Abstract
Lactic acid bacteria (Lactobacillus plantarum KCTC 3103) were fermented to produce gamma-aminobutyric acid (GABA). The conditions of the modified synthetic medium were optimized as 5 g/L glucose, 10 g/L yeast extract, 100 g/L rice bran extract, and 1.0 g/L ascorbic acid for GABA production. Single-step fermentation of cell growth and GABA production with a modified synthetic medium was higher than those with an MRS medium. Two-step fermentation was evaluated by separating the cell growth and GABA production under a modified synthetic medium. The cell concentration of 1.65 g dcw/L produced by the modified synthetic medium was higher than that of 1.0 g dcw/L produced by the MRS medium at 36 h from the first step of two-step fermentation. The highest GABA production of L. plantarum KCTC 3103 was 0.67 g/L with monosodium glutamate addition at 60 h in the second step of fermentation. Two-step fermentation with the modified synthetic medium is suitable for GABA production because of its high GABA productivity and favorable cell growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gamma-aminobutyric acid (GABA) is a ubiquitous non-protein amino acid that is widely distributed in nature, including plants, animals, and microorganisms [1]. GABA-enriched foods have been introduced, including brown rice [2], tea [3], wheat bran [4], soybean [5], and lactic acid bacteria (LAB) fermented food [6]. GABA and some biogenic amines act as the major inhibitory neurotransmitters in the mammalian central nervous system. It has been reported that GABA assists blood flow in the brain, improving memory functions [7], alleviates depressive disorders [8], and lowers blood pressure [9]. Thus, GABA has potential as a bioactive component in food and pharmaceuticals [10].
Substrate costs account for approximately 30–50% of the final product costs; thus, use of a cheap byproduct can be very important to the overall economy of the process. Rice bran is the main byproduct of milling whole rice grain. The byproducts of agricultural crops are of interest because they can be obtained inexpensively from the rice-polishing process in Korea and utilized as extra glutamic acid decarboxylase (GAD, EC 4.1.1.15) [11]. GAD plays a key role in synthesizing GABA in the LAB fermentation process, which catalyzes the irreversible decarboxylation of glutamic acid to produce GABA and carbon dioxide. The glutamate content in the rice bran is 197.3 mg/100 g (wet basis) under sulfosalicylic acid/ethanol extraction [12]. Thus, rice bran extract was used as the culture medium composition for enhanced GABA production via LAB fermentation in this study.
GABA and other amino acids have been determined by high-performance liquid chromatography (HPLC) followed by ultraviolet (UV), fluorescence, or colorimetric detection [13]. Many methods involve pre-column derivatization with orthophthalaldehyde [14], phthaldehyde-2-mercaptoethanol [15], dansyle chloride [16], 2,4,6-trinitro benzene sulphonic acid [13], and 9-fluorenyl-methyl chloroformate [17] for the detection of GABA. In the present study, 2-hydroxynaphthaldehyde was used as a derivatizing reagent. This derivatizing reagent has been used for GABA determination in rice varieties due to its high sensitivity and response to minor GABA and biogenic amines for the formation of Schiff bases [18].
Some of the LAB strains are able to synthesize GABA and are distributed in species including Lactobacillus brevis [15], L. paracasei [19], Lactococcus lactis [20], Streptococcus thermophilus [21], and L. plantarum [6], which showed a potential industrial application due to their high GABA-producing abilities [6]. The GABA-producing LAB species can be increased with a single-step fermentation process by changing the culture conditions, such as the pH, temperature, initial monosodium glutamate (MSG) or glutamic acid concentration, pyridoxal 5’-phosphate (PLP) cofactor concentration, and medium composition [22, 23]. However, an excessive initial MSG concentration of the fermentation substrate can inhibit the cell growth or GABA production, and a low MSG concentration may not satisfy the need for high GABA production [21]. Thus, two-step fermentation was used to enhance the cell growth and GABA production in this study. In the first step, the LAB was grown without MSG to obtain maximum cell growth. Then, in the second step, MSG was added to trigger increased formation of GABA.
