Abstract
The present study investigated the screening of mono and co-culture fungal cultivations for laccase production using extracted lignin as the substrate obtained from cauliflower wastes by two different pretreatment methods. Amongst mono and mixed culture fungal cultivations, monoculture of Aspergillus oryzae exhibited the highest enzymatic activity of 29.7 ± 0.6 U mL−1 under submerged conditions and using alkali extracted lignin as substrate. Under the optimal conditions (pH 4.5, 30 °C, 12 days, 1% (w/v) lignin and 0.5 mM Cu2+ concentration) the maximum laccase activity was estimated to be 41.3 ± 2.8 U mL−1 and production yield of 153.3 ± 2.4 mg L−1. Maximum decolorization of pigment extracted from Aspergillus heteromorphus CBS 117.55 cultivated culture media was achieved by administration of 40 U g−1 of crude enzyme concentration. Thermal and pH stability of crude laccase was observed over wide ranges. The dye decolorization efficiency of crude A. oryzae laccase was studied and Congo Red exhibited maximum decolorization percentage (64 ± 1.3%) at 15 µM, 50 °C and pH 4.5. The kinetic study of different dye (Congo Red) concentrations obtained Vmax and Km values of 0.123 × 10−3 M and 0.724 mol L−1 min−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Depolymerisation of lignin into low molecular weight base chemicals is an important starting point for subsequent valorization of lignocellulosic material as well as lignin. Hydrolysis reactions, catalytic reduction, catalytic oxidation and enzymatic oxidations are the different strategies for depolymerisation of lignin to produce valuable aromatic fine chemicals [1,2,3]. Four unique oxidative extracellular enzymatic systems have been reported to degrade lignin from lignocellulosic biomass which include; lignin peroxidase (LiP, EC 1.11.1.14), manganese peroxidase (MnP, EC 1.11.1.13), versatile peroxidase (VP, EC 1.11.1.16) and laccase (Lcc, EC 1.10.3.2) for opening of phenyl rings [4].
Laccases (benzenediol: oxygen oxidoreductase) are polyphenol oxidase known to catalyse the oxidation of wide range of phenolic compounds and aromatic amines. Laccases contain copper atoms in the catalytic centre and are usually called multicopper oxidases [5]. Due to their potential role in lignin and phenolic compound degradation, laccases are currently widely utilized in biotechnological processes such as dye degradation, bioremediation, biosensor development, effluent detoxification, pulp bleaching, bioplastics and nanocomposites [6]. In addition, laccases have several food based applications such as fruit juice, wine, beer stabilization, food pulp delignification, bakery industry, improvement in food quality and food sensory parameters and as gelling agent [7]. Laccases are widely distributed in nature and fungal laccases are the most frequently studied form of laccase. Laccase producing fungi include ascomycetes, basidiomycetes and deuteromycetes [8].
High production cost of pure lignocellulose degrading enzymes is the major obstacle in the widespread commercialization of enzymatic lignocellulosic biomass hydrolysis and also contributes to our environmental pollution problem. One potential approach to decrease the production cost as well as to offer safe and better environment could be through the utilization of cheap, abundant and renewable lignocellulosic biomass, especially agricultural waste residues as nutrient source for the ligninolytic enzyme producing microbes [9]. Production of enzymes through monoculture cultivation of fungi has been explored widely for its beneficial effects. However, certain limitations such as lower productive yield of enzymes; contamination and not fully expressing of genetic elements has overall negative implications on enzyme yield [10]. Furthermore, lignocellulosic biomass is heterogenous in nature, therefore, mixed fungal cultivations may be utilised as an alternative approach for the complete degradation of waste biomass. Co-culture cultivation is advantageous over monoculture cultivation because it exhibits many advantageous traits such as expression of signalling pathway which might be dormant or silent in one species; synergistic actions of microorganisms for the expression of metabolic pathways; enhanced substrate utilization; production of desired metabolites etc. [11]. Therefore, co-culture or mixed cultivation of fungi as an effective culture method for production of enzymes have been reported by number of authors but still considerable research need to be done to establish its comprehensive mechanism on increased production of enzyme mixtures and to demonstrate its immense potentials. Lignocellulosic biomasses which predominate in the natural biotopes of fungi are utilized as carbon sources and consortium of fungi releases lignocellulosic degrading enzymes in sufficient quantities. Filamentous fungi produce appreciable levels of polysaccharide degrading enzymes [12]. Aspergillus genus namely, Aspergillus niger and Aspergillus oryzae, are the two most important fungi frequently used in the release of enzymes for degradation of recalcitrant biomass [13]. These fungi are frequently used in the production of lignocellulolytic enzymes for its GRAS status, good tolerance to growth maintaining factors [14].
India is the second largest producer of cauliflower in world [15]. Cauliflower has the highest waste index [16] among the other vegetables belonging to Brassicaceae family. Non-edible portion of cauliflower wastes, which contributes to about 45–60% of the total weight of the vegetable, has not been put to any commercial use and thus creates a lot of problem by generating large amount of organic solid wastes. Cauliflower wastes consist of good protein (16.1%), cellulose (16%) and hemicelluloses (8%) [17]. Thus, besides the low cost and higher biomass availability, cauliflower waste could serve as a very promising substrate for microbial production of industrially important enzymes. The use of this inexpensive agro-waste in the production of laccase will bring down their production cost and at the same time reduce environmental pollution due to these wastes generation.
Structural linkages (Cα–Cβ, biphenyl, β-aryl ether bond) of lignin imposes bottleneck in its proper utilization for fine chemicals and it plays a fundamental role in depolymerization of lignin by microorganisms [18]. White rot fungi such as Trametes versicolor, Phanerochaete chrysosporium, Pichia pastoris are especially employed for lignin biodegradation through catabolic process. Catalytic oxidation of lignin is mainly carried by laccase enzyme which possesses the ability to degrade C–C and C–O bonds of lignin [19]. Therefore, aromatic and phenolic inducers such as p-anisidine, 2,5-guaiacol, gallic and ferulic acid, veratryl alcohol enriched media with supplementation of Cu2+ ions are utilized for laccase production. The overpricing of commercial substrates has paved the way for utilization of waste feedstock as a cost effective measure for laccase production. Although variety of lignocellulosic feedstocks were tried for production of laccase [20,21,22], to our knowledge there is no published literature available on the production of laccase using extracted lignins obtained from pretreated cauliflower wastes. Therefore, the current work was designed to investigate the capability of different fungal strains belonging to ascomycetes group in producing laccase using the lignin fractions obtained from pretreated cauliflower wastes (Brassica oleracea var. Botrytis) (CW). Optimization of fermentation conditions for laccase production by the selected fungal species using lignin fractions was also investigated. Characterization of the fungal laccase was also demonstrated for the establishment of its commercial viability. The crude extracellular fungal laccases were applied for fungal pigment removal and synthetic dye decolorization, the prominent source of water pollution caused by dye containing effluent discharge from industries.
Materials and methods
Lignocellulosic biomass collection
CW (leaf and stalk) were collected from the local market of Shibpur, West Bengal, India. Cauliflower stalk and leaf were washed thoroughly in tap water and air dried in a hot air oven (RDHO 50, REMI, India) at a velocity of 2 m s−1 for 24 h. Waste materials were cut, milled and sieved to particle sizes of 0.25 mm for stalk and 0.125 mm for leaf, respectively. The waste substrates, thus, prepared were stored in air tight containers at − 20 °C until needed. Compositional analysis of cauliflower wastes was carried out by different procedures as described in our previous paper [23]. Lignocellulosic composition (cellulose, hemicelluloses and lignin) of CW was determined. Protein content, moisture and ash of CW were determined.
Extraction of lignin fractions from CW
Pretreatment of CW for recovery of maximum amount of lignin was conducted by two different strategies.
