likely to develop feeding difficulties [28].
While the numerous feeding challenges in the CHARGE population are recognized, the early oral sensory experiences and development of feeding skills have not been well documented. This article describes a detailed investigation of the oral sensory and feeding history of a small group of children with CHARGE syndrome and attempts to examine the relationship between these two issues.
Participants and Methods
Six children 3–10 years of age with CHARGE syndrome were identified through hospital records at the Izaak Walton Killam (IWK) Health Centre in Halifax, Nova Scotia, a tertiary care pediatric center for all of the Maritime Provinces of Canada. All children were diagnosed by a geneticist and had negative fluorescence in situ hybridization (FISH) test results for 22q11 deletion. A questionnaire designed to assess the oral sensory and feeding histories was developed and sent to their caregivers. Questions related to oral sensory experiences included sucking on a thumb/finger or pacifier, mouthing of objects, and acceptance of various forms of facial or oral stimulation. Information was also obtained regarding the history of oral and/or nonoral feedings (e.g., duration of bottle feeding and/or tube feeds, age of introduction of cup drinking and spoon feeding, and acceptance of a variety of food textures). This study was approved by the Ethics Review Board of the IWK Health Centre.
Five of six questionnaires were returned. Following receipt of the completed questionnaires, the medical records of the participating children were reviewed to provide additional medical information and to verify or elaborate on the information that had been provided. Caregivers were subsequently contacted if it was necessary to clarify any conflicting information. From this collective information, a detailed description of the oral sensory and feeding histories of these children was developed.
Results
Medical information provided by the parents was generally consistent with that provided in health records; however, the health records provided greater details of clinical and instrumental feeding assessments that were conducted at the tertiary care center. The questionnaires completed by caregivers provided details of feeding history/experiences that were home- or community-based and were not recorded in the tertiary hospital records. One parent was contacted to elaborate on information that was not clearly understood from the questionnaire. Collective documentation of each child’s history follows.
Child A
Child A, a 4-year-old female, was diagnosed with CHARGE syndrome at birth based on the presence of the following major and minor criteria: bilateral posterior choanal atresia, bilateral coloboma of the retina, cranial nerve anomalies (including right facial nerve palsy), characteristic ear anomalies (including bilateral hearing loss), and heart defects. Her medical records indicated documented evidence of aspiration, pneumonia, and gastroesophageal reflux. Since birth, she had been hospitalized approximately 20 times and had undergone 12 surgeries.
Child A was never successfully breast or bottle fed. Due to choanal atresia and clinical observation of poorly coordinated sucking and swallowing, orogastric tube feeds (5–6 bolus feeds per day) were introduced shortly after birth and then changed to nasogastric tube feeds, which continued for six months. As a consequence of persistent gastroesophageal reflux (noted in the medical records), a Nissen fundoplication was performed and a gastrostomy tube (G tube) was inserted at approximately 6 months of age. Continuous G-tube feeds were gradually transitioned to 3 bolus feeds per day, each divided into two sets delivered over approximately 3 minutes, with 1 hour between sets. The parent-reported difficulties during tube feedings included retching, gagging, irritability, and vomiting. These were addressed through formula adjustments, positioning, adjusting the length of time between bolus feeds, and surgical intervention.
At 1 year of age spoon feeding was introduced; however, difficulties with both pureed and mashed/lumpy foods were reported by the caregiver. These difficulties included coughing, gagging, choking, food refusal, and expulsion of food from her mouth. The only food that she consistently took from a spoon was ice cream. At 4 years of age she began to take small sips from a cup. Coughing, choking, and nasal regurgitation with liquids were reported. She had never tried chewable/solid food. A modified barium swallow study completed at 15 months of age identified laryngeal penetration, but there was no evidence of actual aspiration. A repeat study completed at 20 months revealed significant gastroesophageal reflux, but again there was no evidence of aspiration. At the time of questionnaire completion, Child A continued to be fed via G tube (each meal separated into 2 boluses to reduce reflux), with provision of oral stimulation and tastes of substances and textures.
According to the parent, this child did not appear to demonstrate significant aversion to oral or facial tactile stimulation. Suggestions regarding sensory input had been provided by a local feeding team and these were carried out by the caregivers, commencing at about 2 years of age. The child’s oral sensory history included regularly placing her fingers/thumb and sometimes putting objects in her mouth from an early age. She had regularly allowed others to touch her face and enjoyed having her teeth brushed.
Child B
Child B, a male who was 4 years 8 months at the time of this study, was born prematurely at 30 weeks with respiratory distress requiring intubation for the first 3 weeks of life. His initial medical diagnosis of CHARGE syndrome was based on bilateral coloboma, moderate sensorineural hearing impairment, cranial nerve anomalies including right facial palsy, a facial appearance characteristic of CHARGE syndrome, cardiovascular malformations, genital hypoplasia, and renal anomalies. His medical history also included stridor, aspiration, pneumonia, and gastroesophageal reflux. He had frequently been hospitalized and had undergone 19 surgeries.