The objective of this study was to evaluate the effects of the culture medium composition for GABA production—particularly the type of carbon source, nitrogen source, rice bran extract concentration, and ascorbic acid concentration. Two-step fermentation was conducted to demonstrate the feasibility of maximizing the cell growth and GABA production from Lactobacillus plantarum KCTC 3103 through different processes in a 5-L fermentor.
Materials and methods
Chemicals, microbial strain, and culture medium
All chemicals used were of analytical grade and purchased from Sigma-Aldrich Co. (St. Louis, Mo, USA) and Duksan Pure Chemical Co. (Gyeonggi-do, Korea).
Lactobacillus plantarum KCTC 3103 was obtained from the Korean Collection for Type Cultures, Biological Resource Center (Daejeon, South Korea). L. plantarum KCTC 3103 was cultured in MRS broth at 30℃ and 120 rpm for 18 h, and then 5% (w/v) of inoculation was transferred to a 250-mL Erlenmeyer flask containing 100 mL of the modified synthetic medium, which comprised 5 g/L glucose, 10 g/L yeast extract, and 100 g/L rice bran extract, and 1 g/L ascorbic acid (vitamin C). Ascorbic acid was added to the modified synthetic medium after sterile filtration (filter pore size of 0.22 μm). Nutrient supplements of 5 g/L K2HPO4, 0.25 g/L MgSO4, and 10 g/L MSG were added to the modified synthetic medium and mixed together before inoculation. The carbon source, nitrogen source, and nutrient supplements were autoclaved separately at 121 ℃ for 15 min.
Rice bran (Kimpo, Korea) soaked in a tenfold volume of deionized water was extracted at 121 ℃ for 30 min. The extracts were centrifuged at 8000×g for 15 min to eliminate solid fractions, and the supernatants were used as the modified synthetic medium.
Optimization of culture conditions for GABA production
The one-factor-at-a-time (OFAT) strategy varies only one factor at a time and keeping others fixed. OFAT experiments were performed to determine the optimal synthetic medium compositions. The following culture medium conditions for L. plantarum KCTC 3103 were optimized: carbon source and concentration in the range of 1.25–30 g/L; nitrogen source and concentration, 5–50 g/L; rice bran extract concentration, 0–125 g/L; and ascorbic acid concentration, 0.5–4 g/L. Several carbon sources of 20 g/L (sucrose, galactose, glucose, lactose, and fructose) were individually added to a 250-mL Erlenmeyer flask containing 100 mL of the basal synthetic medium with 20 g/L nitrogen, 75 g/L rice bran extract, and 1 g/L ascorbic acid. Under the same conditions, after fixation of the carbon source, nitrogen sources in the basal synthetic medium were replaced with various nitrogen sources of 20 g/L (soytone, yeast extract, tryptone, beef extract, NH4NO3, NH4Cl) to determine the optimal nitrogen source and optimal concentration for GABA production. Other parameters, such as the rice bran extract and ascorbic acid concentrations, were optimized by altering their ranges of the basal synthetic medium compositions.
Two-step fermentation
Two-step fermentations by L. plantarum KCTC 3103 are typically characterized by two distinct steps in a 5-L fermentor (Model BIOCANVAS LF-5, CENTRION Company Ltd., Bucheon, Korea): the cell mass production step and the GABA production step. For the first seed culture, L. plantarum KCTC 3103 was cultured for 24 h in 30-mL MRS medium, and then 5% (w/v) of inoculation was transferred to a 250-mL Erlenmeyer flask containing 150 mL of the same medium and held for 24 h (second seed culture). Cells of the second seed culture were used as the inoculums to the main culture of the 5-L fermentor. Fermentations were carried out at 30 °C and 150 rpm using a 5-L fermentor with a 3-L working volume. The first step of the two-step fermentation process was conducted by inoculating 1.65 g dcw/L of L. plantarum KCTC 3103 until the early stationary phase. Then, MSG was added at a proper concentration for optimal GABA production in the second step of fermentation. Samples were taken periodically to measure the cell growth, residual MSG concentration, and GABA production. The efficiency of GABA conversion was calculated as follows:
where (GABA)inc represents the increase in the GABA concentration (g/L), and (MSG)cons represents the consumption of the total MSG concentration (g/L) during LAB fermentation.
