Abstract
Sago hampas is a starch-based biomass from sago processing industries consisted of 58% remaining starch. This study has demonstrated the bioconversion of sago hampas to volatile fatty acids (VFAs) by Clostridium beijerinckii SR1 via anaerobic digestion. Higher total VFAs were obtained from sago hampas (5.04 g/L and 0.287 g/g) as compared to commercial starch (5.94 g/L and 0.318 g/g). The physical factors have been investigated for the enhancement of VFAs production using one-factor-at-a-time (OFAT). The optimum condition; 3% substrate concentration, 3 g/L of yeast extract concentration and 2 g/L of ammonium nitrate enhanced the production of VFAs by 52.6%, resulted the total VFAs produced is 7.69 g/L with the VFAs yield of 0.451 g/g. VFAs hydrolysate produced successfully generated 273.4 mV of open voltage circuit and 61.5 mW/m2 of power density in microbial fuel cells. It was suggested that sago hampas provide as an alternative carbon feedstock for bioelectricity generation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is a potential feedstock for bioenergy production due to its abundance and renewable. There are many biobased industry products used nowadays are resulted from processing of natural cycle by-products such as biomass, including starch and cellulosic materials. Sago hampas a starch-based biomass resulted from sago starch processing mill that has more than 50% of starch content [1, 2], which a great potential feedstock for production of value-added products. In Malaysia, Sarawak is known as the largest sago starch producer which covers 44,000 tons/year of starch export to Peninsular Malaysia, Japan, Singapore and other Asia countries [3]. Due to the demand, the production of sago starch is expected to increase, subsequently lead to the waste management by the processing mill since current disposal practice is directly release to the river water streams. In general, the sago starch extraction process consist of debarking, pulping and starch extraction process, in which during the pulping process has generated wastewater as well as sago hampas. Based on the composition of sago hampas, it has 58% starch, 32% cellulosic materials and 4% lignin [4, 5]. Recent studies have shown the significant impacts of sago hampas as a substrate for the production of glucose [6], biohydrogen [4], bioethanol [7] as well as bioelectricity [2]. Due to the high amount of starch content, it is beneficial to utilize sago hampas as a substrate for the production of volatile fatty acids (VFA) via anaerobic digestion.
Volatile fatty acids (VFA) are short chain of fatty acids which have less than six carbon atom with aliphatic tail, including acetic acid, propionic acid, butyric acid, valeric acid and caproaic acid. VFAs are the products resulted from the acidogenesis phase in anaerobic digestion of complex organic matters under the absence of oxygen [8, 9]. These VFAs can be produced from all parts of biomass (carbohydrates, lipids, proteins) except for lignin, which potential to be used as a carbon source for bioenergy production [9,10,11]. The new emerging platform (VFA platform) is considered as an essential element to be alternative to sole-dependent sugar platform. Pure sugars such as glucose and sucrose have been commonly used as main carbon sources for the production of value-added products which raises the ethical concern on the use of food [12].
In this study, the potential of VFA platform from sago hampas has been demonstrated on the bioelectricity generation through microbial fuel cell (MFC) system. Microbial fuel cells (MFCs) is a bio-electrochemical device that can generate the electrical current from various types of the chemical through the catalytic oxidation of electrolyte at the anode compartment and chemical reduction at the cathode compartment as a synergic process. The concept of fuel cells is transforming the chemical reactivity into electricity by oxidizing the electron donor in the anode and reducing the electron acceptor in the cathode [13, 14], which provides the electrical power outputs as long as the availability of sufficient fuels and oxidants. In reviews, several VFAs including acetate [15, 16], propionate [17] and butyrate [18]are suitable as a carbon sources for bioelectricity generation. At the same time, few studies also have been demonstrated the application of mixed VFAs in MFC [19,20,21].
However, the VFAs platform is deliberated as a new emerging technology approach, thus to realize its advantages, crucial effort is needed to contribute in better understanding on its processes [22]. Furthermore, the utilization of sago biomass would be promising due to the fact that no pretreatment is required. Therefore, this study was aimed to enhance the production of VFAs sago hampas as carbon source and subsequently demonstrate the bioelectricity generation from VFAs hydrolysate from sago hampas in MFCs.
Materials and methods
Sample collection and preparation
Substrate used in this study was starch-based biomass resulted from the starch processing mill known as sago hampas. Sago hampas was collected from River Link Sago Resources Sdn. Bhd., Mukah Sarawak, Malaysia. The collected sample was left in the porous bag for 1–2 days to eliminate the remaining water, then further subjected to the drying process using dry oven (Labwit, China) at 60 °C for overnight. The dried powdered sago hampas was kept in the sealed container at room temperature for further experiment.
Culture preparation
Inoculum used in this was a single culture, Clostridium beijerinckii SR1 obtained from Assoc Prof Dr Madihah Md Salleh, Universiti Teknologi Malaysia, Skudai, Johor, Malaysia [23]. One mL aliquot of the stock culture was inoculated into 125 mL serum bottle, with 100 mL working volume of oxygen free and sterilized growth medium, pH 5.5. Reinforced clostridia medium (RCM) was used as a growth medium. A 37 g of RCM (Merck, Denmark) was dissolved in 1000 mL distilled water. The inoculation was done at 37 °C for 24 h at 150 rpm using the incubator shaker (Labwit, China). Fresh inoculum was prepared for each fermentation of VFA.