Alkali pretreatment by sodium hydroxide (NaOH)
The extraction of lignin with alkali was carried out according to Saito et al. [22] with a little modification. 1 g of cauliflower leaf and stalk was mixed separately with 1 M NaOH solution in 1:10 ratio in 250 mL Erlenmeyer flasks. The solutions were autoclaved at 120 °C for 30 min. The mixtures were filtered through Whatman No.1 filter paper to separate solid and filtrate or black liquor. The separated solids were oven dried at 55 ± 5 °C for 16 h. To the liquid fraction, 12 M acetic acid was added to adjust the pH to 5.0 for precipitation of xylan fraction. The remaining liquid portion was adjusted to pH 2.0 for precipitation of lignin. The precipitated lignins thus obtained were designated as alkaline extracted lignin [Ak-L (leaf) and Ak-L (stalk)]. Later these filtered and washed Ak-L was freeze-dried (Eyela FDU-1110, Japan) and kept in − 30 °C for further analysis.
Hydrothermal pretreatment (HTP) with Na acetate as catalyst
The extraction of lignin by HTP with catalyst was carried out according to Wu et al. [24] with a slight modification. 1% (w/v) cauliflower leaf and stalk was mixed separately with deionised water in 1:10 ratio and 0.1 M Na acetate as catalyst. The mixtures were autoclaved at 120 °C for 30 min. The mixtures were filtered through Whatman No.1 filter paper to separate solid and filtrate or black liquor. The separated solids were oven dried for 16 h at 55 ± 5 °C. To the liquid fraction, 12 M acetic acid was added to adjust the pH to 5.0 for precipitation of xylan fraction. The remaining liquid portion was adjusted to pH 2.0 for precipitation of lignin. The lignin thus obtained was termed as catalyst assisted hydrothermally extracted lignin [HT-L (leaf) and HT-L (stalk)]. Later these filtered and washed HT-L was lyophilized (Eyela FDU-1110, Japan) and kept in − 30 °C for further analysis.
The lignin recovered from both the extraction methods was calculated according to the following formula
Estimation of acid soluble and insoluble lignins
Acid insoluble lignin (AIL) content of Ak-L and HT-L was determined according to TAPPI method. Acid soluble lignin (ASL) was estimated spectrophotometrically (JASCO V 630 UV–Vis Spectrophotometer, USA) at 208 nm by NREL method [25].
A lignin sample extracted from the CWs without alkaline and catalyst assisted HTP pretreatments was also prepared for comparison and designated as control lignin [C-L (leaf) and C-L (stalk)].
Microorganisms
Three lyophilized filamentous fungal strains Aspergillus oryzae (MTCC 3782), Aspergillus niger (MTCC 9687) and Candida intermedia (MTCC 1404) were purchased from IMTech, Chandigarh, India. A small amount of pellet of each fungal strain was dissolved in 30 mL of sterilized growth medium specified for each fungus (Czapek yeast extract agar for Aspergillus oryzae (1 × 106), Malt extract agar for Aspergillus niger (1 × 106) and Malt yeast extract agar for Candida intermedia (1 × 106), respectively). All the flasks were incubated at 30 °C for 48 h in stationary condition.
Stock cultures of pure fungal strains were maintained on the agar slants prepared with the above mentioned specified media and all the slants were incubated at 30 °C to obtain sufficient growth in stationary condition. Well grown fungal strains were preserved at 4 °C. Each strain was subcultured in specific growth media every fortnight to keep them viable. The fungal cultures were maintained as a suspension of vegetative cells and hyphal fragments in 15% (v/v) sterile glycerol at − 30 °C for long-term preservation in stationary condition.
All the media components (Malt extract, yeast extract, peptone, agar) were purchased from HiMedia Laboratories Pvt. Ltd., India. All the chemicals (NaOH, ZnSO4, CuSO4, MgSO4, MnSO4, HCl, H2SO4 and guaiacol) used were of analytical grade and procured from Merck, India. Synthetic dyes (Congo Red, Coomassie Brilliant Blue, Indigo carmine and Rhodamine B) were procured from Sigma–Aldrich Pvt. Ltd, India.
Inoculum preparation
The inoculum was prepared by growing the fungal strains under submerged fermentation in 150 mL Erlenmeyer flasks in stationary condition containing 50 mL of sterile Czapek Yeast extract for Aspergillus oryzae (1 × 106), Malt extract and Malt Yeast extract media for Aspergillus niger (1 × 106) and Candida intermedia (1 × 106), respectively. Each flask was inoculated with 7 mm agar plugs cut from the periphery of actively growing colonies of 5-day-old culture of fungal strains grown on respective agar plates followed by incubation (RHI 80, REMI, India) at 29 °C for 72 h under stationary condition until sufficient vegetative cells growth was observed. Fungi were co-cultivated by inoculating 2 µL of vegetative cells suspension on PDA plate with a distance of 3 cm by mixing the vegetative cells and inoculating it in the centre. The plates were incubated at 29 °C for 7 days under stationary condition.
Laccase production from lignin fractions by submerged fermentation
Fermentation for laccase production was carried out using lignin fractions obtained from pretreated CW under submerged condition using both monoculture and mixed culture of the selected fungal strains.
In first set of experiment, fermentation was conducted separately in 250 mL of Erlenmeyer flasks containing 1 g (dry weight, w/v) of Ak-L and HT-L extracted from cauliflower leaf and stalk as substrate and 150 mL of culture medium to produce laccase under submerged fermentation in stationary condition. The composition of culture medium consisted of peptone 3.0 g L−1, KH2PO4 0.6 g L−1, ZnSO4 0.001 g L−1, CuSO4 0.001 g L−1, MgSO4⋅7H2O 0.5 g L−1, MnSO4 0.05 g L−1 and CaCl2 0.1 g L−1, pH 5.5. The flasks were autoclaved at 121 °C for 15 min, cooled and then inoculated separately with vegetative cells suspension containing actively growing mycelia of A. oryzae, A. niger and C. intermedia. In the second set of experiment, fermentation was carried out using vegetative cells suspension of co-cultures of the above mentioned fungi (adjusted according to specific growth rates) under the same conditions as described above. All the flasks were incubated (RHI 80, REMI, India) at 30 °C under stationary condition and enzymatic extraction was carried out after 12 days of fermentation. A control media was prepared by growing the different fungal strains in basal media supplemented without any traces of lignin under the previously described culture conditions. All the submerged fermentations were carried out in triplicate.
Extraction of enzyme
After 12 days of incubation period, the enzyme secreted during submerged fermentation using mono and co-culture of selected fungi was recovered by extraction with sodium acetate buffer (0.1 M, pH 5) under shaking at 150 rpm for 1 h at room temperature. The extract was separated by centrifugation (R-12C, REMI, India) at 3000g for 20 min at 4 °C in refrigerated centrifuge (RA-2314, REMI Lab World, India). Supernatant was collected, freeze-dried (Eyela FDU-1110, Japan) and stored at − 30 °C for subsequent use as crude enzyme sample.
Optimization of culture conditions for laccase production
To study the optimal incubation period for the maximum laccase production, the production medium was prepared and inoculated by the selected fungus as mentioned before followed by incubation at 29 °C for 16 days in stationary condition. The culture was harvested periodically at every 48 h of interval and the culture filtrate was concentrated and used to determine laccase activity.
To evaluate the effect of various parameters (temperature, pH, Cu2+ and lignin concentrations) of culture condition on laccase production by the tested fungus, the fermentation medium was prepared, inoculated and incubated by the tested fungus as previously described. The effect of temperature, pH, Cu2+concentrations and lignin concentrations optima on the laccase production was investigated by incubating the flasks at different temperatures (20°, 25°, 30°, 35 °C), different pH (2.0–4.0 acetate buffer, 6.0–7.0 phosphate buffer) and CuSO4 concentrations (0.5, 1.0,1.5 and 2.0 mM). Different amounts of lignin fractions ranged from 0.5 to 2.5% w/v were also added to study the effect of lignin concentrations upon laccase production.