For the first 2 months of life, Child B received intravenous parenteral nutrition, after which bottle feeds were attempted. Due to limited success with bottle feeding, this was supplemented with oral gavage tube feeding until a modified barium swallow at 3 months of age identified nasal regurgitation and gastroesophageal reflux, as well as tracheal aspiration. At that time, a nasogastric tube was inserted as Child B had become aversive to having tubes passed for intermittent oral gavage feeds. From 4 to 7 months of age he continued to receive small amounts of formula by bottle feeds, with supplementation through the tube, until a modified barium swallow suggested that oral feeding was not safe due to observed aspiration and significant reflux. Nasojejunal (NJ) tube feedings were initiated at 7 months and continued until approximately 8 months of age when a Nissen fundoplication was performed and G and J tubes were inserted. Continuous J-tube feeds were subsequently given until 16 months of age, when he was switched from 12-hour continuous J-tube to G-tube feedings. However, two weeks later he developed aspiration pneumonia and was then switched from a 12-hour to a 24-hour feeding schedule. During the next two years, Child B gradually transitioned from continuous feeds to primarily bolus feeds via G tube; however, periodically experienced gastroesophageal reflux with coughing, vomiting, gagging, and retching noted by the parent.
A videofluoroscopic swallow study performed at 3 years 8 months of age indicated functional swallowing abilities, with no evidence of aspiration. However, during clinical feeding assessment, he displayed severe oral-aversive behaviors, particularly related to food intake. While he would play with food, he would not put it in his mouth, lick, or taste it. When others would attempt to give him food, he would clamp his mouth shut or block the approach of a finger or spoon to his mouth. The psychologist on the feeding team prescribed a stringent behavioral program to facilitate his acceptance of food/liquid orally, which was subsequently implemented by his caregivers in conjunction with support from a local team of professionals. This resulted in gradual success with oral feeding. According to the caregiver report, he subsequently learned to drink from a cup and orally swallow purees and chewable/solid foods with minimal difficulties. By 4 years 10 months of age, Child B received all of his nutrition through oral feeds and the G-tube site was surgically closed. A persistent problem with oral retention of food was reported in that he would sometimes hold food in his mouth for as long as 30 minutes before swallowing it.
The oral sensory history of this child reflects early tactile sensitivity. Between the ages of 6 months and 3 years he refused to allow anyone to touch his face, particularly the oral area. Although he regularly used a pacifier, he would accept only the type that he had used in the neonatal nursery and would not tolerate any commercially available soothers. Attempts were consistently made to provide oral/facial stimulation during early tube feedings, such as sucking on a pacifier, stroking his lips, rubbing his cheeks, and using a vibrating toothbrush.
Child C
Child C, a female who was 9 years old at the time of this study, was diagnosed with CHARGE syndrome based on the presence of bilateral coloboma of the retina, characteristic external ear anomalies, profound bilateral sensorineural hearing loss, a tracheoesophageal fistula, distinctive facies, and minor cardiovascular malformations. She was hospitalized more than 15 times and had numerous surgeries.
This child experienced early feeding difficulties evidenced by coughing and increased congestion associated with nipple feeds, resulting in identification of the tracheoesophageal fistula. Oral (bottle) feeding was resumed when she was 1 week old, following surgical repair. Hospital records noted her to be a poor feeder immediately following the operation but she gradually began to nipple well and gain weight. However, her mother reported that “some days after feeding she would vomit everything back up.” There was no documentation of this child ever receiving medication for reflux (she was not followed through the tertiary care center during her first few years of life). Spoon feeding had been attempted prior to one year 6 months of age but was not successful until this time due to constant gagging. The transition to mashed/lumpy and chewable/solid foods also presented problems, evidenced by food refusal, holding food in the mouth to prevent swallowing, expelling food from her mouth, choking, and gagging. The mother reported that a videofluoroscopic swallowing study was conducted by a local feeding team at 2 years 6 months of age and revealed no abnormalities in the swallow. However, the child continued to drink from a bottle and ate only pureed foods until she was 3 years old. Cup drinking (with a spill-proof top) was introduced between the ages of 3 and 4. Responses to introduction of the cup included squirming, temper tantrums, throwing the cup, refusing to open her mouth, holding liquid in her mouth without swallowing, gagging, and expelling the liquid from her mouth. At the time of questionnaire completion, Child C was successfully eating all textures and trying more foods than she would in the past. She demonstrated no apparent texture preferences but showed a preference for sweet-tasting foods. She continued to experience some reflux, but was not receiving any medication. She was reported to be a slow eater who ate only small amounts at a time.