Anaerobic digestion of sago hampas
The production of volatile fatty acids (VFA) was subjected to anaerobic fermentation using modified RAMM medium [24]. The modified RAMM medium consist (in g/L) of yeast extract (1), KH2PO4 (0.27), K2HPO4 (0.35), NH4Cl (0.53), MgCl.6H2O (0.1) and CaCl2.2H2O (0.075). The RAMM medium also contained 2% (w/v) of substrate and NaHCO3 (10 g/L) which is act as an alkaline buffer. The medium was prepared anaerobically in 125 mL serum bottle before it was autoclaved at 121 °C for 15 min. The 2-bromoethanesulfonate, BES (12 mM) (methane inhibitor [25]), trace element solution (DSMZ 320, 0.1% v/v) and vitamin solution (DSMZ 503, 0.1% v/v) were prepared separately and subsequently transferred into the modified RAMM medium, aseptically and anaerobically. The medium was freshly prepared prior to the fermentation.
The production of VFA was done by initially transferred 10% (v/v) of freshly prepared inoculum (C. beijerinckii SR1) into 100 mL working volume RAMM medium, and further fermented at 37 °C and 150 rpm for 6 days. The sampling was done daily for triplicates and subjected to the analytical analysis.
Enhancement of VFAs production from sago hampas using one-factor-at-a-time approach
The effect of several physical factors affecting the production of volatile fatty acids has been studied using one-factor-at-a-time (OFAT) approach. Table 1 illustrates the factors that have been studied, as well the range of the factors. Based on Table 1, the effect of sago hampas concentration was done by ranging the substrate loading from 1 to 7% (w/v). Furthermore, different concentration of yeast extract was also investigated by varied the concentration of yeast extract from 0.5 g/L up to 5.0 g/L. The experiment to determine the effect of additional inorganic nitrogen was conducted by the addition of 2 g/L of different inorganic nitrogen sources, as shown in Table 1. The pH of all experiments was adjusted to pH 6.0 using 1 M NaOH and 1 M HCl. Each parameters tested were done in triplicates.
MFC construction and operation
The construction of MFC system was based on the study by Jenol et al. [2]. The proton exchange membrane (PEM) was pretreated using boiling 3% (v/v) H2O2 for 1 h, followed 1 M H2SO4 for 1 h, before it was rinsed with boiling deionized water for 1 h. The pretreatment of PEM was based on the method described by Basu et al. [26]. The electrodes (1.5 × 1.5 cm of the surface area) used in the anode and cathode compartment were the carbon cloth (5% wet-proofing) (Fuel Cell Earth, Massachusetts, USA) and 20% platinum on Vulcan—carbon cloth (Fuel Cell Store, USA), respectively. All the possible leakages at the anode compartment were sealed using epoxy. The electrodes were connected with external wire connected to data acquisition system (LabJack U12 Series, LabJack Corporation, US). The MFC chambers were filled with 200 mL working volume of fermentation medium. The fermentation medium containing 1 g/L of carbon source (unless otherwise stated) was transferred aseptically into the anode compartment before it was sparged with nitrogen gas for 15 min to provide anaerobic condition. Ten percent (v/v) of C. beijerinckii SR1 was inoculated into the anode compartment contained in the fermentation medium prior to the fermentation. In the cathode compartment, 50 mM K3Fe(CN)6 was mixed into the medium acted as the terminal electron acceptor. During the fermentation period, one aliquot mL of sample was drawn out from the sampling port in 24-h time interval. The collected sample was subjected to analytical analysis, including substrate concentration, pH and cell growth.
Analytical analysis
Starch content was determined using method described by Nakamura [27]. Cellulose, hemicellulose, and lignin composition of sago hampas determined based on the method by Goering and Van Soest [28]. The hydrolysate obtained from the anaerobic digestion of sago hampas was subjected to the VFAs determination using the method proposed by Ibrahim et al.[29] and Jenol et al.[4]. The cell concentration is term of dry cell weight (DCW) was based on method explained by Ibrahim et al.[29], where by the pellet was measured at 680 nm. The concentrations of VFAs was measured by filtrate samples through 0.45 µM nylon membrane syringe filter. A gas chromatograph (BP-21, Shimadzu, Japan) equipped with capillary column flame ionization detector. The column was set at 60 °C, injector at 150 °C and detector at 200 °C. Cell voltages (V) were measured across a fixed external circuit resistor (300 Ω) using a data acquisition system (LabJack U12 Series, LabJack Corporation, US) that recorded every 10 min. A box plot was used to analyse the voltage recorded from each batches of MFCs operation was based on the method explained by McGill et al. [30].
Results and discussion
Production of volatile fatty acids by Clostridium beijerinckii SR1
Using commercial glucose and starch
The anaerobic digestion of sago hampas by C. beijerinckii was done for the production of volatile fatty acids (VFAs) using commercial glucose and starch. In this study, commercial starch used was a soluble starch. Figure 1 illustrates the VFAs production using 2% (w/v) of commercial glucose and starch cultured in anaerobic condition of C. beijerinckii SR1. This preliminary work was performed to determine the ability of C. beijerinckii SR1 in the bioconversion of carbon sources (commercial glucose and starch) to VFAs.
Based on Fig. 1a, C. beijerinckii SR1 has successfully produced 4.96 g/L of total VFAs from 20 g/L of glucose at 96 h of the fermentation time. On the other hand, the same situation was observed in commercial starch, where the highest total VFAs (5.93 g/L) recorded at 96 h of the fermentation time. On the other hand, the VFAs production by C. beijerinckii SR1 was determined slightly higher in commercial starch as compared to commercial glucose. C. beijierinckii SR1 was seemed to degrade 93% of total starch supplemented in the fermentation medium. This situation is further explained by Md Salleh et al. [31] the conversion of starch with the addition of water is derived by 1 g-starch/L is converted to 1.1 g-glucose/L. The result obtained suggested that C beijerinckii SR1 has the ability to directly utilize the starch in the fermentation. This matter is due to the fact that Clostridium sp. has the ability to secrete the amylolytic enzyme to degrade the starch [2, 4, 7, 31].