The specific growth rate (μ) of L. plantarum KCTC 3103 during exponential growth and the dry cell weight of the final biomass at stationary phase were determined. The specific growth rate (μ) of LAB was calculated as follows:
where μ is specific growth rate (h−1), and X0 and X are the initial and final dry cell weight at times t0 and t, respectively.
Pre-column derivatization process with 2-hydroxynaphthaldehyde
Five working solutions of each GABA and MSG were prepared via the procedures reported by Hayat et al. [18]. Each working solution of 1 mL was treated with 1 mL of 2-hydroxynaphthaldehyde (2.5% in methanol), followed by the addition of 0.5 mL of a borate-NaOH buffer (pH 8.0) in a 5-mL volumetric flask. The resultant mixture was heated at 85 ℃ for 15 min in a water bath, and the solution was allowed to cool at room temperature. The volume was adjusted to 5 mL with methanol, and the solution was kept at 4 ℃ until analysis.
Analytical methods
The MSG and GABA concentrations in the fermentation broths were determined using an HPLC system (Agilent 1200 Series; Agilent Inc., Santa Clara, CA, USA) equipped with a UV detector. After derivatization, the solution (5 μL) was injected on a SUPERSIL ODS I-C18 column (250 × 4.6 mm) with an acetonitrile (A) and 0.1% acetic acid (pH 4.8) (B) gradient system (at 0 min, solvent B was 75%; at 2 min, solvent B was 75%; at 32 min, B was 40%; at 45 min, B was 75%) with a flow rate of 0.8 mL/min. The UV detection was based on the photodiode array and was performed at a wavelength of 254 nm. The identification of each peak was based on a comparison of the dilutions of the standard solutions. The cell growth was monitored by measuring OD600 using a UV–visible spectrophotometer (EMC-18PC-UV, EMCLAB®, Germany). The OD600 value was converted to the cell concentration using the standard curve between OD600 and the dry cell weight with the ratio of OD600 = 1.0 corresponding to 0.15 g dcw/L. Each sample was analyzed in triplicate, and the mean values were calculated.
Results and discussion
Effects of carbon and nitrogen sources for GABA production
Fermentation was performed with 100 mL of the basal synthetic medium containing 20 g/L of each carbon source, 20 g/L yeast extract, 75 g/L rice bran extract, and 1 g/L ascorbic acid in a 250-mL Erlenmeyer flask under anaerobic conditions for 72 h. The nutrient supplements and 10 g/L MSG were added to the basal synthetic medium and mixed together prior inoculation. L. plantarum KCTC 3103 was pre-cultured for 24 h until the OD600 reached 11.0, and then 5% (1.65 g dcw/L) of LAB were inoculated into 100 mL of the basal synthetic medium.
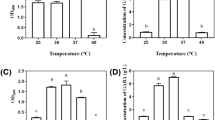
As shown in Fig. 1, the effect of the basal synthetic medium composition, e.g., the carbon (Fig. 1A and B) and nitrogen (Fig. 1C and D) sources, on the GABA production by L. plantarum KCTC 3103 was investigated. Fermentation at 72 h was increased with the maximum concentration of GABA (data not shown). Figure 1A shows the GABA production of L. plantarum KCTC 3103 under the different carbon sources, including sucrose and fructose at 72 h of fermentation. Among these carbon sources, glucose produced the highest GABA production of L. plantarum KCTC 3103 (reaching 0.34 g/L at 72 h), followed by sucrose (0.28 g/L), galactose (0.29 g/L), lactose (0.28 g/L), and fructose (0.16 g/L). Thus, glucose was selected as a carbon source for GABA production of L. plantarum KCTC 3103. As shown in Fig. 1B, the GABA production increased as the glucose concentration increased from 1.25 to 30 g/L. The highest GABA production (0.46 g/L) and efficiency of GABA conversion (EGC = 6.7%) were obtained with L. plantarum KCTC 3103 at a glucose concentration of 5 g/L. A further increase in the glucose concentration reduced the GABA production from 0.46 to 0.32 g/L for L. plantarum KCTC 3103. These results indicate that low concentrations of glucose can significantly enhance the GABA production of L. plantarum KCTC 3103. Similarly, Wang et al. [22] reported that high concentrations of glucose may have adverse effects on GABA synthesis in fermentation. The high concentrations of glucose aggravate the harshness of the environment because LAB metabolizes glucose into low-molecular weight organic acids. Thus, the glucose concentration of 5 g/L was selected as the optimal condition for GABA production in this study.