Extracellular laccase assay
Laccase activity was determined by the oxidation of guaiacol according to Kalra et al. [26]. The reaction mixture contained 0.2 mL of crude enzyme extract with protein content of 1.12 mg mL−1, 1 mL of sodium acetate buffer (10 mM, pH 4.2) and 1 mL of guaiacol (2 mM) (ɛ = molar coefficient for guaiacol 6740 M−1 cm−1) solution. Development of reddish brown colored due to the oxidation of guaiacol by laccase resulted an increase in absorbance at 470 nm. A470nm change was related to the rate of oxidation of 2 mM guaiacol in 10 mM sodium acetate buffer (pH 4.2). A control and blank was prepared with the aforementioned reagents where culture filtrate was replaced by sodium acetate buffer and distilled water respectively. The mixture was incubated at 30 °C for 15 min and absorbance was measured spectrophotometrically (JASCO V 630 UV Vis Spectrophotometer, USA). One international unit of enzyme activity is defined as the amount of enzyme which leads to the oxidation of 1 µmol of guaiacol equivalent/min under the assay conditions.
where A = absorbance, V = total mixture volume (mL), v = enzyme volume (mL), t = incubation time and ɛ = extinction coefficient for guaiacol (0.6740 µM cm−1).
where \(k = {\text{specific}}\;{\text{constant}}.\)
Protein assay
The protein content of sample was estimated using Lowry’s method [27], bovine serum albumin was used as the standard.
Fungal biomass determination
The fungal biomass was harvested by pouring the suspension through Whatman No.1 filter paper after 72 h of submerged fermentation in stationary condition. The fungal mycelia collected was washed with deionized water until clear filtrate was obtained. The obtained mycelia was freeze-dried in lyophilizer (Eyela FDU-1110, Japan). The biomass amount was gravimetrically measured and reported as grams of dried biomass per litre (g L−1).
Characterisation of crude laccase
The characteristics of crude laccase were studied over wide range of pH and temperature. The pH stability was determined by oxidation of 2 mM guaiacol over a wide pH range of 3.0–9.0. The pH of the reaction mixture was adjusted with different buffers (pH 3.0 acetate buffer, pH 4.0–7.0 phosphate buffer, pH 8.0 and 9.0 Tris–HCl buffer) and incubated at optimum temperature of laccase for 60 min for determination of residual activity. The thermal stability was determined under the previously described assay conditions within a temperature range of 30–90 °C at optimum pH of laccase for determination of residual activity.
Application of crude fungal laccase
The capability of crude fungal laccase in decolorization of fungal pigment and synthetic dyes were assessed.
Fungal pigment decolourization assay
Extraction of fungal pigment
The pigment (anthocyanin, ɛ = molar extinction coefficient, 28,000 M−1 cm−1) production by Aspergillus heteromorphus CBS 117.55 isolated from oil (rice bran oil industry) contaminated soil was carried out in 250 mL Erlenmeyer flasks containing Czapek Dox broth using 1 mL vegetative cell suspension containing 1 × 106 vegetative cell mL−1 of Aspergillus heteromorphus as inoculums in stationary condition. After 25 days of incubation period at 30 °C under stationary condition, pigment production was observed as reddish brown in the culture filtrate and was collected by centrifugation (R-12C, REMI, India) (5000g, 15 min). The pigment was collected through extraction with distilled water and stored for further studies. The pigment was quantified by UV–Vis spectroscopy (JASCO-V630, USA) in a wide range from 300 to 700 nm. The pigment secreted in culture supernatant was further used in pigment removal assay using crude laccase.
Decolorization efficiency of A. oryzae crude laccase
Different concentrations of crude laccase (10–70 U g−1) were added to 100 µg of aforementioned fungal pigment and were incubated at room temperature for 40 min. A blank and control sample was prepared parallel by adding sodium acetate buffer (pH 4.5) and distilled water instead of laccase under the similar condition. The absorption of pigment was measured spectrophotometrically (JASCO V 630 UV Vis Spectrophotometer, USA) at 400 nm, which was the specific wavelength for the fungal pigment extracted in this study. Pigment decolorization by fungal laccase was determined by the decrease in decolorization percentage (%) evaluated using the following equation:
Decolorization of fungal pigment on ageing paper
Pieces of ageing paper (10 × 15 cm) (old newspapers that has yellowish appearance) were inoculated by 2 mL vegetative cells suspension of isolated Aspergillus heteromorphus CBS 117.55 containing 2 × 106 vegetative cell mL−1. The paper was over grown with the fungal mycelia and secreted pigment. After every 7 days of interval, the pigmented paper was then treated with the optimum concentration of laccase obtained from previous experiment for removal of fungal pigment and incubated at room temperature. Decolorization of fungal pigment on ageing paper was carried out for 1 month. The percentage of decolorization pigment (%) was calculated as mentioned before.
Decolorization of synthetic dyes by crude laccase
To evaluate the ability of crude A. oryzae laccase to decolorize the selected synthetic dyes, reaction mixtures containing 0.5 mL of each selected dye solution, 40 U g−1 of crude laccase dissolved in 50 mM of sodium acetate buffer, pH 4.5 were incubated at room temperature (25 °C) at static condition. After withdrawal of the reaction mixtures at different intervals decolorization of dyes was measured spectrophotometrically (JASCO V 630 UV Vis Spectrophotometer, USA) by monitoring the decrease in absorption maxima of each dye (Congo Red, λ = 500 nm; Coomassie Brilliant Blue, λ = 595 nm; Indigo carmine, λ = 612 nm and Rhodamine B, λ = 544 nm). Experiments were carried out in triplicate and in parallel heat inactivated laccase was used in control reactions.
Crude laccase obtained maximum decolorization of the synthetic dye was further optimized for maximizing the dye decolorization percentage.
Optimization of different parameters affecting dye decolorization
The effect of different parameters on the dye decolorization was investigated by incubating the flasks at different temperatures (30, 40, 50, 60, 70 and 80 °C), pH of the reaction mixture was adjusted with different buffers (pH 3.0 glycine–HCl buffer, pH 4.0 acetate buffer, 5.0–6.0 citrate buffer, 7.0 phosphate buffer) and different dye concentrations ranging from 5 to 20 µM.
Dye decolorization kinetics
The kinetic study of different parameters associated with dye decolorization, mainly, the relative velocity [Vo] and different dye concentrations [S] were performed. Michaelis–Menten curve was drawn by plotting relative velocity against substrate (dye) concentrations. Furthermore, Lineweaver–Burk plot was applied to obtain the kinetic constants (Km and Vmax) of different dye concentrations.
Statistical analysis
All experiments were done in triplicate and standard deviation was determined. To determine the significance, the data were analysed by one-way ANOVA using Origin 2018 software. Tukey test was performed for p value determination. Values of p < 0.05 were considered as significant value.
Results and discussion
Lignin extraction yield
In the present experimental work, different pretreatment processes namely, alkali and catalyst assisted hydrothermal were deployed for extraction of lignin from CW. The data in Table 1 depicted that total lignin recovered from cauliflower leaf and stalk by the aforementioned processes and reached upto 10% for leaf (ASL 3.8% and AIL 6.2%) and 13% for stalk (3.4 and 9.6%). Alkali pretreatment method is the preferred process because the alkali acts on the aryl β–O–4´ ether linkages and cleaves 5–5 phenyl and β–β´ aryl bonds. Alkaline pH efficiently increased the swelling of the cell wall structure and forms free phenolic groups which cause the fragmentation of the lignin from the feedstock structure [28, 29]. Kaur et al. [29] investigated the extraction of lignin from sugarcane bagasse by administering different concentrations of alkali (1, 5 and 10%) and achieved maximum lignin yield (3.52 g/20 g of biomass) at 10% alkali concentration. The alkali extraction method removed lignin by partially dissolving lignin and hemicellulose by the cleavage of bond between these two macromolecular structures. Alkali treatment also hydrolysed the uronic, acetic and hydrocinnamic acid esters [30].