As demonstrated by Awg-Adeni et al. [6], the starch granule placement is on the outer layer of the substrate suggested the amylolytic enzyme easier to access the starch. Since the bioconversion of starch, glucose was the main fermentable sugar produced by the action of amylolytic enzyme during the fermentation, followed by the low amount of cellobiose and mannose. This situation was in agreement with the previous studies employing the starch as substrate [4, 31]. Thus, glucose produced was utilized by the bacteria to undergo the acidogenesis process at the early stage. As explained by Xue et al. [32], the clostridia has efficiently consumed glucose as compared to other types of pentoses and hexoses due to the carbon catabolite repression (CCR) in their metabolism. The observed situation was in agreement Ibrahim et al. [29], whereby the aforementioned authors found out that glucose was intensively consumed during 24 h of fermentation while C. butyricum EB6 producing the acetic and butyric acid. This is supported by several works related to Clostridia sp.[1, 4, 29, 33].
This situation was associated with the growth of C. beijerinckii SR1 during the fermentation (Fig. 1b). It is observed that this particular bacteria has entered the log phase from hour 48 until 96. This situation observed was slightly different as reported in the literatures [1, 29, 34], whereby in their study the Clostridia sp. required shorter lag phase. Based on Gheshlaghi et al. [35], Clostridia sp. has two distinctive phases; acidogenesis and solventogenesis. In the first phase (acidogenesis), the cells grow rapidly and form the carboxylic acids, which mainly acetate as well as butyrate. From this study, it can be observed that the production of VFAs was increased proportionally with the increment of the cell density of C. beijerinckii SR1 (Fig. 1b).
The changes in pH (Fig. 1b) was indirectly proportional to the cell growth and VFAs production. The lowest pH recorded for all sets of experiment was almost comparable, which was 4.6. Since the anaerobic fermentation of Clostridium sp. consists of acidogenesis, the phase where the VFAs were produced, therefore, the pH of fermentation medium will significantly dropped, in which further affect the cell concentration inversely due to acidic condition of the fermentation medium resulted from the production of VFAs. This may explained that too acidic condition might be toxic to the cell, further slightly decrease the cell concentration This situation was observed to be supported by previous studies involving Clostridium sp.[4, 33, 36,37,38]. In all works, the final pH observed was dropped to below pH 5, which indicates the production of acids contributed to more than onefold pH dropped. This situation was explained by Gheshlaghi et al. [35], in which the formation of carboxylic acids (acetate and butyrate) by C. beijerinckii SR1 were excreted out from the cell into the fermentation medium. Husin et al. [1] found the final pH was dropped to pH 4.9 after 24 h of fermentation using C. acetobutylicum ATCC824 for the production of biobutanol. In other cases, the pH was dropped to 4.26 [29], 4.4 [4, 39] and 4.72 [33]. As explained by Liu et al. (2016), the reduction in pH of fermentation medium was accompanied by the ATP synthesis through substrate-level phosphorylation. Substrate-level phosphorylation represents the formation of ATP from ADP and a phosphorylated intermediate, rather than from ADP and inorganic phosphate as is done in oxidative phosphorylation.
However, the pH was observed to be slightly increased after 96 h of the fermentation, from pH 4.6 increased to 4.8. This situation explained by the adaptive response of the cell culture to the lower pH medium resulted from the production of VFAs, in which further resulted the acids in the medium re-entered the cell body to act as an inducer for the formation of solvents (acetone, butanol and ethanol); during solventogenesis. The similar pattern in pH changes was observed in Liu et al. [40] with the increment in pH during stationary phase, indicating the solventogenesis phase has taken place. This situation is further explained by Yang et al. [41] in which the undissociated acids were the major driving force for the cell to enter the solventogenesis process, which required the cell to induce the extracellular acids to trigger the metabolic pathway. Therefore, this study suggested that the production of VFAs by C. beijerinckii SR1 has the highest production around 96 h of the anaerobic digestion of sago hampas.
Using sago hampas as carbon source
In this study, sago hampas used contained 58% of starch content and 34.4% of cellulosic materials. The composition of sago hampas has seemed to be in agreement with previous studies [1, 2, 42]. Further, C. beijerinckii SR1 was subjected to the production of VFAs using sago hampas as a carbon source. Figure 2 showed the VFAs production using 4% (w/v) of sago hampas cultured in anaerobic condition during fermentation process. C. beijerinckii SR1. It should be noted that the sago hampas was directly utilized as carbon source without any gelatinization step as compared in the hydrolysis process of sago hampas [4, 6]. Since the starch content in sago hampas accounted 58% (w/w), an initial substrate loading was set to 4% (w/v) for the production of VFAs from sago hampas. The amount of substrate loading used was based on the composition of starch content in sago hampas, since 2% of carbon source used for VFA production [24].