Effects of the carbon source (A), glucose concentration (B), nitrogen source (C), and yeast extract concentration (D) on GABA production by L. plantarum KCTC 3103. The initial basal synthetic medium composition was 20 g/L carbon, 20 g/L nitrogen, 75 g/L rice bran extract, 1 g/L ascorbic acid, 10 g/L MSG, and nutrient supplements. The pH was 6.0, the temperature was 30 °C, and fermentation was conducted for 72 h
As shown in Fig. 1C and D, optimization of the nitrogen sources and concentrations was performed to increase the GABA production of L. plantarum KCTC 3103 after 72 h in the flask culture. Figure 1C indicates that the highest GABA production (0.46 g/L) and EGC (7.3%) were obtained with yeast extract at 20 g/L. Soytone, tryptone, beef extract, NH4NO3, and NH4Cl exhibited GABA production of 0.28, 0.35, 0.32, 0.05, and 0.04 g/L, respectively. Thus, yeast extract was selected as the nitrogen source in this study. As shown in Fig. 1D, the GABA contents of L. plantarum KCTC 3103 increased as the yeast extract concentration increased to 10 g/L and then decreased with a further increase in the yeast extract concentration from 20 to 50 g/L. The highest GABA content (0.50 g/L) was obtained with L. plantarum KCTC 3103 at yeast extract concentration of 10 g/L for 72 h. At a relatively low concentration of yeast extract (< 10 g/L), GABA formation increased with the yeast extract concentration, but when the concentration exceeded 10 g/L, the increase in the GABA production was almost negligible. These results are consistent with the findings of the following studies. Kook et al. [23] reported that yeast extracts with concentrations of 3–10 g/L were the optimal nitrogen sources, according to a comparison of the culture conditions and GABA productivity. Thus, 10 g/L yeast extract was selected for subsequent experiments.
Effects of rice bran extract and ascorbic acid sources on GABA production
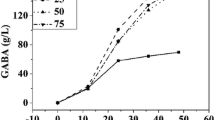
The effects of the additional rice bran extract and ascorbic acid concentrations on the GABA production were examined, as shown in Fig. 2. In previous studies, rice bran extract has often been used as an additional medium constituent to improve the GABA production and cell growth [24, 25]. As shown in Fig. 2A, the basal synthetic medium with rice bran extract significantly improved the GABA production. Addition of 100 g/L rice bran extract increased the GABA concentration to 0.54 g/L with an EGC of 9.3% at 72 h, and further addition of rice bran extract up to 125 g/L caused no significant increase in the GABA concentration or EGC. A control experiment without rice bran extract addition (0 g/L) was conducted under the same conditions of the basal synthetic medium for 72 h. The GABA concentration was 0.04 g/L with an EGC of 0.6%. These results suggest that the rice bran extract addition enhanced the GABA production and has significant potential for the development of a cost-effective synthetic medium for the mass production of GABA [25]. Thus, the rice bran extract concentration of 100 g/L was selected as a suitable synthetic medium condition.