Parent report indicated evidence of early tactile defensiveness. While the child regularly used a pacifier between birth and 3 years of age, she seldom sucked her thumb/fingers and never mouthed objects during that time. She seldom allowed anyone to touch her face. While she regularly had her teeth brushed, she did not like to have the toothbrush touch her back molars and would close her mouth if the brush reached too far back. This child did not receive any therapeutic feeding services.
Child D
Child D, a 4-year-old female, received a diagnosis of CHARGE syndrome at 2 weeks of age. Her diagnosis was based on coloboma of the left eye, unilateral choanal atresia, profound bilateral sensorineural hearing loss, right facial palsy, cardiac anomalies, and developmental delay. Her medical history included aspiration, pneumonia, and gastroesophageal reflux. She had been hospitalized numerous times and had undergone 13 surgeries.
Bottle feeding was attempted with Child D during the first few days after birth; however, hospital records noted progressive difficulties due to clinical evidence of a poor suck and swallow. Therefore, tube feeds became the primary method of formula delivery, with periodic attempts at bottle feeding provided during the subsequent six months. A barium swallow study completed when she was approximately 1 month old revealed no evidence of aspiration and only mild reflux. Feeds were provided via an orogastric (OG) tube until she was 2 months of age, when a G tube was inserted. Bolus G-tube feeding continued until she was 6 months of age, but was changed to a J tube due to frequent regurgitation and subsequent identification of laryngeal pooling and aspiration during a modified barium swallow study. Attempts at oral (bottle) feeding were discontinued at this time. A repeat videofluoroscopic swallow study at 10 months revealed silent aspiration which prohibited the reintroduction of oral feedings. When cup drinking was introduced at 3 years of age, she experienced a wet/gurgly vocal quality, with coughing and oral and nasal regurgitation. Another videofluoroscopic swallow study conducted when she was 3 years 6 months of age revealed no evidence of frank aspiration but showed pooling of barium in the valleculae and pyriform sinuses. Continuous J-tube feedings were provided until she was 3 years 9 months old, at which time she underwent fundoplication surgery due to persistent reflux. At this time the G tube began to be used again and a transition to bolus feeds was attempted. An additional video- fluoroscopic swallow study conducted when Child D was 4 years of age found that the pharyngeal swallow had improved (i.e., no evidence of aspiration or pooling), and there was no evidence of nasal regurgitation. Attempts were then made to reintroduce oral feeds but were essentially unsuccessful. Episodes of irregular breath holding, coughing, choking, gagging, vomiting, crying, food/liquid refusal, and expulsion of food/liquid from her mouth were reported with liquids, purees, mashed/lumpy, and chewable/solid foods. Although she might readily open her mouth to accept food, she was reported to then refuse it or hold it in her mouth to prevent swallowing. Her caregiver indicated that she “doesn’t seem to taste” but demonstrated some preference for foods such as crackers, cookies, and breads. At the time of questionnaire completion, Child D would eat only small amounts orally and relied on 12-hour overnight tube feeding to meet her nutritional needs. Bolus feeds had not been tolerated well, with nausea, irritability, retching, gagging, reflux, and vomiting reported.
This child’s parents had consistently attempted to provide oral stimulation since birth, with limited success. Although she did use a pacifier, she seldom sucked her fingers/thumb, mouthed objects, or allowed others to touch her face. When attempts were made to touch her facial/oral area, she reacted by gagging, crying, and/or turning her head away. She seldom allowed anyone to brush her teeth, disliked toothpaste, and had daily temper tantrums around tooth brushing time.
Child E
Child E, a male who was 5 years of age at the time of this study, was diagnosed with CHARGE syndrome based on the presence of bilateral coloboma of the iris and retina, unilateral choanal stenosis, cranial nerve anomalies, bilateral sensorineural hearing loss, genital hypoplasia, renal anomalies, and cardiovascular malformations. Since birth he had been hospitalized approximately 6 times and had undergone several surgeries.
Breast feeding was attempted from birth to approximately 3 weeks of age. Hospital records reported discontinuation of oral feeds due to observed sucking difficulty and poor weight gain. Nasogastric tube feeding was started and continued until 5 months of age. Bottle feeding was initiated at 3 months of age, with gradual progress to weaning from the tube. He began to take pureed food orally at 5 months of age, with gagging the only difficulty reported. Bottle feeding was gradually replaced by cup drinking when he was 3 years of age. Some of the reported difficulties encountered with early cup drinking were coughing and refusing and throwing the cup. According to a caregiver report, the child would open his mouth readily to accept food and had never experienced significant difficulties swallowing liquids, purees, mashed/lumpy foods, or chewable/solid foods. He had not exhibited any taste preferences. He never underwent feeding or swallowing assessment or therapy, and there was no reported or documented evidence of nasal regurgitation or gastroesophageal reflux or aspiration.
The oral sensory history of this child suggests some degree of oral/facial hypersensitivity. Although he never used a pacifier or explored objects orally, he often placed his fingers, fist, or thumb in his mouth at an early age. He seldom allowed others to touch his face. He did not like others to brush his teeth and was resistant to having his back molars cleaned.