Based on Fig. 2a, the highest VFAs produced from sago hampas by C. beijerinckii SR1 was 5.04 g/L at 96 h. As compared to the commercial starch, the production of VFAs from sago hampas was slightly lower with almost comparable VFAs yield. It was observed that the difference in the butyric acid production of commercial starch (5.00 g/L) was slightly higher as compared to sago hampas (4.15 g/L). However, the acetic acid production was determined to be almost comparable for both set of experiments, in which 0.89 and 0.93 g/L from sago hampas and commercial starch, respectively. The difference in VFAs production observed was might be due to the solubility of the substrate, since the commercial starch is solubilized in the medium. However, the VFAs production from sago hampas is deemed to have almost comparable as compared to commercial glucose (4.96 g/L). In comparison, this study shows slightly lower in VFAs production as compared to Ibrahim et al. [29] with total VFAs production recorded was 6.6 g/L using glucose by C. butyricum EB6. The major difference in VFAs production by Clostridia sp. is due to the carbon source used, thereby glucose [29] is simpler carbohydrate as compared to starch (This study). In conjunction to that, 9.64 g/L of total VFAs was obtained by Husin et al. [1] from 50 g/L of sago hampas hydrolysate using C. acetobutylicum ATCC824. On the other hand, Park et al. [24] has successfully demonstrated the VFAs production in range of 3.35–7.09 g/L from rice straw with various concentration of NaOH pretreatment was applied. All in all, even though that sago hampas hold a potential value to be as an alternative substrate to replace the commercial carbon source for the production of VFAs, the enhancement in production should be done.
The effect additional glucoamylase enzyme (Dextrozyme) as enzyme-assisted process was further investigated for VFAs production from sago hampas. Based on Fig. 2a, the production of VFAs was drastically increased as compared to sago hampas alone. The increment of 24% was observed by the additional of 2 U/mL of Dextrozyme. This situation could be explained by the synergic act of the additional amylolytic enzyme to the fermentation has improved the production of VFAs. This is supported by the growth profile of C. beijerinckii SR1 (Fig. 2b) in enzyme-assisted VFA production was slightly better as compared to sago hampas alone. Even though Clostridia sp. has been reported to have the ability to secret the amylase for the degradation of starch [31, 43], it might not contributed in fully degradation of starch in sago hampas. This situation is supported by the amount of leftover starch content in sago hampas only and enzyme-assisted sago hampas (Table 2) is 5.26 and 3.93 g/L, respectively. Based on reviews, 5.56 U/mL of glucoamylase activity was used for the hydrolysis of sago hampas into fermentable sugar [4, 6]. Thus, this study suggested that the additional of glucoamylase into the fermentation medium is beneficial to assist Clostridia sp. to degrade starch in sago hampas.
Table 2 summarize the production of VFAs by C. beijerinckii SR1. Based on the results obtained, the VFAs production from enzyme-assisted sago hampas is slightly higher as compared to commercial starch and sago hampas only. In comparison to that, a comparable VFAs production (6.25 g/L) was obtained in this study as compared to the previous study (6.00–7.09 g/L) [24, 29]. In conjunction, this study has shown better in VFAs production as compared to Md Salleh et al. [31] by more than threefold of increment whereby using 30 g/L of sago hampas by C. acetobutylicum P262. Husin et al. [1] has managed to obtain around 10 g/L of total VFAs from sago hampas using simultaneous saccharification fermentation process by C. acetobutylicum ATCC824. The difference from the aforementioned authors with this study is mainly due to the utilization of cellulosic materials in the sago hampas projected to higher production of VFAs. In this study, it is interesting to mention that the production of butyric acid observed from starch was slightly higher as compared to glucose (as acetic acid is higher than butyric acid). This situation might be related to the metabolism route taken by this strain which significantly affects the concentration of acetic and butyric acid production. However, further investigations are needed to justify the actual mechanism of each routes.
The total starch consumed by C beijerinckii SR1 in sago hampas was 17.58 g/L. The highest yield recorded was in the production of VFAs from sago hampas with enzyme-assisted system (0.324 g/g) resulted from 19.3 g/L of total starch consumed during the fermentation, which were 7.5 and 12.9% higher as compared to commercial starch and sago hampas only, respectively. The difference in VFAs production and yield might be due to the additional glucoamylase into the medium, which assist the production of VFAs as well as increase the yield of the VFAs produced from sago hampas. It is interesting to mention that the yield from sago hampas only was slightly higher by 7.9% as compared to the commercial glucose. In review, the VFAs yield of 0.34 g/g was achieved by Park et al. [24] using 20 g/L of rice straw pretreated with 2% sodium hydroxide. It is similar result was observed from Ibrahim et al. [29] when using 20 g/L of glucose resulted 0.33 g/g of VFAs by C. butyricum EB6. All in all, even though there is few research focusing on the production of VFAs from the degradation of biomass, this study has contributed the additional knowledge in this area of interest. Therefore, the potential value of sago hampas in the production of VFAs has successfully been identified. To further improve the VFAs production from sago hampas, the fermentation factors affecting the production should be determined using one-factor-at a-time (OFAT).
Physical factors affecting VFAs production
Enhancement of VFAs production from sago hampas was done by determining the factors affecting the anaerobic digestion of sago hampas via one-factor-at-a-time (OFAT). The physical factors that have been studied in this present work, including sago hampas concentration, yeast extract concentration and the additional of inorganic nitrogen.