Effects of the rice bran concentration (A) and ascorbic acid concentration (B) on GABA production by L. plantarum KCTC 3103. The basal synthetic medium composition was 5 g/L glucose, 10 g/L yeast extract, various rice bran extract concentrations, 1 g/L ascorbic acid, 10 g/L MSG, and nutrient supplements. The pH was 6.0, the temperature was 30 °C, and fermentation was conducted for 72 h
The effect of the addition of ascorbic acid on the GABA production of the basal synthetic medium was assessed with concentrations of 0.5–4.0 g/L at 30 °C and 150 rpm for 72 h, as shown in Fig. 2B. The maximum GABA production at an ascorbic acid concentration of 1.0 g/L was 0.54 g/L with an EGC of 9.3% at 72 h. However, a further increase in ascorbic acid concentration over 1.0 g/L resulted in a decrease in the GABA production from 0.54 to 0.09 g/L for L. plantarum KCTC 3103. These results indicate that increasing the ascorbic acid concentration can have a negative effect on the GABA production and cell growth (data not shown). Accordingly, the harshness of the environment was aggravated by the high concentration of ascorbic acid, which was harmful to the strain and inhibited GABA synthesis. Thus, for efficient GABA production, it is necessary to reduce the concentration of ascorbic acid. Similar results were obtained by Yao et al. [26] in an investigation of the effects of vitamins such as pantothenic acid, vitamin C, and biotin on the growth of Lactobacillus helveticus. They suggested that these vitamins were related to the synthesis of fatty acids and participated in the energy supply of the cells. Therefore, 1.0 g/L ascorbic acid was selected as the optimal synthetic medium condition for GABA production.
Comparison of cell growth and GABA production between single- and two-step fermentation processes
The single- and two-step fermentation profiles were compared using an MRS medium (pure culture) containing 30 g/L MSG at 30 °C and a pH of 6.0 for 144 h. As shown in Fig. 3, the fermentation profiles of the single- and two-step processes were obtained and compared with regard to the cell growth and GABA production. The effects of the cell growth and GABA production on single-step fermentation were evaluated with and without MSG, and the results are presented in Fig. 3A. The growth of L. plantarum KCTC 3103 without MSG had no lag time and entered a stationary phase in 36 h. For the strain L. plantarum KCTC 3103, the highest cell concentration and specific growth rate were 1.68 g dcw/L and 0.10 h−1, respectively. However, L. plantarum KCTC 3103 with 30 g/L MSG exhibited no substantial cell growth. The highest cell concentration and specific growth rate were 0.89 g dcw/L and 0.08 h−1), respectively. The GABA concentration after 144 h of fermentation was 0.19 g/L. These results indicate that the MSG concentration inhibited both the cell growth and the GABA production via the single-step fermentation. Similar results were obtained for excessive MSG [21, 27]. They suggest that the superfluous addition of MSG can inhibit cell growth and reduce GABA accumulation. The opposite effect was observed for Lactobacillus brevis CRL1942 [28]; the optimal MSG content was 270 mM (45.6 g/L) for GABA production. This result indicates that the optimal concentrations of MSG and glutamic acid for GABA production differ among microorganisms. From the results of a comparison between the cases with and without MSG addition, this inhibition can be overcome using two-step fermentation.
As shown in Fig. 3B, compared with Fig. 3A, two-step fermentation was performed to assess its effectiveness for increasing the cell growth and GABA production in L. plantarum KCTC 3103. In the first step, L. plantarum KCTC 3103 exhibited a maximum cell concentration of 1.70 g dcw/L and specific growth rate of 0.10 h−1. Then, GABA production was performed with 30 g/L MSG addition at the following 108 h of culture as the second step. The maximum concentration of GABA was 0.34 g/L at 120 h. These results indicate that two-step fermentation is advantageous over single-step fermentation for enhancing the cell growth and GABA accumulation. In several studies, co-culturing with different strains has been employed to promote GABA synthesis in two-step fermentation [29,30,31]. Lim et al. [32] reported that TRSF flour (turmeric and roasted soybean flour) was fermented by the two microorganism strains Bacillus subtilis HA and Lactobacillus plantarum K154. The GABA accumulation resulting from co-fermentation was up to 1.78% higher than that for the single-fermentation process. Therefore, the use of two-step fermentation should significantly enhance the cell growth and GABA production of L. plantarum KCTC 3103.