Another newly adopted pretreatment method; hydrothermal process with Na acetate as catalyst has been applied for efficient lignin extraction. Table 1 also represented the yield of isolated lignin fractions from hydrothermally pretreated cauliflower wastes using the sodium acetate as catalyst. The total isolated lignin content was found to be 8.63% for leaf (3% ASL and 5.63% AIL) and 12.63% for stalk (2.3% ASL and 10.33% AIL) which was higher than lignin obtained from CW without pretreatment [1.43% for stalk (0.45% ASL and 1% AIL) and 1.2% for leaf (0.44% ASL and 0.75% AIL)]. Catalyst assisted HTP affects the biomass composition, reducing mainly lignin content by cleavage of ester linkages joining the phenolic acids by liberation of more hydronium ions. The nucleophilic acyl substitution of ester bonds normally takes place during reaction with an alkaline salt. The alkaline pH of the fractionated solution increases the hydrophilicity of lignin and this promotes the lignin solubilization [31]. Chen et al. [31] has investigated the effect of hydrothermal treatment followed by post treatment with 70% ethanol containing 1% NaOH for separation of lignin from compact structure of wheat straw. They achieved maximum lignin yield up to 12.93% which was much higher than that of lignin obtained without HTP (3.87%). They correlated increase in hydrothermal severity factor (increasing reaction temperature) with increased recovery of lignin. Wu et al. [24] who obtained lignin fractions by the extraction of cotton stalk under catalytic HTP in the presence of different metal chlorides (AlCl3, FeCl3, CrCl3 and ZnCl2) and they observed that lignin fractions obtained were relatively higher when compared with sample treated without catalyst addition. Hydrothermal pretreatment catalysed with AlCl3 was found to be the most efficient method in the production of relatively high (18.5%) and pure lignin component from cotton stalk. They also reported that the lignins obtained by catalytic HTP consisted mainly of β–O–4' ether linkages with a few amount of β–β' and β-5' carbon–carbon bonds.
The efficiency of lignin recovery solely depends upon the species of plant material chosen as the raw material for lignin extraction [2]. Lignin from angiosperms (Brassica family vegetables) is mainly intertwined in the feedstock framework by phenylpropanoid units. These units are generated by radical coupling of hydroxycinnamyl alcohols named monolignols, coniferyl alcohol, sinapyl alcohol and p-coumaryl alcohol [32]. Few literatures [33] have reported on extraction of lignin from brassica family vegetables but these are potential plant species for isolation of lignin.
Laccase production from lignin fractions by submerged fermentation
The growing interest of production of in-house low-cost enzymes by microbial cultivation on low-cost substrates can be a resource of cleaner and greener processes to produce many industrially important end products. Cauliflower waste an easily available inexpensive agricultural residue used in the present study was rich in carbon sources and other nutrients indicating that this agricultural waste could be used as an appropriate substrate that would provide all the essential nutrients for the growth of ligninolytic microorganisms.
In the present study, monoculture and co-culture of three selected fungal strains were grown on different culture media containing isolated lignins (Ak-L and HT-L) obtained from cauliflower stalk and leaf as carbon source(s) with the aim to investigate fungal growth and subsequent enzyme production. These results were then compared with fermentations using untreated biomasses as substrate and presented in Table 2. It was observed that in the present study, in monoculture cultivation, Ak-L leaf exhibited highest amount of laccase activity (29.7 ± 0.6 U mL−1) by A. oryzae with protein concentration and biomass weight of the mycelia were 2.54 mg mL−1 and 1.23 g DM L−1, respectively, whereas least activity (4.4 U mL−1) with protein concentration and biomass weight of the mycelia were 0.12 mg mL−1 and 0.27 g DM L−1, respectively, was shown by A. oryzae when cultivated on untreated cauliflower leaf. Table 2 also depicts that other monoculture fungal strains had also shown substantial laccase activity (27 U mL−1 by A. niger and 24.6 U mL−1 by C. intermedia while cultivated on HT-L leaf). Fungal laccases are the most frequently studied form of laccases and laccases from white rot fungi or wood rot fungi are considered as the most common sources for extracellular fungal laccases production [9, 34].
Basidiomycetes or white rot fungi are the chiefly utilized microbial sources for production of laccase for biodegradation of waste biomass. However, present study highlights the efficiency of ascomycetes group of fungi for effective degradation of isolated lignin for release of laccase extracellularly. Data presented in Table 2 clearly indicated that enhanced production of laccase by Aspergillus sp. and Candida sp. was observed with lignin fractions obtained from pretreated CW compared to the untreated one. Therefore, Ak-L and HT-L were used as the substrate for extracellular laccase production by mono and co-culture of the tested fungi.
Kumar et al. [17] screened and isolated laccase producing fungal isolate from natural habitat using bromophenol blue and 2, 2′-Azinobis 3-ethyl-benzothiazoline 6-sulfonate (ABTS) as substrates. Isolated Aspergillus flavus exhibited maximum laccase activity of 23.1 U mL−1 after 12 days of fermentation which was lower in comparison to the maximum enzyme activity obtained with Aspergillus oryzae in the present study. El Monssef et al. [20] isolated laccases producing fungus Trichoderma harzianum from biodeteriorated ancient paper and parchment and demonstrated that isolated T. harzianum decolorized yellow colored fungal pigment extracted from Aspergillus terreus and Aspergillus ochraceus containing aromatic ring and phenols. In another study, Singh et al. [35] investigated laccase production in various media and in the presence and absence of lignocellulosic biomass supplementation such as rice straw, wheat bran and sugarcane bagasse by Aspergillus heteromorphus. They found that anaerobically treated distillery spent medium induced better laccase production than the mineral media and highest laccase activity was obtained after supplementation of mineral medium with rice straw. The difference in enzyme production might be due to the composition of polysaccharides and the presence of higher amount of aromatic polyphenolic groups in the substrates which served as positive inducers of ligninases. It was explicitly demonstrated that laccase activity was increased and induced by the addition of xenobiotic compound such as lignin [36]. Lignin which is a natural inducer of laccase biosynthesis is made up of phenolic and non-phenolic compounds. Cauliflower wastes which is an abundant source of lignocellulosic biomass contain lignin may be used as substrate as well as inducer of fermentation for efficient production of ligninases (laccase) during secondary metabolism of fungus. From Table 2, it is clear that laccase activity in culture with untreated cauliflower wastes remained low as compared to Ak-L and HT-L supplemented media. Aromatic compounds which are released due to fungal degradation of lignocellulosic biomass mediated by laccase are potent inducers of ligninolytic enzymes. Thus, Ak-L and HT-L supplemented media was better laccase inducer media than medium containing untreated CW. One possible explanation for the differences found in laccase production might be due to increased availability of aromatic compounds in the Ak-L and HT-L supplemented media.
Ranieri et al. [37] constructed stable integrative recombinant strain of Kluyveromyces lactis with Lcc1 laccase of the fungus Trametes trogii using the pyruvate decarboxylase promoter (KlPDC1) as an expression system and observed that the secreted extracellular recombinant laccase exhibited better enzyme properties than the native enzyme. Very few studies have focused on the production of laccase by Candida intermedia and according to our results C. intermedia exhibited significant laccase production of 24.6 U mL−1 with protein concentration and biomass weight of the mycelia were 1.76 mg mL−1 and 1.17 g DM L−1, respectively, without any genetic manipulations while cultivated on HT-L leaf.