Sago hampas concentration
Figure 3 illustrates the VFAs production using different concentrations of sago hampas by C. beijerinckii SR1 in anaerobic fermentation process. Concentration of nitrogen source, which is yeast extract was fixed at 1 g/L for all substrate concentration. Carbon sources concentration was crucial to fermentation process as it effects production rate, product yield and total cost of whole process [31, 44]. Figure 3 illustrated the VFAs produced from different concentration of sago hampas at 96 h, since all concentration of carbon sources showed the maximum total VFAs being produced during the fermentation. It is interesting to mention that C. beijerinckii SR1 employed has been determined to produced 4.64 ± 0.08 U/mL of maximum glucoamylase activity, which the key enzyme in conversion of starch in sago hampas into glucose. Even though the theoretical yield of VFAs from glucose has not yet fully understood, the acetic acids and glucose are both share the same number of electrons, which is 8 per molecules, while butyric acid has 20 per molecules [24]. This matter is due to the similarity role of VFAs to glucose, which is they both can be used for cell formation, cell maintenance as well as formation of products [45]. Park et al. [24] has reported that the yield of VFAs over glucose was 0.35 g/g using 20 g/L of pretreated rice straw by mixed culture.
It was observed that 3% (w/v) of sago hampas successfully produced higher total VFAs (6.99 g/L) as compared to others. Interestingly, the remaining starch determined at the end of fermentation was 0.37 g/L, which makes the total of 98% of starch degraded during the fermentation by C. beijerinckii SR1. The production of VFAs was improved by 38.7% as compared to 4% (w/v) sago hampas (Table 2). Nevertheless, the production of VFAs was gradually increase for the anaerobic digestion of sago hampas using 10–50 g/L. However, 70 g/L of sago hampas loading has produced lower total VFAs production. As for the concentration of 10–50 g/L of substrate, the increase in the availability of carbon source to be converted into VFA was directly proportional. However, further increase in substrate concentration would affect the viscosity of the fermentation medium, which further decrease the dynamic equilibrium of the system and productivity. This situation was in supported by previous study, where the utilization of > 60 g/L of substrate loading has reduced the fermentation performance due to the pseudoplastic behaviour exhibited by sago starch which made the fermentation medium became more viscous [1, 6, 31]. In addition, the starch granules inside complex material might be difficult to liberate by the enzymes of C. beijerinckii SR1 at higher concentration of substrate thus producing less total VFAs [6].
On the contrary, in this study, it is found out that the butyric acid production was observed to be higher as compared to acetic acid. This situation might be due to the ability of C. beijerinckii SR1 in producing biobutanol in its metabolic pathway. This is further explained by Desai et al. [46] that butyrate is a preferential intermediate in Clostridia sp. for biobutanol formation. This situation was observed the same for all set of sago hampas concentration loading. Butyrate is known as the inducer for Clostridia sp. in the production of biobutanol in solventogenesis [47]. Butyrate produced by Clostridium sp. was used back by the bacterium in their metabolic pathway for the production of acetoacetate from acetoacetyl-CoA and butyryl-CoA for the production of butyraldehyde as an intermediate product for n-butanol. While acetate was derived from acetyl-CoA with the presence of phosphotransacetylase (Pta). Therefore, the concentration of butyric acid excreted out the cell is higher as compared to acetic acid. This phenomenal was observed to be in agreement in most studies related to Clostridia sp.[1, 31, 36, 48].
Yeast extract concentration
Based on the result obtained in Fig. 4, the higher VFAs production recorded was at 3 g/L yeast extract (YE) loading with the total production of 7.27 g/L of total VFAs. Since the total starch consumed was 17.02 g/L, the VFAs yield recorded was 0.427 g/g, in which the increment of 1.04-fold as compared to previous experiment (3% substrate loading with 1 g/L of YE). It is also observed that the production of VFAs was gradually increase with the increment of YE concentration from 0.5–3 g/L. However, the production of VFAs was declined by 4.3% in 5.0 g/L of YE concentration as compared to 3.0 g/L of the YE loading. This situation is due to the C/N ratio (for 5.0 g/L) is 3.48, which considerably low for the fermentation has led to the limitation of carbon as suggested by Md Salleh et al. [31].
Study by Khani et al. [49] shown that nitrogen sources play a significant role as their nutrient was directly linked to the cell proliferation and metabolites biosynthesis. Yeast extract (YE) was an organic nitrogen sources that commonly used in fermentation industries and was a favoured in producing VFAs and other by-products [24, 44]. YE has provided various growth factors, including amino acids, minerals and vitamins that promote good growth of microorganism. As demonstrated by Md Salleh et al. [31], different types of starchy biomass have different influential relation with YE. The aforementioned author has obtained higher max cell density when using starch from sago hampas (2.49 g/L) as compared to tapioca (1.12 g/L), potato (1.89 g/L) and Corn (2.04 g/L). However, different pattern in VFAs production was observed, whereby higher VFAs production was obtained from potato (3.44 g/L) as compared to sago hampas (1.51 g/L). Since sago hampas has lower degree of polymerization (2.45) [50] correlates with enhanced growth of microorganism, which further increase the production of value-added products. This situation indicates that higher degree of polymerization of starch in potato has negative correlation with YE since it will contributes to higher accumulation of acids which eventually inhibit the growth of microorganism [31, 51, 52].
In comparison to that, this study showed higher total VFAs production as compared to Wang et al. [44] whereby the aforementioned authors obtained 4.34 and 5.27 g/L of total VFAs from 3 g/L of YE using waste potato starch (10 g/L) and molasses (22.5 g/L), respectively, as a carbon source by C. butyricum W5. Since the organic nitrogen source, such as yeast extract has great influenced in cell growth as well as acid production [31, 49, 53], the optimum C/N ratio of fermentation should be further understood. This matter is due to the fact that organic nitrogen has certain growth factor (including peptides and free amino acids) that could be simply absorbed by the cell to improve in anaerobic fermentation [54] Table 3 illustrates the summary of VFAs production from different concentration of YE loading.