Summary/Comparison of Children
For comparison purposes, the feeding-related medical histories, oral sensory experiences, and feeding histories of these children are summarized in Tables 1–3. All of the children in this study had issues surrounding feeding. All experienced sucking difficulty in infancy, and all but one required early tube feeding. With the exception of Child E, parents reported episodes of gagging, coughing, and food refusal. All were late to begin drinking from a cup and most demonstrated aversive behaviors when cup drinking was introduced. Two of the five children continued to be dependent on tube feeding past the age of 4 years.
Child A and Child D appear to have the most chronic/severe difficulties with feeding. However, while these two children had similar medical and feeding histories, they appear to have had significantly different oral sensory histories. It is interesting to note that Child A seems to have had greater acceptance of oral/facial tactile stimulation but has been more reluctant to accept oral feeding. On the other hand, Child D has demonstrated significant oral hypersensitivity but would accept a variety of foods orally.
Child E had the relatively least difficulty with oral feeding, while Child C would also be considered less severely affected. However, both of these children had demonstrated some evidence of oral/facial sensitivity and aversion and limited oral exploration. Child C, who never required tube feeding, demonstrated difficulty transitioning from a bottle to a cup, accepting a spoon, and transitioning from pureed to textured foods. While Child E experienced nasogastric tube feeds during the first 5 months and went through a lengthy period before complete transition to cup drinking, transitions to other textures were reportedly not difficult.
Child B experienced severe difficulties with oral feeding from 0 to 4 years of age, in conjunction with oral tactile defensiveness and limited oral exploration. In spite of this, he was able to successfully eat by mouth following an intensive behavioral therapeutic approach to feeding.
Discussion
Children with CHARGE syndrome present with a myriad of feeding problems that are extremely variable within the population. Likewise, their early oral sensory experiences appear to differ widely and are seemingly unpredictable. All but one of the children in our study showed difficulty with initial food acceptance and transitions to new utensils and textures. This indicates a high prevalence of oral sensory issues in the population studied.
Feeding difficulties arise from a constellation of interrelated medical, environmental, nutritional, and social variables [24]. Unfortunately, only a general neurologic evaluation was conducted with these children; therefore, we were unable to obtain precise delineation of specific cranial nerve anomalies in this group. However, the children who presented with the most severe feeding problems (A, B, and D) have more apparent dysfunction of the relative cranial nerves (CN V, VII, IX, and/or X) than those with milder difficulties (C and E). This is evidenced by the presence of facial palsy in Children A, B, and D, implicating the presence of a CN VII anomaly. Their documented history of poorly coordinated sucking and swallowing, nasal regurgitation, aspiration, and severe gastroesophageal reflux requiring Nissen fundoplication indicates likely involvement of CN X as well as possible involvement of CN V and/or IX. Neither Child C nor Child E experienced facial palsy or history of nasal regurgitation or aspiration. Their early sucking behavior may have been inefficient but was never described as poorly coordinated. Child E had no reported evidence of gastroesophageal reflux, while Child C’s mother reported episodes of reflux following feeding with no documentation of any medical intervention.
Developmental delay or disability is frequently cited in the literature as being linked with a high prevalence of feeding disorders [10,22]. While many of the children in this study exhibited early psychomotor delay, it is not felt that the variability in their feeding profiles can be accounted for by the presence of cognitive deficits. Raqbi et al. [8] followed 21 children with CHARGE syndrome over a period of approximately 7 years and found that while psychomotor milestones were severely delayed for all children, intellectual outcome was satisfactory for half of the population studied. While developmental assessments were not part of the current investigation, those researchers familiar with this group of children would suggest that the cognitive/intellectual abilities of all of these children would fall at minimum into the normal range. Interestingly, one of the children with the most severe feeding difficulties has shown an excellent intellectual outcome, while another child demonstrating more severe psychomotor and speech and language delays had relatively less severe feeding difficulties.
The cardiovascular malformations diagnosed in this group are variable and do not appear to account for severity of feeding difficulties. Child E, with relatively milder feeding difficulties, had both major and minor cardiovascular malformations. His need for nasogastric feeding during the first 5 months of life may be explained by his cardiac problems. While cardiovascular conditions do not inherently cause oral-motor feeding problems, their impact is often related to increased effort involved in feeding resulting in lengthy/inefficient feeding, fatigue, and poor weight gain [17]. The cardiac anomalies do not appear to have had a major impact on this child’s long-term feeding success. His relative ease with transitions to textured foods is likely reflective of the fact that he did not experience gastroesophageal reflux. In a study of children with 22q11.2 deletion [29], a history of gastroesophageal reflux, esophagitis, and/or constipation was more predictive of feeding difficulties than cardiac anomalies. For Child C, the presence of a tracheoesophageal fistula caused serious feeding concerns requiring immediate surgical intervention. However, this child’s long-term feeding issues were likely more impacted by her history of gastroesophageal reflux.