Influence of fungal co-cultivation on extracellular enzyme production was also investigated in the present study. According to our results for Ak-L and HT-L extracted from pretreated cauliflower stalk, mixed cultivation of the three selected fungal strains resulted in noticeable laccase production (19.9 U mL−1 from Ak-L stalk and 18.7 U mL−1 from HT-L leaf) but mixed cultivation produced a little less laccase activity than individual culture. Amongst the mono and co-culture fungal strains, A. oryzae exhibited maximum laccase activity (29.7 ± 0.6 U mL−1) with protein concentration and biomass weight of the mycelia were 2.54 mg mL−1 and 1.23 g DM L−1, respectively, while cultivated on Ak-L leaf (Table 2). Therefore, further studies were conducted by utilizing crude laccase obtained from A. oryzae as enzyme source. In contrast, the study carried out by Du et al. [38] demonstrated that mixed culture Shiraia bambusicola and Phoma sp. BZJ6 significantly enhanced the laccase yield (4680 ± 19 U L−1) and NO, O2− and H2O2 are necessary signal molecules to induce laccase synthesis. Their studies also showed that mixed culture effectively enhanced accumulation of phenolic compounds in cells and suggested that there might be some relationship between the total phenolics/flavonoids/signalling molecules and induction of laccase synthesis in the mixed culture. Similarly, Pan et al. [39] showed that the mixed cultivation of Coprinopsis cinerea Okayama 7 and Gongronella sp. w5 improved laccase yield probably due to activation of silent laccase gene and certain resistance to adverse environments might have been produced by increased laccase production. Kannaiyan et al. [40] observed that laccase yield was also increased in the mixed cultivation of Dichomitus squalens and Ceriporiopsis subvermispora. In addition, Hailei et al. [41] suggested that glucose starvation in the co-culture of Trametes versicolor and Candida sp. HSD07A led to increase in laccase yield. However, several studies have found that not all fungi in co-cultivation increase the laccase yield [42, 43]. Though co-cultivation did not increase the enzyme yield in comparison to monoculture cultivation, however, the enzyme yield was slightly lower than laccase yield obtained from A. oryzae cultivation. In natural habitat, diverse groups of microorganisms collectively inhabit the same niche which can act as an emerging source for a significant yield of laccase. Further work was conducted on A. oryzae as this fungal strain was found to be maximum producer of extracellular laccase over other monoculture and co-culture in quantitative enzyme assay.
Time course study for production of crude laccase by A. oryzae
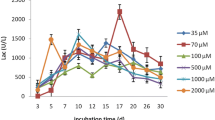
Incubation time is one of the predominant factors for maximum laccase production from extracted lignins obtained from pretreated cauliflower wastes. A gradual increase in enzyme activity was observed up to 12 days of fermentation period where the maximum enzyme activity was found to be of 29.7 ± 0.6 U mL−1 (Fig. 1). Enhanced laccase production occurred in the secondary phase of fungal metabolism with the advancement of incubation period. After 12 days of fermentation, a gradual decrease in enzyme production was observed due to the exhaustion of available nutrients in the culture media or weak mycelial growth. It is evident from the biomass yield measured at different time interval that the accumulation of end products such as low molecular weight compounds such as vanillin, syringaldehyde, p-coumaric acid, ferulic acid released after extracted lignin utilisation by the fungi inhibits further biomass growth. The trend observed in the current study was similar to the observations reported by Kumar et al. [17]. They observed that maximum extracellular laccase production was achieved after 12 days of fermentation (17.39 U mL−1) by Aspergillus flavus isolated from natural habitat. In another study, Sidhu et al. [44] conducted optimization of laccase production by Scytalidium lignicola isolated from decayed wood and reported that maximum extracellular laccase production (16.9 U mL−1) was achieved after 7 days of incubation period. In another investigation Jaber et al. [21] reported the effect of incubation period on laccase production by five different fungi using rubber wood dust as substrate and observed that highest laccase production was obtained by Trichoderma muroiana after 4 days of fermentation period (5.8 U mL−1). Sathishkumar et al. [45] investigated the time course profile study of laccase production by Pleurotus florida NCIM 1243 by utilizing banana peel, mandarin peel and cantaloupe peel as substrate under solid state fermentation and reported that maximum laccase activity (5.4 U g−1) was detected after 10 days of fermentation period.
Optimization of culture conditions for effective laccase production
The optimization of laccase production (temperature, pH and lignin concentrations) was conducted using the crude extract obtained with A. oryzae because it showed the highest enzyme activity for Ak-L leaf under the evaluated submerged fermentation conditions.
Optimization of initial pH
pH is an important parameter that affected the growth and subsequent enzyme production in fermentation. The results of the present study demonstrated maximum enzyme activity and production yield (35.3 ± 1.2 U mL−1 and 142.4 ± 2.5 mg L−1, respectively) was achieved at pH 4.5 (Fig. 2a) and decrease in enzyme activity was observed at higher pH. This might be due to the fact that weak mycelial growth (0.84 g DM L−1) at higher pH may not support the production of laccase. Jaber et al. [21] reported similar result of maximum laccase activity and biomass weight (5.8 U mL−1) achieved at pH 4.5 by T. muroiana using rubber wood dust as substrate. Optimization studies of extracellular laccase production were also carried out by Sidhu et al. [44] and they demonstrated that maximal laccase production (21.7 U mL−1) was attained at pH 6.0 by the fungi Scytalidium lignicola. Nadeem et al. [46] also observed similar trend of laccase production in pH range of 4.5 to 6.5 by Pleurotus ostreatus. These findings revealed that there is high correlation between pH of culture media and enzyme production by microorganisms and optimum value of pH depends on the nature of substrates as reactions for laccases varied with different substrates. Fungal laccases typically exhibits pH optima in the acidic range [7].
Optimization of temperature
Incubation temperature is a critical factor which affects the metabolic processes of the microorganism for both the fungal growth and laccase production which are sensitive to temperature. Study on the effect of temperature revealed that the highest laccase activity and production yield (36.9 ± 1.8U mL−1 and 146.6 ± 3.3 mg L−1, respectively) by A. oryzae was achieved at 30 °C (Fig. 2b). This result was similar to other reports on laccase production by both Jaber et al. [21] and Sidhu et al. [44] who found that ideal temperature for laccase production was 30 °C by both the fungi T. muroiana and Scytalidium lignicola. Fungi belonging to ascomycetes group mostly achieved maximum mycelium growth as well as highest enzyme activity in temperature range of 25–30 °C [47].
Optimization of Cu 2+ concentration
The laccase production can be improved or enhanced by addition or supplementing the culture media with different inducers, most prominently being Cu2+ supplementation. In the present study, different Cu2+ concentrations were studied and maximum laccase activity (37.4 ± 1.2 U mL−1) and production yield (149.9 ± 1.2 mg L−1) were obtained at 0.5 mM Cu2+ concentration. The biomass content and protein content (Fig. 2c) of A. oryzae also increases after Cu2+ supplementation of culture media. Increase in Cu2+ concentration decreases the fungal mycelium growth and furthermore, decreases the enzyme activity which might be due to the toxic effect of higher Cu2+ concentration upon fungal physiology [48]. Optimization studies of extracellular laccase production were also carried out by Sidhu et al. [44] and they demonstrated that maximal laccase production (22.6 U mL−1) by the fungi Scytalidium lignicola was obtained by addition of 2 mM CuSO4. The presence of Cu2+ in culture medium has effect on the active sites of laccase by combining with free ω-carboxylic anions situated on acidic amino acid residues which promote the catalytic efficiency of laccase. Another effect copper ions has is the facilitation of electron transfer near enzyme surface and increases its activation [49].
Optimization of lignin concentration
Non-natural substrates such as ABTS, guaiacol, syringaldehyde are commonly supplemented for laccase production but it raises the production cost of the enzyme. Therefore, the lignocellulosic enriched substrates are utilized for laccase production in cost effective manner. In the present study, the effect of various lignin concentrations upon laccase secretion by the different fungal species was evaluated for achieving maximum yield of laccase. The media was supplemented with varied lignin (0.5%–2.5% w/v) concentrations. Highest laccase activity and production yield (39.7 U mL−1 and 151.6 ± 3.5 mg L−1, respectively) was achieved with 1% (w/v) of lignin concentration (Fig. 2d). Lignin is interlinked with cellulose and hemicelluloses by phenylpropanoid units. It is composed of p-hydroxycinnamic acid, syringyl and guaiacyl bonds. The phenolic subunits of lignin are degraded by laccases due to the phenoxy radical formation by subtraction of one electron from phenolic-OH groups of lignins. This radical formation leads to oxidation of Cα–Cβ and subsequent cleavage of aryl–alkyl bonds [18]. The presence of lignin acts as inducer and activates the Lcc1 gene of Aspergillus genera results in an increased laccase production yield [50].