Based on Table 3, the result obtained shows that the increment in C/N ratio has proportional increment in cell density of C. beijerinckii SR1. On the other hand, it is also can be observed that the increase in total VFAs produced has negative effect on the maximum cell density (except for 5 g/L of YE as per discussed earlier). This situation is explained by Khani et al. [49] and Md Salleh et al. [31], since high acid formation in the fermentation medium gave “acid-crush” effect contributed to lower bacteria growth [49, 55]. As suggested by Md Salleh et al. [31], lower in C/N ratio (3.6) has contributed to higher total VFAs production (3.05 g/L). Based on this study, slightly higher C/N ratio (5.8) was observed to give higher total VFAs production (7.27 g/L) as compared to the aforementioned author. On the other hand, Park et al. [24] found out that the optimal C/N ratio and the presence of inhibitors (furfural and HMF) has greatly affect the production of VFAs from rice straw by consortia. All in all, this study suggested that the optimum C/N ratio for VFAs production from sago hampas by C. beijerinckii SR1 is in the range of 3.48–17.4, with the optimum C/N is 5.8.
Addition of inorganic nitrogen sources
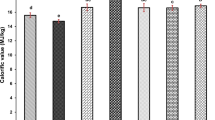
The development and production of bacteria usually depend on the availability of nutrient, including inorganic nitrogen in the medium. When considering the growth, nitrogen is becoming the limiting factor; therefore, the assimilation and metabolism of the presence nitrogen in the medium is one of the crucial factors for the cell to growth, further produce the desired products. Thus, this study has been conducted to investigate the effect of additional of inorganic nitrogen sources to improve the production of VFAs by C. beijerinckii SR1 using sago hampas as a substrate. Figure 5 illustrates the production of VFAs from 2 g/L of various types of inorganic nitrogen sources.
Volatile fatty acids production has complex relationship with the nitrogen sources due to their different in synthetic origin [56, 57]. Based on the result obtained, the additional of ammonium nitrate in the fermentation medium has produced higher total VFAs (7.69 g/L) as compared to ammonium chloride (6.09 g/L) and ammonium sulphate (7.16 g/L). This situation was also can be observed in the study made by Md Salleh et al. [31], where the ammonium nitrate has given slightly better performance in terms of cell growth of C. acetobutylicum (5 and 24% improvement) and the production of VFAs (1.5-fold and 1.2-fold) as compared to ammonium chloride and ammonium sulphate, respectively. It was observed that by the addition of ammonium nitrate, the production of VFAs was improved by 5.8%. At the same instant, the VFAs yield recorded was also improved for additional ammonium nitrate (0.451 g/g) by 0.1.07-fold as compared to YE only (0.421 g/g). From this result, it is suggested that the mixture of organic and inorganic nitrogen is necessary by the anaerobic digestion of sago hampas by C. beijerinckii SR1 to bacterial growth as well as product formation. This matter can be partially explained by the anions from ammonium salts supplied by NH4NO3 could be metabolized by Clostridia sp. with a positive effect during the accumulation of acids [58].
The additional of ammonium nitrate as inorganic nitrogen into the anaerobic fermentation of Clostridia sp. has supportive effect specifically on growth of the microorganism as well as amylase production [59] and overcome the risk of insufficient assimilable nitrogen source which leads to lower biomass yields and consequently slower fermentation rates [57]. Furthermore, Wang et al. [44] has demonstrated by utilizing ammonium nitrate as inorganic nitrogen source has significantly decreased the lag phase for C. butyricum W5, in which increase the productivity of product of interest and increase the production of acetic and butyric acids. Thus, these findings have supported the results obtained in this study in the utilization of inorganic nitrogen source, such as ammonium nitrate could possible support the growth of the bacterium and further it proved that this nitrogen is suitable nitrogen source for the production of VFAs. The use of the cheaper carbon source as well as nitrogen source would greatly enhance the potential of this technology to commercialization.
Bioelectricity generation from VFAs-based sago hampas hydrolysate
The power density generated in MFCs by C. beijerinckii SR1 from commercial VFAs (acetate and butyrate) and VFAs-based sago hampas hydrolysate (VFABSHH) was conducted in MFCs. Since VFABSHH contained acetate and butyrate, C. beijerinckii SR1 was subjected to use commercial acetate and butyrate to determine the feasibility of this strain to generate bioelectricity in MFCs. Figure 6 shows the bioelectricity generation performance by C. beijerinckii SR1 from acetate, butyrate and VFABSHH.
Based in Fig. 6a, C. beijerinckii SR1 has generate slightly higher OVC from acetate as compared to butyrate. The OVC obtained from acetate was 297.9 mV, which is 9% higher as compared to butyrate (273.4 mV). Addition to that, the maximum power density generated (Fig. 6b) from acetate (78.4 mW/m2) was found higher as compared to butyrate (71.6 mW/m2). This situation was in agreement to various studies [19, 60,61,62]. In comparison to the study made by Chae et al. [63], this study shown better maximum power density for both acetate (64.3 mW/m2) and butyrate (51.4 mW/m2), in which 21.9 and 39.2% of enhanced power density, respectively. The consistent results were observed in Liu et al. [62], where higher power density was obtained by acetate (506 mW/m2) as compared to butyrate (305 mW/m2). This situation was observed by Desai et al. [46] in Clostridium sp., where the acetate has significant impact in ATP generation during cell growth which provides the free energy to the cell, meanwhile the butyrate was reutilized for the formation of acetone and butanol. All things considered, C. beijerinckii SR1 is suggested to be able to generate the bioelectricity from VFAs.