The severity of feeding difficulties in these children does not appear to correlate well with their oral sensory experiences. For example, Child A, one of the most severely affected with respect to feeding problems, accepted a variety of forms of stimulation in the oral and facial area. Child D on the other hand, also one of the more severely affected, seldom accepted oral or facial tactile stimulation. The fact that this latter child accepted a pacifier is not surprising. Wolf and Glass [18] note that children with oral hypersensitivity often become fixated on one object which is seen as safe and is accepted in the mouth. Child B, who also used a pacifier on a regular basis, would accept only one specific type.
The variable sensory experiences of these children are challenging to elucidate but are likely reflective of the complex nature of sensorimotor development. In the typically developing child, oral sensorimotor skills advance in conjunction with neuromotor maturity, improved muscle control, and psychosocial discrimination [17]. For children with complex medical concerns such as those seen in CHARGE syndrome, it is apparent that an intricate relationship exists between underlying organic/medical issues, sensory–perceptual deficits, oral–motor dysfunction, behavior/learning, and parent child interaction. For many of these children, cranial nerve anomalies may have had a significant and direct impact on their sensorimotor development. However, additional factors need to be considered relative to their medical conditions and subsequent interventions, which are known to have an association with oral–sensory-based feeding issues [18]. All of these children had serious cardiac concerns. Rommel et al. [30], in a study of 700 children who presented to their tertiary care institution with feeding problems, found a strong association between isolated cardiac conditions and oral–sensory-based feeding problems. Wolf and Glass [18] suggest that infants who are chronically ill early in life may direct all their resources toward survival. As such, any increase in sensory stimulation may be extremely stressful and a pattern of hypersensitive or aversive responses may arise. In addition, children who have had negative and/or traumatic oral–facial experiences in association with medical treatment may learn a pattern of defensiveness to stimulation in and around the oral area which may persist well beyond the end of the medical interventions and become reflected in oral hypersensitive and aversive responses. This phenomenon would certainly be applicable to the group of children in this study, all of whom experienced numerous surgeries requiring intubation. All required a least a short period of oral gavage or nasogastric feedings, the latter of which may have required repeated reinsertions and aversive facial stimulation associated with taping the tube to the cheek. This may explain why Child E, who reportedly became aversive to NG-tube changes, seldom accepted touch to his face.
From an experiential point of view, early oral exploration is considered a vital part of normal development and prepares children for the sensations they will encounter when introduced to solid foods [20]. While the extent of oral stimulation provided to the children in this study was not known, it appears that a number of them experienced limited early oral exploration in conjunction with oral hypersensitivity and oral/facial tactile defensiveness. It also appears that a number of these children may have missed the critical or sensitive period for the acquisition of oral feeding skills [27]. Four of these children had minimal experience with bottle feeding in infancy, while those that were bottle fed continued to do so well beyond the typical age of introduction of cup drinking. Child E, who is the only child who was spoon fed at an appropriate age, was also the only child who did not have difficulty transitioning to other textured foods.
Field et al. [22] have predicted that children with the most numerous and/or severe medical conditions are likely to have more severe feeding problems due to negative feeding experiences. For several of the children in this study, their early feeding experiences were compromised by oral-motor and/or sensory deficits and were associated with coughing, choking, nasal regurgitation, aspiration, and/or gastroesophageal reflux. These experiences may have resulted in fear and aversion associated not only with eating but with any oral stimulation. In an earlier study, Blake et al. [31] noted that the children with the most severe feeding problems had bilateral choanal atresia. Only one child in this study was born with bilateral choanal atresia (Child A), who also experienced relatively more severe feeding difficulties. While the long-term impact of this condition on her feeding development is not known, it may be speculated that her inability to breathe adequately through her nose during early attempts to feed would have been extremely stressful. This child’s mother noted “I’ve often wondered about the effect of this [choanal atresia] on her swallow, etc.” This child’s negative experiences were likely heightened by recurrent gastroesophageal reflux, as was the case for many of the other children. Pain from esophagitis is a common sequela of gastroesophageal reflux due to recurrent contact between acid gastric contents and the esophageal mucosa [18]. Zangen et al. [32] have speculated that a history of gastritis and esophagitis, combined with past medical treatment, may contribute to the creation of pathological pain pathways in infants with central nervous system (CNS) disorders. This might explain the heightened food aversion or refusal in the subgroup of children in this study who experienced gastroesophageal reflux. The three most severely affected children in this study underwent Nissen fundoplication. While this surgical procedure may reduce or prevent vomiting, it does not alter the presence of abdominal pressure against the lower esophageal sphincter; hence, these children may have continued to gag, choke, and retch following surgery [33].