The enzyme activity obtained under the above mentioned optimized culture conditions was 41.3 ± 2.8 U mL−1, which is ninefold higher than enzyme activity obtained in control media. Moreover, total protein content and biomass content obtained under optimized culture conditions were 14.2 ± 1.5 g L−1and 3.9 g DM L−1. The laccase production yield obtained under optimized culture condition was noted to be 153.3 ± 2.4 mg L−1 than control media, which is lower than theoretical laccase production yield of 567.9 ± 5.2 mg L−1.
Characterization of crude laccase
Aspergillus oryzae extracellular laccase prepared from Ak-L of pretreated cauliflower leaf was employed for characterization studies. The pH stability of crude extracellular laccase was studied and it was found that crude laccase was stable in acidic (78.2% for pH 5) to neutral pH (37.3% for pH 7) (3.0–7.0) for 60 min. Enzyme stability was comparatively low at alkaline pH (11.3% for pH 8 and 7.3% for pH 9) and crude enzyme lost more than 50% of its relative activity at alkaline pH (8.0–9.0) (Fig. 3). The stability of laccase at acidic pH makes it suitable additive for industrial applications. Kumar et al. [17] reported that activity of laccase secreted by Aspergillus flavus was significantly influenced by pH and highest relative activity of the enzyme was found to be 91.4% after 1 h of incubation at pH 5.0 and retained 86.0% of its relative activity even after 4 h of incubation. El Monseef et al. [20] and More et al. [51] noted highest relative activity of purified laccase obtained from T. harzianum and Pleurotus sp. was at pH 5.0 and 4.5, respectively. Shrestha et al. [52] studied the pH stability of purified laccase extracted from lignin supplemented culture media cultivated with Ganoderma lucidum CDBT1. They reported maximum stability of purified laccase at pH 5.0 for 180 min and have noted a decrease in relative activity by 93% at pH 8.0 after 180 min of incubation.
Impact of temperature on crude laccase activity was evaluated at different temperatures. It was stable at wide temperature range (30–50 °C). The relative activity of 83.2% was noted at 50 °C after 60 min of incubation and the least relative activity was reported at 70 °C (22.1%). After 50 °C the extracellular laccase activity decreased proportionally with increase in temperature and the crude enzyme lost 50.2% of its relative activity beyond 50 °C which might be due to denaturation of the three dimensional structure of enzyme.
Kalra et al. [26] investigated the laccase production by cultivation of rice bran with fungal isolates obtained from water samples. They achieved thermal stability of crude laccase at 40 °C similar to the experimental results reported in the present study. Kumar et al. [17] observed thermal stability of laccase produced by Aspergillus flavus over wide temperature range (25–50 °C). They achieved maximum relative activity of enzyme at 25 °C (91.4%) after 1 h and retained 86% of relative activity even after 4 h of incubation.
According to Nawaz et al. [53], vegetables belonging to brassicaceae family especially, cauliflower waste is a natural source of bioactive components especially polyphenols. Therefore, lignin extracted from this substrate resulted in maximum lignin yield and thus, served as the positive inducer for laccase production.
Application of crude fungal laccase
Decolorization of fungal pigment by crude laccase
Laccase has varied biotechnological applications among which the most promising being the decolourization of pigment or dye. In the present study, decolourization efficiency of crude extracellular laccase was determined on fungal pigment produced by Aspergillus heteromorphus CBS 117.55 isolated and identified in laboratory. The maximum decolourization percentage was obtained with 40 U g−1 (85.9 ± 1.2%) of crude extracellular laccase. Increase in laccase concentration upto 40 U g−1 increased the pigment decolourization efficiency of the enzyme (Fig. 4). Further increase in laccase concentration decreased decolourization percentage. El Monssef et al. [20] investigated the effect of different laccase concentrations on decolourization of pigments isolated from five different fungi. They achieved maximum dye decolourization (90.71%) with partially purified laccase obtained from Aspergillus terreus at the concentration of 200 µL. Konkol et al. [54] also demonstrated the potentiality of laccase enzyme produced by Trametes versicolor as decolorizing agent for the decolorization of red brown stains produced by Serratia marcescens on Isamu Noguchi’s marble sculpture Slide Mantra (1991).
Mostly laccases from white rot fungi are utilised for decolourization of chemical pulps and textiles. However, decolorization efficiency of extracellular laccase released by Aspergillus oryzae have not been reported earlier to the best of knowledge available. The laccase enzyme will provide safe and clean environmental solution for decolourization of pigments produced by microorganisms on historic monuments and also for dye decolourization in paper, pulp and textile industries.
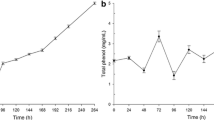
In the present study, the decolorization of fungal pigment on deteriorated paper by crude A. oryzae laccase enzyme was recorded at 7 days of interval during 30 days of incubation. The decolorization percentage of treated paper was determined and presented in Fig. 5 revealed that laccase enzyme was highly effective on decolorization of fungal pigment on biodeteriorated paper. The highest percentage of decolorization of 48.5% was obtained after 28 days of incubation followed by decolorization percentage of 12.6 and 3.2% after 14 and 7 days of incubation, respectively.
Dye decolorization of different synthetic dyes by A. oryzae laccase
Effluents mixed with dyes discharged from textile, paper and pulp industries are the main contributing factor for polluting the water bodies. Dyes are recalcitrant in nature, which affect their biodegradability and thus, remain at large in wastewater discharged from industries. Therefore, mitigation of this ever-increasing environmental concern needs proper disposal of effluents without any residual dye or its toxic byproducts. Laccases obtained from fungi have been reported in several literatures [45, 55] for its dye decolorization efficiency as biological means for bioremediation of polluted waters.
In this study, maximum decolorization percentage was achieved with 10 µM of Congo Red (54.1%) after 24 h of incubation followed by Indigo Carmine (48%), Coomassie Brilliant Blue R (39%) and Rhodamine B (39%) (Fig. 6). Bankole et al. [56] evaluated the decolorization potency of Peyronellaea prosopidis laccase against azo dye Scarlet RR dye and achieved 58% decolorization by crude laccase after 5 days of incubation. Moilanen et al. [57] tested the decolorization ability of crude laccases from Cerrena unicolor and Trametes hirsuta against synthetic dyes Remazol Brilliant Blue R (RBBR), Congo Red, Lanaset Grey and Poly R-478. Both C. unicolor and T. hirsuta were able to decolorize Congo red (91%) after 19.5 h of incubation period. Forootanfar et al. [55] studied the decolorization of six synthetic dyes using three fungal laccases from A. oryzae and T. versicolor and Paraconiothyrium variabile. They achieved color removal of synthetic dyes, methylene blue (53%) and RBBR (26%) after 3 h of incubation but could not decolorize other dyes mainly, Congo Red. Furthermore, most of the studies reported on addition of redox mediators (syringaldehyde, hydroxybenzotriazole, ABTS) for increasing the laccase efficacy, however, in this study appreciable dye decolorization percentage were achieved without addition of any redox mediator.
Fungal laccases are Cu2+ containing multioxidase enzymes and are potent in degrading xenobiotic compounds such as synthetic dyes. It degraded azo dyes such as Congo Red by non-specific free radical mechanism yielding non-toxic phenolic compounds as end product [58].
Optimization of different parameters for dye decolorization by A. oryzae laccase
Optimization of dye decolorization (temperature, pH and dye concentrations) was conducted using the crude extract of laccase obtained from A. oryzae.
The effect of pH on dye decolorization was studied over wide pH range (3.0–7.0) (Table 3) and the highest dye decolorization value (54 ± 1.2%) was achieved at pH 4.5. The maximum decolorization percentage was achieved at temperature 50 °C (62 ± 1%). The effect of Congo Red concentrations (5, 10, 15, 20, 25, 30 µM) revealed that maximum dye decolorization was obtained at dye concentration of 15 µM (64 ± 1%). The decolorization percentage obtained under the optimized parameters was 64 ± 1%, which are 8 folds higher than decolorization percentage obtained in control (8%).