Two-step fermentation on GABA production using 5-L fermentor
Two-step fermentation was performed at 30 °C and 150 rpm using a 5-L fermentor with a 3-L working volume. The single- and two-step fermentation profiles are shown in Fig. 4. Single-step fermentation (control experiment) was conducted under an MRS medium at the same MSG concentration and with the same operating conditions for 168 h, as shown in Fig. 4A. The growth of L. plantarum KCTC 3103 with an MSG concentration of 30 g/L resulted in the maximum biomass production (1.0 g dcw/L), with a specific growth rate of 0.08 h−1, and reached the stationary phase at 36 h. The cell growth tended to slow, and MSG was consumed slowly until 168 h. Thus, the single-step fermentation using the MRS medium produced a GABA concentration of 0.21 g/L after 120 h of fermentation. As shown in Fig. 4B, compared with Fig. 4A, single-step fermentation was conducted using a modified synthetic medium under the optimized conditions described. L. plantarum KCTC 3103 with the MSG concentration of 30 g/L did not grow well under the modified synthetic medium. The initial pH of the medium was 6.0, but decreased to pH 4.2 during fermentation. The highest cell concentration and specific growth rate were 1.19 g dcw/L and 0.09 h−1, respectively. However, a high GABA concentration of 0.55 g/L was observed at 72 h of fermentation comparing to that of GABA concentration (Fig. 4A). Therefore, single-step fermentation under MRS and modified synthetic media not only slows the cell growth and fermentation but also reduces the GABA yield.
Fermentation profiles of the single- and two-step processes showing the cell growth and GABA production (30 °C, 150 rpm, 168 h): single-step with MRS medium (A); single-step with modified synthetic medium (B); two-step with modified synthetic medium (C) in 5-L fermentor under anaerobic conditions. The vertical line in (C) indicates the start of the second step of fermentation
As shown in Fig. 4C, the effect of the two-step fermentation was examined using a modified synthetic medium containing 30 g/L MSG for cell growth and GABA production. The use of the modified synthetic medium and two-step fermentation with L. plantarum KCTC 3103 reduced the lag time compared with the results shown in Fig. 4A and B. The first step of the two-step fermentation for optimal cell production was conducted until the early stationary phase at 36 h, as indicated by the vertical line (Fig. 4C). In the second step, MSG was added to trigger an increased concentration of GABA for the following 60 h. During fermentation in the second step (addition of MSG), the pH of the medium increased from 3.9 to 4.4 owing to the MSG decarboxylation, which alkalized the culture medium. When MSG is converted to GABA via decarboxylation by GAD, the release of carbon dioxide coincides with the consumption of hydrogen (H+), increasing the pH of the medium [33]. Wang et al. [22] reported the pH was gradually increased from 3.3 to 5.3 during the fermentation process, a pH range that favors glutamate decarboxylation by GAD. And then, GADs of LAB show optimal activity at pH 4.0–5.0 [34].
The growth of L. plantarum KCTC 3103 reached the stationary phase (first step) at 36 h with a cell concentration of 1.65 g dcw/L and a specific growth rate of 0.10 h−1. The GABA concentration initially increased to 0.67 g/L and then decreased to 0.54 g/L at the end of the fermentation. Similar results were obtained for GABA production [35,36,37]. These reports indicate that L. plantarum, which was isolated Kimchi, honeybees, and yogurt, had a GABA production of 0.2–0.7 g/L in MRS broth when proper MSG concentrations were used for the substrate. Furthermore, these results indicate the degradation of GABA in the fermentation, which had a negative effect on the GABA production [34]. For some bacteria, such as Escherichia coli and Listeria monocytogenes, the molecular mechanisms of GABA degradation have been clarified [38, 39]. GABA is degraded to succinic semialdehyde by the major GABA-degradative enzyme GABA aminotransferase (GABA-AT, EC 2.6.1.19) and is later converted to succinic acid during the catalysis of succinate semialdehyde dehydrogenase (EC 1.2.1.16) for entry into the TCA cycle. Compared with the control group, the two-step fermentation using the modified synthetic medium enhanced the cell growth and GABA productivity in the 5-L fermentor.