Children with medically based feeding disorders are at high risk of developing behavioral feeding problems attributable to motivational or skill deficits [24]. Maladaptive behaviors are relatively common among children with severe feeding disorders [24], and the CHARGE population is not likely immune to this phenomenon. Behaviors described by the parents of the children in this study included food refusal, gagging, breath holding, holding food in the mouth without swallowing it, and expulsion of food from the mouth. This avoidance of food, while initially adaptive, often persisted even after the precipitating factor(s) had resolved and it was considered safe for the children to eat orally. Parent–child interaction may play a role in the maintenance of these behaviors. The biopsychosocial model of feeding development considers physiological, behavioral, and social factors all contributing to the development of severe feeding problems and “an ongoing process of mutual feedback and adaptation” of the parent and child [34]. The child’s physiological status and nutritional status, temperament, or history of traumatic or negative oral/feeding experiences may indirectly affect the caregiver–child relationship by influencing both the caregiver’s responses to the child and his/her anxiety about the child’s feeding development [35]. From the time the child is born, parent–child interaction is initially stressed by the parent’s inability to provide the basic caregiving involved in feeding [34]. Parental responses to a child’s avoidance of foods or oral/facial tactile stimulation may intensify the child’s maladaptive behaviors, resulting in further reactions of the child that lead to greater avoidance behaviors [24,35].
Motivation to eat was likely an issue for the three children who experienced long-term G-tube feedings. Given the limited amount of oral feeding and the length of time these children were on tube feedings, it is unlikely that any link between sensations in the mouth and sensations in the stomach were ever established. While ensuring adequate nutrition and hydration, the tube feedings would likely decrease any hunger-driven desire to eat orally [36]. This would be particularly pertinent when continuous (versus bolus) feeds were provided with no opportunity to develop a hunger/satiation cycle [18]. It is unknown whether any of the children experienced anosmia or altered sense of smell, but its presence might have further diminished any appetite-related desire to eat from an early age [37].
Conclusion
From the histories of these children it can be seen that feeding disorders associated with CHARGE syndrome initially have an organic or underlying medical or neurologic basis and may be maintained over time by remaining sensory and oral-motor skill deficits as well as the acquisition of maladaptive behavioral patterns. The majority of children with CHARGE syndrome will eventually become oral eaters [6], although it should be understood that this is often a long and gradual process. All of the five children in this study were accepting some foods orally at the time of investigation, while only three were completely orally fed. For children who have been tube fed for a lengthy period of time, there is a continuum from nonoral to oral feeding [38]. Early management from the time of identification of feeding difficulties is important, with prevention or minimization of oral defensiveness as a primary goal [6,23,37]. All of the parents of children who were fed via G tube in this study reported having provided their children with some sort of oral sensory stimulation throughout their child’s development.
A child will be ready to take greater variety of foods in larger amounts only when a number of conditions are met. These include resolution of the precipitating problem(s) (e.g., tracheoesophageal fistula, cardiac condition, or presence of aspiration), with an extended period of good health status; swallowing safety; adequate oral-motor skills; ability to comfortably take sufficient bolus feeds and develop a sense of hunger; and demonstration of interest/readiness on the part of the child and parent [38]. These conditions should be assessed by a multidisciplinary feeding team involving the parent and with the use of videofluoroscopic swallow studies to document swallowing safety when this is an area of concern. Helping children with the transition from gastrostomy tube feeds to oral feeding is a veritable challenge and requires a coordinated multidisciplinary approach [33]. This is demonstrated in the case of Child B, where the use of consistent behavioral intervention [35] facilitated successful oral feeding following evidence of the appropriate readiness factors. Unfortunately, for the two other tube-fed children, their reported histories indicated that some outstanding readiness issues remained. The mother of Child A reported that her child was interested in the social aspects of eating but did “not feel safe enough.” This child continued to experience nasal regurgitation with oral intake. Child D, who showed significant interest and oral motor ability in eating, continued to require continuous tube feeds because of severe gastroesophageal reflux. This impeded the introduction of bolus feeds and prohibited significant reduction in tube feeding. On a positive note, caregivers of both of these children indicated that it was likely their child would eventually eat orally.
Limitations and Future Directions
This study represents a preliminary investigation into the relationship between oral sensory experiences and feeding issues in a small number of children with CHARGE syndrome. Limitations of the study involve the nature of retrospective data collection and reliance on parent recall. In the medical histories of these children, we were unable to obtain precise delineation of specific cranial nerve anomalies or complete information regarding early investigation and medical management of gastroesophageal reflux. Further information on type, intensity, and duration of feeding therapy would also be helpful to determine what interventions have been most successful with this population. These issues might best be investigated through a more detailed prospective study involving a greater number of participants, while exploration of early intervention techniques (medical and/or behavioral) may be successful in reducing or eliminating sensory and tactile aversion and facilitate the prevention and management of feeding difficulties in this population.