Kinetic study of laccase catalysed azo dye decolorization
The kinetic study of crude laccase decolorized Congo Red indicated that the linear relationship between the initial velocity [Vo] and dye concentration [S] (Fig. 7) is a first order reaction. Based on Michaelis–Menten and Lineweaver–Burk plot for laccase decolorized Congo Red, Km and Vmax values obtained were 0.123 × 10−3 M and 0.724 mol L−1 min−1, respectively. Rezai et al. [59] investigated the laccase catalysed decolorization of Acid Blue 92 and also performed the kinetic study. They obtained Km and Vmax values of 0.48 mM and 227 mM−1 min−1 mg−1, respectively. Zilly et al. [49] studied the kinetic parameters of decolorization of Remazol Brilliant Blue R (RBBR) using crude laccase from Ganoderma lucidum cultivated on solid state medium using yellow passion fruit waste as substrate and they obtained Km and Vmax values of 0.602 × 10−3 M and 0.089 mol L−1 min−1.
Laccase is a highly adaptable oxidoreductase group of enzymes containing copper atoms in its catalytic centre for degradation of polyphenolic enriched substrates. Several scientific researches has demonstrated isolation of novel laccase producing microorganisms especially, belonging to white rot fungi group through utilization of agro-wastes or synthetic substrates and study of different parameters for optimum yield of laccase. The results of this present work will be of great importance in the economic production of laccase with high yield from agro-industrial waste by ascomycetes group of fungi (A. oryzae). Production of laccase utilizing extracted lignins, a polyphenolic enriched substrate from cauliflower wastes will be an innovative approach in a cost effective manner through providing a scope for cyclic economy of using unused portions of kitchen/domestic wastes. Purification of enzymes is an integral process in exhibiting profound enzyme activity with less inhibition on reaction mechanism due to the absence of undesirable products but it accrues additional costs for enzyme production from microbial source and also increases the overall operational cost. However, the present study effectively demonstrated the efficiency of crude laccase with better production yield and effective manifestation of relative activity in extreme pH and temperature displayed the decolorization of fungal pigment as well synthetic dyes which is the potent source of pollution of waterways and rivers.
Conclusion
The present study showed the potential of cauliflower wastes as economically viable substrate for isolation of lignin and subsequent production of fungal laccases, the ligninolytic oxidases involve in lignin degradation of waste feedstock for release of potential bioactive components of polyphenolic nature. Significantly, higher laccase activity was observed for lignin fractions isolated from alkali and catalytic hydrothermal pretreated cauliflower wastes rather than for untreated wastes. In view of the results obtained it can be concluded that A. oryzae could be considered the most important source of thermostable fungal laccase followed by A. niger while cultivated on alkali lignin isolated from cauliflower leaf. Production and preparation of laccase from the fungi under study using inexpensive and easily available agricultural waste is financially and environmentally very attractive for biotechnological and food application. Under the optimum culture condition, maximum laccase activity and laccase production yield were estimated to be 41.3 ± 2.8 U mL−1 and 153.3 ± 2.4 mg L−1, respectively. The stability of crude in-situ laccase obtained from monoculture cultivation (A. oryzae) was observed over wide pH (4–7) and temperature (30–50 °C) range, which holds potentiality in terms of industrial relevance. Fungal laccases are Cu2+ containing multioxidase enzymes and are potent in degrading xenobiotic compounds such as synthetic dyes decolorization. The kinetic study of crude laccase decolorized Congo Red indicated that the linear relationship between the initial velocity [Vo] and dye concentration is a first order reaction. The results will be of great importance in the laccase production using lignin isolated from agro-industrial waste, an enriched source of polyphenolic compounds by ascomycetes group of fungi (A. oryzae) to avoid the use of inducer that would involve cost effective production of laccase.
Crude laccase production from lignins extracted from cauliflower wastes though exhibited better activity and yield, however, has its limitations. Purification and immobilization of laccase was not performed and possess limitations in enzyme specificity, catalytic efficiency and stability. Moreover, crude laccase displayed better fungal pigment and synthetic dyes decolorization but not at par in comparison with other scientific studies. Biorefinery concept has arisen the possibility of utilization of byproducts for laccase production without the help of mediators. Furthermore, more extrapolative research work is needed in the optimization of submerged fermentation parameters for the improved recovery of laccase to increase its commercial viability. The present study provided added knowledge about upcycling of brassica wastes for extraction of lignin, a polyphenolic enriched substrate for laccase production under optimized conditions through cultivation with filamentous fungi. More research work is needed for the establishment of laccase and circular economy concept for mitigation of environmental pollution through minimization of domestic wastes.
References
Chapman J, Ismail A, Dinu C (2018) Industrial applications of enzymes: recent advances, techniques, and outlooks. Catalysts 8(6):238
Li SH, Liu S, Colmenares JC, Xu YJ (2016) A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem 18(3):594–607
Nanayakkara S, Patti AF, Saito K (2014) Chemical depolymerization of lignin involving the redistribution mechanism with phenols and repolymerization of depolymerized products. Green Chem 16(4):1897–1903
Peralta RM, da Silva BP, Côrrea RCG, Kato CG, Seixas FAV, Bracht A (2017) Enzymes from basidiomycetes—peculiar and efficient tools for biotechnology. Biotechnology of microbial enzymes. Academic Press, pp 119–149
Moreno LF, Feng P, Weiss VA, Vicente VA, Stielow JB, de Hoog S (2017) Phylogenomic analyses reveal the diversity of laccase-coding genes in Fonsecaea genomes. PLoS ONE 12(2):e0171291
Roopan SM (2017) An overview of natural renewable bio-polymer lignin towards nano and biotechnological applications. Int J Biol Macromol 103:508–514
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res 2014:1–21
Gonzalez JC, Medina SC, Rodriguez A, Osma JF, Alméciga-Díaz CJ, Sánchez OF (2013) Production of Trametes pubescens laccase under submerged and semi-solid culture conditions on agro-industrial wastes. PLoS ONE 8(9):e73721
Khedkar MA, Nimbalkar PR, Chavan PV, Chendake YJ, Bankar SB (2017) Cauliflower waste utilization for sustainable biobutanol production: revelation of drying kinetics and bioprocess development. Bioprocess Biosyst Eng 40(10):1493–1506
Zhou C, Dong A, Wang Q, Yu Y, Fan X, Cao Y, Li T (2017) Effect of common metal ions and anions on laccase catalysis of guaiacol and lignocellulosic fiber. BioResources 12(3):5102–5117
Ijoma GN, Tekere M (2017) Potential microbial applications of co-cultures involving ligninolytic fungi in the bioremediation of recalcitrant xenobiotic compounds. Int J Environ Sci Technol 14(8):1787–1806
Ferreira FL, Dall’Antonia CB, Shiga EA, Alvim LJ, Pessoni RAB (2018) Sugarcane bagasse as a source of carbon for enzyme production by filamentous fungi. Hoehnea 45(1):134–142
Hu HL, den Brink J, Van Gruben BS, Wösten HAB, Gu JD, de Vries RP (2011) Improved enzyme production by co- cultivation of Aspergillus oryzae and with other fungi. Int Biodeterior Biodegrad 65:248–252
Koziróg A, Otlewska A, Gapińska M, Michlewska S (2019) Influence of gemini surfactants on biochemical profile and ultrastructure of Aspergillus brasiliensis. Appl Sci 9(2):245
Pankar SA, Bornare DT (2018) Studies on cauliflower leave powder and its waste utilization in traditional product. Int J Agric Eng 11:95–98