Conclusions
In this study, L. plantarum KCTC 3103 was capable of higher cell growth and GABA production rates with a modified synthetic medium than with an ordinary MRS medium under a two-step fermentation process. The one-factor-at-a-time (OFAT) experiments were carried out using various levels of carbon and nitrogen sources, rice bran concentrations, and ascorbic acid concentrations to optimize the medium composition. The optimal conditions for GABA production were determined to be 5 g/L glucose, 10 g/L yeast extract, 100 g/L rice bran extract, and 1.0 g/L ascorbic acid. Single-step fermentation produced lower cell and GABA concentrations, and two-step fermentation increased the cell concentration and enhanced the GABA production owing to the separation of the cell growth and GABA production into different steps in the L. plantarum KCTC 3103 fermentation. This fermentation process should allow the efficient production of LAB and GABA from the basal synthetic medium.
References
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzyme 10:67–79
Jannoey P, Niamsup H, Lumyong S, Tajima S, Nomura M, Chairote G (2010) γ-aminobutyric acid (GABA) accumulations in rice during germination. Chiang Mai J Sci 66:2600–2605
Wang HF, Tsai YS, Lin ML, Ou ASM (2006) Comparison of bioactive compounds in GABA tea and green tea produced in Taiwan. Food Chem 96:648–653
Youn YS, Park JK, Jang HD, Rhee YW (2011) Sequential hydration with anaerobic and heat treatment increases GABA (γ-aminobutyric acid) content in wheat. Food Chem 129:1631–1635
Park KB, Oh SH (2007) Production of yogurt with enhanced levels of γ-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol 98:1675–1679
Park SJ, Kim DH, Kang HJ, Shin MH, Yang SY, Yang JW, Jung YH (2021) Enhanced production of γ-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT Food Sci Technol 137:110443
Kalueff A, Nutt DJ (1996) Role of GABA in memory and anxiety. Depress Anxiety 4:100–110
Okada T, Sugishita T, Murakami T, Murai H, Saikusa T, Horino T, Onoda A, Kajimoto O, Takahashi R, Takahashi T (2000) Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J Jpn Soc Food Sci Technol 47:596–603
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H (2003) Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 57:490–495
Li H, Cao Y (2010) Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39:1107–1116
Wang L, Xu DX, Lv YG, Zhang H (2010) Purification and biochemical characterization of a novel glutamate decarboxylase from rice bran. J Sci Food Agric 90:1027–1033
Oh SJ, Kim HS, Lim ST, Reddy CK (2019) Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem 271:187–192
Clark G, O’Mahony S, Malone G, Dinan TG (2007) An isocratic high performance liquid chromatography method for the determination of GABA and glutamate in discrete regions of the rodent brain. J Neurosci Methods 160:223–230
Sawai Y, Yamaguchi Y, Miyama K, Yoshitomi H (2001) Cyclic treatment of anaerobic incubation increases the content of γ-aminobutyric acid in tea shoots. J Amino Acids 20:331–334
Lee BJ, Kim JS, Mi KY, Lim JH, Kim MY, Lee SM, Jeong MH, Ahn CB, Je JY (2010) Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. J Food Chem 122:271–276
Mazur R, Kovalovska K, Hudecin J (2011) Change in selectivity of gamma-aminobutyric acid formation effected by fermentation condition and microorganisms. J Microbiol Biotechnol Food Sci 1:164–171
Roohinejad S, Mirhosseini H, Saari N, Mustafa S, Alias I, Hussin ASM, Hamid A, Manap MY (2009) Evaluation of GABA, crude protein and amino acid composition from different varieties of Malaysian’s brown rice. Aust J Crop Sci 3:184–190
Hayat A, Jahangir TM, Khuhawar MY, Alamgir M, Siddiqui AJ, Musharraf SG (2014) Simultaneous HPLC determination of gamma amino butyric acid (GABA) and lysine in selected Pakistani rice varieties by pre-column derivatization with 2-Hydroxynaphth aldehyde. J Cereal Sci 60:356–360
Komatsuzaki N, Shima J, Kawamoto S, Momose H, Kimura T (2005) Production of gamma-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22:497–504
Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, Gobbetti M (2007) Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 73:7280–7290
Yang SY, Lu FX, Lu ZX, Bie XM, Jiao Y, Sun LJ, Yu B (2008) Production of γ-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids 34:473–478
Wang Q, Liu X, Fu J, Wang S, Chen Y, Chang K, Li H (2018) Substrate sustained release-based high efficacy biosynthesis of GABA by Lactobacillus brevis NCL912. Microb Cell Fact 17:80–88
Kook MC, Cho SC (2013) Production of GABA (gamma aminobutyric acid) by lactic acid bacteria. Korean J Food Sci Anim Resour 33:377–389
Eamarjharn A, Theerakulkait C, Thanachasai S (2016) Effect of incubation time, buffer type and concentration on gamma-aminobutyric acid (GABA) production using Khao Dawk Mali 105 rice bran. Agric Nat Resour 50:80–84
Kook MC, Seo MJ, Cheigh CI, Pyun YR, Cho SC, Park H (2010) Enhanced production of γ-aminobutyric acid using rice bran extracts by Lactobacillus sakei B2–16. J Microbiol Biotechnol 20:763–766
Yao C, Chou J, Wang T, Zhao H, Zhang B (2018) Pantothenic acid, vitamin C, and biotin play important roles in the growth of Lactobacillus helveticus. Front Microbiol 9:1194–1202
Zhuang K, Jiang Y, Feng X, Li L, Dang F, Zhang W, Man C (2018) Transcriptomic response to GABA-producing Lactobacillus plantarum CGMCC 1.2437T induced by L-MSG. PLoS One 13:e0199021
Villegas JM, Brown L, de Giori GS, Hebert EM (2016) Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942.; isolated from quinoa sourdough. LWT-Food Sci Technol 67:22–26
Park EJ, Garcia CV, Youn SJ, Park CD, Lee SP (2019) Fortification of γ-aminobutyric acid and bioactive compounds in Cucurbita moschata by novel two-step fermentation using Bacillus subtilis and Lactobacillus plantarum. LWT-Food Sci Technol 102:22–29
Sanchart C, Watthanasakphuban N, Boonseng O, Nguyen TH, Haltrich D, Maneerat S (2018) Tuna condensate as a promising low-cost substrate for glutamic acid and GABA formation using Candida rugosa and Lactobacillus futsaii. Process Biochem 70:29–35
Kwon SY, Garcia CV, Song YC, Lee SP (2016) GABA-enriched water dropwort produced by co-fermentation with Leuconostoc mesenteroides SM and Lactobacillus plantarum K154. LWT-Food Sci Technol 73:233–238
Lim JS, Garcia C, Lee SP (2016) Optimized production of GABA and γ-PGA in a turmeric and roasted soybean mixture co-fermented by Bacillus subtilis and Lactobacillus plantarum. Food Sci Technol Res 22:209–217
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
Cui Y, Miao K, Niyaphorn S, Qu X (2020) Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int J Mol Sci 21:995–1114
Park SY, Lee JW, Lim SD (2014) The probiotic characteristics and GABA production of Lactobacillus plantarum K154 isolated from Kimchi. Food Sci Biotechnol 23:1951–1957
Tajabadi N, Ebrahimpour A, Baradaran A, Rahim RA, Mahyudin NA, Manap MYA, Abu Bakar F, Saari N (2015) Optimization of gamma-aminobutyric acid production by Lactobacillus plantarum Tak-Apis362 from honeybees. Molecules 20:6654–6669
Zarei F, Nateghi L, Eshaghi MR, Abadi MET (2018) Optimization of gamma-aminobutyric acid production in probiotics extracted from local dairy products in west region of Iran using MRS broth and whey protein media. Appl Food Biotechnol 5:233–242
Dover S, Halpern YS (1972) Control of the pathway of γ-aminobutyrate breakdown in Escherichia coli K-12. J Bacteriol 110:165–170
Feehily C, O’Byrne CP, Karatzas KAG (2013) Functional γ-aminobutyrate shunt in Listeria monocytogenes: role in acid tolerance and succinate biosynthesis. Appl Environ Microb 79:74–80
Acknowledgements
This work was supported by a research grant from Hankyong National University in the year of 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, N.Y., Kim, SK. & Ra, C.H. Evaluation of gamma-aminobutyric acid (GABA) production by Lactobacillus plantarum using two-step fermentation. Bioprocess Biosyst Eng 44, 2099–2108 (2021). https://doi.org/10.1007/s00449-021-02586-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02586-8