Then, the VFABSHH was subjected as a carbon source in bioelectricity generation by C. beijerinckii SR1. From the result obtained, the OVC recorded and the maximum power density were 273.4 mV and 61.5 mW/m2. As compared to commercial acetate and butyrate, the OVC generated was almost comparable to both carbon source, however the maximum power density generated was slightly lower. It is interesting to mention that the VFABSHH used in this study was a mixture of VFAs produced from anaerobic digestion of sago hampas. In comparison to Li et al. [64], corn stalk fermentation effluent containing VFAs mixture has generated stable OVC of 551 mV with 391 A/m3 of current density. The huge difference in OVC generation as compared to this study is mainly due to the acclimatized mixture culture used in the aforementioned authors, in which increase the synergically and ability of mixture culture for the generation of bioelectricity. Since that there are limited information on the generation of bioelectricity from VFAs-based biomass hydrolysate in the literature, the integration of mixture VFAs towards the power density is yet fully understood.
In comparison, this study shows higher PD generation as compared to Min et al. [65], whereby the aforementioned author used 2.82 g/L of acetate as a main carbon source to generate 40 mW/m2 by G. metallireducens. Geobacter sp. is a well-known bioelectricity producer and been employed in several studies [66,67,68]. On the other hand, higher PD (195 mV/m3) has been recorded by Li et al. [69], which using mixed culture from food waste leachate inoculated in 12 g/L of total VFAs in food waste. The aforementioned author also reported that the dominant microbial community involved in the mixed culture from food waste leachate are Clostridia sp. and Bacteroides sp., which further explained to have symbiosis effect in the generation of bioelectricity from food waste. The great difference in PD as compared to this study was mainly due to the fact that this study was employed single wild culture, C. beijerinckii SR1 for bioelectricity generation.
Conclusions
As a conclusion, the anaerobic digestion of sago hampas by C. beijerinckii SR1 has successfully produced a comparable amount of total VFAs production with commercial glucose in which 5.04 and 4.96 g/L of total VFAs, respectively, that contained 18% of acetic acid and 82% of butyric acid. Enhanced total VFAs has obtained using 3% (w/v) of initial sago hampas loading, 3.0 g/L of initial yeast extract and addition of 2 g/L of ammonium nitrate resulted 7.69 g/L of the total VFAs produced, which 52.6% of increment recorded. Thus, the application of VFAs hydrolysate obtained was demonstrated on bioelectricity generation resulted 273.4 mV of open voltage circuit generated and 61.5 mW/m2 of power density recorded.
Abbreviations
- C/N:
-
Carbon per nitrogen
- PEM:
-
Proton exchange membrane
- OFAT:
-
One factor at a time
- RCM:
-
Reinforced clostridium media
- VFAs:
-
Volatile fatty acids
- YE:
-
Yeast extract
References
Husin H, Ibrahim MF, Kamal Bahrin E, Abd-Aziz S (2019) Simultaneous saccharification and fermentation of sago hampas into biobutanol by Clostridium acetobutylicum ATCC 824. Energy Sci Eng 7:66–75. https://doi.org/10.1002/ese3.226
Jenol MA, Ibrahim MF, Bahrin EK et al (2019) Direct bioelectricity generation from sago hampas. Molecules 24:2397
Awg-Adeni DS, Abd-Aziz S, Bujang KB, Hassan MA (2010) Bioconversion of sago residue into value added products. African J Biotechnol 9:2016–2021
Jenol MA, Ibrahim MF, Yee PL et al (2014) Sago biomass as a sustainable source for biohydrogen production by Clostridium butyricum A1. BioResources 9:1007–1026
Linggang S, Yee PL, Wasoh MH, Abd-Aziz S (2012) Sago pith residue as an alternative cheap substrate for fermentable sugars production. Appl Biochem Biotechnol 167:122–131. https://doi.org/10.1007/s12010-012-9592-0
Awg-Adeni DS, Bujang KB, Hassan MA, Abd-Aziz S (2013) Recovery of glucose from residual starch of sago hampas for bioethanol production. Biomed Res Int. https://doi.org/10.1155/2013/935852
Awg-Adeni DS (2015) Bioethanol production from residual starch of sago (Metroxylon sago) hampas. Doctoral thesis, Universiti Putra Malaysia, Selangor, Malaysia. Retrieved from http://psasir.upm.edu.my/id/eprint/66550/1/FBSB%202015%2026%20IR.pdf
Poh PE, Chong MF (2009) Development of anaerobic digestion methods for palm oil mill effluent (POME) treatment. Bioresour Technol 100:1–9. https://doi.org/10.1016/j.biortech.2008.06.022
Huang W, Huang W, Yuan T et al (2016) Volatile fatty acids (VFAs) production from swine manure through short-term dry anaerobic digestion and its separation from nitrogen and phosphorus resources in the digestate. Water Res 90:344–353. https://doi.org/10.1016/j.watres.2015.12.044
Sawatdeenarunat C, Sung S, Khanal SK (2017) Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour Technol 237:139–145. https://doi.org/10.1016/j.biortech.2017.02.029