Wadhwa M, Bakshi MPS, Makkar HPS (2015) Wastes to worth: value added products from fruit and vegetable wastes. CAB Int 1–25
Kumar R, Kaur J, Jain S, Kumar A (2016) Optimization of laccase production from Aspergillus flavus by design of experiment technique: partial purification and characterization. J Genet Eng Biotechnol 14(1):125–131
Pollegioni L, Tonin F, Rosini E (2015) Lignin degrading enzymes. FEBS J 282(7):1190–1213
Adekunle AE, Guo C, Liu CZ (2017) Lignin-enhanced laccase production from Trametes versicolor. Waste Biomass Valor 8(4):1061–1066
El Monssef RAA, Hassan EA, Ramadan EM (2016) Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann Agric Sci 61(1):145–154
Jaber SM, Shah UKM, Asa’ari AZM, Ariff AB (2017) Optimization of laccase production by locally isolated Trichoderma muroiana IS1037 using rubber wood dust as substrate. BioResources 12(2):3834–3849
Saito K, Horikawa Y, Sugiyama J, Watanabe T, Kobayashi Y, Takabe K (2016) Effect of thermochemical pretreatment on lignin alteration and cell wall microstructural degradation in Eucalyptus globulus: comparison of acid, alkali, and water pretreatments. J Wood Sci 62(3):276–284
Majumdar S, Naha A, Bhattacharyya DK, Bhowal J (2019) Effective delignification and decrystallization of cauliflower wastes by using dilute phosphoric acid for efficient enzymatic digestibility to produce fermentable sugars. Biomass Bioenerg 125:169–179
Wu M, Zhao D, Pang J, Zhang X, Li M, Xu F, Sun R (2015) Separation and characterization of lignin obtained by catalytic hydrothermal pretreatment of cotton stalk. Ind Crops Prod 66:123–130
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. LAP 1617:1–16
Kalra K, Chauhan R, Shavez M, Sachdeva S (2013) Isolation of laccase producing Trichoderma spp. and effect of pH and temperature on its activity. Int J Chem Environ Technol 5(5):2229–2235
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Li M, Foster C, Kelkar S, Pu Y (2012) Structural characterization of alkaline hydrogen peroxide pretreated grasses exhibiting diverse lignin phenotypes. Biotechnol Biofuels 5(1):38
Kaur R, Uppal SK (2015) Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid Polym Sci 293(9):2585–2592
Grimaldi MP, Marques MP, Laluce C, Cilli EM (2015) Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol Biofuels 8(1):205
Chen X, Li H, Sun S, Cao X (2016) Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci Rep 6:39354
Wang Y, Chantreau M, Sibout R, Hawkins S (2013) Plant cell wall lignification and monolignol metabolism. Front Plant Sci 4:220
Bunzel M, Seiler A, Steinhart H (2005) Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. J Agric Food Chem 53(24):9553–9559
Osma JF, Moilanen U, Toca-Herrera JL, Rodríguez-Couto S (2011) Uses of laccases in the food industry. FEMS Microbiol Lett 318(1):27–34
Singh A, Bajar S, Bishnoi NR, Singh N (2010) Laccase production by Aspergillus heteromorphus using distillery spent wash and lignocellulosic biomass. J Hazard Mater 176(1–3):1079–1082
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67(3):369–385
Ranieri D, Colao MC, Ruzzi M, Romagnoli G, Bianchi MM (2009) Optimization of recombinant fungal laccase production with strains of the yeast Kluyveromyces lactis from the pyruvate decarboxylase promoter. FEMS Yeast Res 9(6):892–902
Du W, Sun C, Wang J, Xie W, Wang B, Liu X, Fan Y (2017) Conditions and regulation of mixed culture to promote Shiraia bambusicola and Phoma sp. BZJ6 for laccase production. Sci Rep 7(1):17801
Pan K, Zhao N, Yin Q, Zhang T, Xu X, Fang W, Xiao Y (2014) Induction of a laccase Lcc9 from Coprinopsis cinerea by fungal coculture and its application on indigo dye decolorization. Biores Technol 162:45–52
Kannaiyan R, Mahinpey N, Kostenko V, Martinuzzi RJ (2015) Nutrient media optimization for simultaneous enhancement of the laccase and peroxidases production by coculture of Dichomitus squalens and Ceriporiopsis subvermispora. Biotechnol Appl Biochem 62(2):173–185
Hailei W, Guangli Y, Ping L, Yanchang G, Jun L, Guosheng L, Jianming Y (2009) Overproduction of Trametes versicolor laccase by making glucose starvation using yeast. Enzyme Microb Technol 45(2):146–149
Baldi A, Jain A, Gupta N, Srivastava AK, Bisaria VS (2008) Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxins: a first report. Biotechnol Lett 30(9):1671
Díaz R, Alonso S, Sánchez C, Tomasini A, Bibbins-Martinez M, Díaz-Godínez G (2011) Characterization of the growth and laccase activity of strains of Pleurotus ostreatus in submerged fermentation. BioResources 6(1):282–290
Sidhu AK, Darade SB, Bhavsar PP, Gaikwad VB, Patil SN (2017) Isolation, screening and optimization for laccase production by Scytalidium lignicolapesante under submerged fermentation. Int J Curr Microbiol Appl Sci 6(4):2477–2491
Sathishkumar P, Murugesan K, Palvannan T (2010) Production of laccase from Pleurotus florida using agro-wastes and efficient decolorization of reactive blue 198. J Basic Microbiol 50(4):360–367
Nadeem A, Baig S, Sheikh N (2014) Mycotechnological production of laccase by Pleurotus ostreatus P1 and its inhibition study. J Anim Plant Sci 24(2):492–502
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzyme Res 2010:1–10
Tychanowicz GK, Souza DFD, Souza CG, Kadowaki MK, Peralta RM (2006) Copper improves the production of laccase by the white-rot fungus Pleurotus pulmonarius in solid state fermentation. Braz Arch Biol Technol 49(5):699–704
Zilly A, da Silva C-M, Bracht A, De Souza CGM, Carvajal AE, Koehnlein EA, Peralta RM (2011) Influence of NaCl and Na2SO4 on the kinetics and dye decolorization ability of crude laccase from Ganoderma lucidum. Int Biodeterior Biodegrad 65(2):340–344
Yang J, Li W, Ng TB, Deng X, Lin J, Ye X (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8:832
More SS, Renuka PS, Malini S (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res (Article ID 248735)
Shrestha P, Joshi B, Joshi J, Malla R, Sreerama L (2016) Isolation and physicochemical characterization of laccase from Ganoderma lucidum-CDBT1 isolated from its native habitat in Nepal. BioMed Res Int (Article ID 3238909)
Nawaz H, Shad MA, Muzaffar S (2018) Phytochemical composition and antioxidant potential of brassica. In: Brassica germplasm: characterization, breeding and utilization. Intechopen 7
Konkol N, McNamara C, Sembrat J, Rabinowitz M (2009) Enzymatic decolorization of bacterial pigments from culturally significant marble. J Cult Herit 10(3):362–366
Forootanfar H, Moezzi A, Aghaie-Khozani M, Mahmoudjanlou Y (2012) Synthetic dye decolorization by three sources of fungal laccase. Iran J Environ Health 9(1):27
Bankole PO, Adekunle AA, Obidi OF, Chandanshive VV (2018) Biodegradation and detoxification of Scarlet RR dye by a newly isolated filamentous fungus, Peyronellaea prosopidis. Sustain Environ Res 28(5):214–222
Moilanen U, Osma JF, Winquist E, Leisola M (2010) Decolorization of simulated textile dye baths by crude laccases from Trametes hirsuta and Cerrena unicolor. Eng Life Sci 10(3):242–247
Sarkar S, Banerjee A, Halder U, Biswas R, Bandopadhyay R (2017) Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv Sci Eng 2(4):121–131
Rezaei S, Tahmasbi H, Mogharabi M, Ameri A, Forootanfar H, Khoshayand MR, Faramarzi MA (2015) Laccase-catalyzed decolorization and detoxification of Acid Blue 92: statistical optimization, microtoxicity, kinetics, and energetic. J Environ Health Sci Eng 13(1):31
Acknowledgements
Authors are thankful to Indian Institute of Engineering Science and Technology, Shibpur (Formerly Bengal Engineering and Science University, Shibpur), West Bengal, India for providing facilities for the completion of this research work. Research Scholar (S. Majumdar) is grateful to UGC for providing financial support as Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
Both the authors contributed equally to this research work.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majumdar, S., Bhowal, J. Studies on production and evaluation of biopigment and synthetic dye decolorization capacity of laccase produced by A. oryzae cultivated on agro-waste. Bioprocess Biosyst Eng 45, 45–60 (2022). https://doi.org/10.1007/s00449-021-02638-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02638-z