Given the high prevalence of reflux in children with CHARGE syndrome, we recommend referral to a pediatric gastroenterology specialist in the neonatal period as soon as the diagnosis of CHARGE is made. Followup should involve a multidisciplinary feeding team and parents should be made aware of the potential for long-term feeding issues.
References
B Hall (1979) ArticleTitleChoanal atresia and associated multiple anomalies J Pediatr 95 IssueID3 395–398 Occurrence Handle469662
RA Pagan JM Graham SuffixJr J Zonana SL Yong (1981) ArticleTitleColoboma, congenital heart disease, choanal atresia with multiple anomalies: CHARGE association J Pediatr 99 IssueID2 223–227 Occurrence Handle6166737
KD Blake SL Davenport BD Hall MA Hefner RA Pagon MS Williams AE Lin JM Graham (1998) ArticleTitleCHARGE association: An update and review for the primary pediatrician Clin Pediatr 37 159–174
Issekutz KA, Graham JM, Prasad C, Smith IM, Blake KD: An epidemiological analysis of CHARGE syndrome: preliminary results from a Canadian study. Am J Med Genet (accepted August 2004)
Lawland C: CHARGE association/syndrome: looking ahead (Web document). Published by the Canadian Pediatric Surveillance Program (CPSP) 2003 Resources, (cited 11 January 2004). < http://www.cps.ca/english/CPSP/Resources/CHARGE.htm>
AS Harvey PM Leaper A Bankier (1991) ArticleTitleCHARGE association: clinical manifestations and developmental outcome Am J Med Genet 39 IssueID1 48–55 Occurrence Handle10.1002/ajmg.1320390112 Occurrence Handle1867265
MS Abi Daoud J Gradstein KD Blake (2002) ArticleTitleCHARGE into the adolescent and adult decade Pediatr Child Health 7 IssueIDSuppl A 27A
F Raqbi C Le Bihan MP Morisseau–Durand P Dureau S Lyonnet V Abadie (2003) ArticleTitleEarly prognostic factors for intellectual outcome in CHARGE syndrome Dev Med Child Neurol 45 483–488 Occurrence Handle10.1017/S0012162203000896 Occurrence Handle12828403
S Bazyk (1990) ArticleTitleFactors associated with the transition to oral feeding in infants fed by nasogastric tubes Am J Occup Ther 44 IssueID12 1070–1078 Occurrence Handle2126165
KA Burklow AN Phelps JR Schultz K MacConnell C Rudolph (1998) ArticleTitleClassifying complex pediatric feeding disorders J Pediatr Gastroenterol Nutr 27 IssueID2 143–147 Occurrence Handle10.1097/00005176-199808000-00003 Occurrence Handle9702643
AL Tellier V Cormier–Daire V Abadie J Amiel D Bonnet et al. (1998) ArticleTitle CHARGE syndrome: report of 47 cases and review Am J Med Genet 76 402–409 Occurrence Handle10.1002/(SICI)1096-8628(19980413)76:5<402::AID-AJMG7>3.0.CO;2-O Occurrence Handle9556299
G Roger M-P Morisseau–Durand T Abbeele ParticleVan Den R Nicollas J-M Triglia P Narcy V Abadie Y Manac’h E-N Garabedian (1999) ArticleTitleThe CHARGE association: the role of tracheotomy Otolaryngol Head Neck Surg 125 IssueID1 33–38
AE Lin JR Siebert JM Graham SuffixJr (1990) ArticleTitleCentral nervous system malformations in the CHARGE association Am J Med Genet 37 304–310 Occurrence Handle10.1002/ajmg.1320370303 Occurrence Handle2260555
KD Blake IM Russell–Eggitt DW Morgan JM Ratcliffe RK Wyse (1990) ArticleTitleWho’s in CHARGE? Multidisciplinary management of patients with CHARGE association Arch Dis Child 65 217–223 Occurrence Handle2317068
CMD Lawland KD Blake C Prasad JM Graham SuffixJr (2003) ArticleTitleThe cranial nerve anomalies of CHARGE association/syndrome (A/S) Pediatr Child Health 8 IssueIDSuppl B 26B, 2003
RN Ichord (1994) Neurology of swallowing disorders. DN Tuchman RS Walter (Eds) Disorders of feeding and swallowing in infants and children Singular San Diego 37–52
JC Arvedson L Brodsky (2002) Pediatric swallowing and feeding: Assessment and management EditionNumber2 Singular Publishing New York
LS Wolf RP Glass (1992) Feeding and swallowing disorders in infancy: Assessment and management Therapy Skill Builders San Antonio, TX
A Staiano (2003) ArticleTitleFood refusal in toddlers with chronic diseases J Pediatr Gastroenterol Nutr 37 225–227 Occurrence Handle12960640
SE Morris MD Klein (2001) Pre-feeding skills: a comprehensive resource for feeding development Therapy Skill Builders San Antonio, TX
S Strudwick (2003) ArticleTitleGastro-oesophageal reflux and feeding: the speech and language therapist’s perspective Int J Pediatr Otorhinolaryngol 67S1 S101–S102 Occurrence Handle10.1016/j.ijporl.2003.08.039
D Field M Garland K Williams (2003) ArticleTitleCorrelates of specific childhood feeding problems J Pediatr Child Health 39 299–304 Occurrence Handle10.1046/j.1440-1754.2003.00151.x
LA Newman (2000) ArticleTitleOptimal care patterns in pediatric dysphagia Semin Speech Lang 21 IssueID4 281–291 Occurrence Handle10.1055/s-2000-8382 Occurrence Handle11085253
RL Babbitt TA Hoch DA Coe (1994) Behavioral feeding disorders. DN Tuchman RS Walter (Eds) Disorders of feeding and swallowing in infants and children Singular San Diego 77–95
R Mannikam JA Perman (2000) ArticleTitlePediatric feeding disorders J Clin Gastroenterol 30 IssueID1 34–46 Occurrence Handle10.1097/00004836-200001000-00007 Occurrence Handle10636208
CD Rudolph DT Link (2002) ArticleTitleFeeding disorders in infants and children Pediatr Clin North Am 49 IssueID1 97–112 Occurrence Handle10.1016/S0031-3955(03)00110-X Occurrence Handle11826810
RS Illingworth MB Lister (1964) ArticleTitleThe critical or sensitive period, with special references to certain feeding problems in infants and children J Pediatr 65 839–848 Occurrence Handle14244090
K Northstone P Emmett F Nethersole InstitutionalAuthorNamethe ALSPAC Study Team: (2001) ArticleTitleThe effect of age of introduction to lumpy solids on foods eaten and reported feeding difficulties at 6 and 15 months J Hum Nutr Dietet 14 43–54 Occurrence Handle10.1046/j.1365-277X.2001.00264.x
PS Eicher DM McDonald–McGinn CA Fox DA Driscoll BS Emanuel EH Zackai (2000) ArticleTitleDysphagia in children with 22q11.2 deletion: unusual pattern found on modified barium swallow J Pediatr 137 IssueID2 158–165 Occurrence Handle10.1067/mpd.2000.105356 Occurrence Handle10931405
N Rommel A-M DeMeyer L Feenstra G Veerman–Wauters (2003) ArticleTitleThe complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution J Pediatr Gastroenterol Nutr 37 75–84 Occurrence Handle10.1097/00005176-200307000-00014 Occurrence Handle12827010
KD Blake D Brown (1993) ArticleTitleCHARGE association looking at the future—the voice of a family support group Child Care Health Dev 19 395–409 Occurrence Handle9098398
TZ Zangen C Ciarla S Zangen C Di Lorenzo AF Flores et al. (2003) ArticleTitle Gastrointestinal motility and sensory abnormalities may contribute to food refusal in medically fragile toddlers J Pediatr Gastroenterol Nutr 37 287–293 Occurrence Handle12960651
MM Palmer (1998) ArticleTitleWeaning from gastrostomy tube feeding: commentary on oral aversion Pediatr Nurs 25 IssueID5 475–478
PH Casey (1983) ArticleTitleFailure to thrive: a reconceptualization Dev Behav Pediatr 4 IssueID1 63–66
ME Kerwin (1999) ArticleTitleEmpirically supported treatments in pediatric psychology: severe feeding problems J Pediatr Psychol 24 IssueID3 293–314 Occurrence Handle10.1093/jpepsy/24.3.193
KG Byars KA Burklow K Ferguson T O’Flaherty K Santoro et al. (2003) ArticleTitleA multicomponent behavioral program for oral aversion in children dependent on gastrostomy feedings J Pediatr Gastroenterol Nutr 37 473–480 Occurrence Handle14508219
Abadie V: Swallowing and Feeding in CHARGE syndrome, Paper presented at the 4th International CHARGE Syndrome Conference. Houston, TX, 1999
Morris SE: Children with feeding tubes. Part 3: Making the transition to oral feeding. (Web document). Published by New Visions, 2000 (accessed 10 June, 2004). < http://www. new-vis.com/fym/papers/p-feed 15.htm>
Acknowledgments
The authors would like to thank the caregivers of the children in our study for their time and effort in participating in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was conducted at Dalhousie University in collaboration with the Izaak Walton Killam (IWK) Health Centre.
Rights and permissions
About this article
Cite this article
Dobbelsteyn, C., Marche, D.M., Blake, K. et al. Early Oral Sensory Experiences and Feeding Development in Children with CHARGE Syndrome: A Report of Five Cases. Dysphagia 20, 89–100 (2005). https://doi.org/10.1007/s00455-004-0026-1
Issue Date:
DOI: https://doi.org/10.1007/s00455-004-0026-1