Jin X, Li X, Zhao N et al (2017) Bio-electrolytic sensor for rapid monitoring of volatile fatty acids in anaerobic digestion process. Water Res 111:74–80. https://doi.org/10.1016/j.watres.2016.12.045
Lee WS, Chua ASM, Yeoh HK, Ngoh GC (2014) A review of the production and applications of waste-derived volatile fatty acids. Chem Eng J 235:83–99. https://doi.org/10.1016/j.cej.2013.09.002
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381
Toczyłowska-Mamińska R, Szymona K, Król P et al (2018) Evolving microbial communities in cellulose-fed microbial fuel cell. Energies 11:1–12. https://doi.org/10.3390/en11010124
Jung S, Regan JM (2007) Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl Microbiol Biotechnol 77:393–402. https://doi.org/10.1007/s00253-007-1162-y
Borole AP, Hamilton CY, Vishnivetskaya T et al (2009) Improving power production in acetate-fed microbial fuel cells via enrichment of exoelectrogenic organisms in flow-through systems. Biochem Eng J 48:71–80. https://doi.org/10.1016/j.bej.2009.08.008
De Cárcer DA, Ha PT, Jang JK, Chang IS (2011) Microbial community differences between propionate-fed microbial fuel cell systems under open and closed circuit conditions. Appl Microbiol Biotechnol 89:605–612. https://doi.org/10.1007/s00253-010-2903-x
Yu J, Park Y, Kim B, Lee T (2015) Power densities and microbial communities of brewery wastewater-fed microbial fuel cells according to the initial substrates. Bioprocess Biosyst Eng 38:85–92. https://doi.org/10.1007/s00449-014-1246-x
Freguia S, Teh EH, Boon N et al (2010) Microbial fuel cells operating on mixed fatty acids. Bioresour Technol 101:1233–1238. https://doi.org/10.1016/j.biortech.2009.09.054
Teng SX, Tong ZH, Li WW et al (2010) Electricity generation from mixed volatile fatty acids using microbial fuel cells. Appl Microbiol Biotechnol 87:2365–2372. https://doi.org/10.1007/s00253-010-2746-5
Choi JDR, Chang HN, Han JI (2011) Performance of microbial fuel cell with volatile fatty acids from food wastes. Biotechnol Lett 33:705–714. https://doi.org/10.1007/s10529-010-0507-2
Chang HN, Kim NJ, Kang J, Jeong CM (2010) Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol Bioprocess Eng 15:1–10. https://doi.org/10.1007/s12257-009-3070-8
Md Salleh M (2016) Application of fed batch system in the production of biobutanol by Clostridium sp using lemongrass leaves hydrolysate. In: 2016 Korean Society for Biotechnology Spring Conference and International Symposium, Korea, Apr 2016. Korean Society for Biotechnology
Park GW, Kim I, Jung K et al (2015) Enhancement of volatile fatty acids production from rice straw via anaerobic digestion with chemical pretreatment. Bioprocess Biosyst Eng 38:1623–1627. https://doi.org/10.1007/s00449-015-1387-6
Zhou Z, Meng Q, Yu Z (2011) Effects of methanogenic inhibitors on methane production and abundances of methanogens and cellulolytic bacteria in in vitro ruminal cultures. Appl Environ Microbiol 77:2634–2639. https://doi.org/10.1128/AEM.02779-10
Basu S, Agarwal A, Pramanik H (2008) Improvement in performance of a direct ethanol fuel cell: effect of sulfuric acid and Ni-mesh. Electrochem commun 10:1254–1257. https://doi.org/10.1016/j.elecom.2008.05.042
Nakamura LK (1981) Lactorbacillus amylovorus, a new starch-hydrolyzing species from cattle waste-corn fermentations. Int J Syst Bacteriol 31:56–63
Goering HK, Van Soest PJ (1970) Forage fiber analyses: apparatus, reagents, procedures, and some applications (No. 379). Agricultural Research Service, US Department of Agriculture, Washington, DC
Ibrahim MF, Abd-Aziz S, Razak MNA et al (2012) Oil palm empty fruit bunch as alternative substrate for acetone-butanol-ethanol production by Clostridium butyricum EB6. Appl Biochem Biotechnol 166:1615–1625. https://doi.org/10.1007/s12010-012-9538-6
McGill R, Tukey JW, Larsen WA (1978) Variations of box plots. Am Stat 32:12–16. https://doi.org/10.1080/00031305.1978.10479236
Md Salleh M, Ariff AB, Sahaid KM et al (2001) Direct fermentation of gelatinized sago starch to acetone-butanol-ethanol by Clostridium acetobutylicum. World J Microbiol Biotechnol 17:567–576. https://doi.org/10.1023/A:1012351112351
Xue C, Zhao J, Chen L et al (2017) Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnol Adv 35:310–322. https://doi.org/10.1016/j.biotechadv.2017.01.007
Linggang S, Phang LY, Wasoh H, Abd-Aziz S (2013) Acetone-Butanol-Ethanol production by Clostridium acetobutylicum ATCC 824 using sago pith residues hydrolysate. Bioenergy Res 6:321–328. https://doi.org/10.1007/s12155-012-9260-9
Sun X, Atiyeh HK, Adesanya YA et al (2019) Enhanced acetone-butanol-ethanol production by Clostridium beijerinckii using biochar. ASABE Annu Int Meet. https://doi.org/10.13031/aim.201900256
Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781
Ibrahim MF, Linggang S, Jenol MA et al (2015) Effect of buffering system on acetone-butanol-ethanol fermentation by Clostridium acetobutylicum ATCC 824 using pretreated oil palm empty fruit bunch. BioResources 10:3890–3907. https://doi.org/10.15376/biores.10.3.3890-3907
Dürre P (2008) Fermentative butanol production: bulk chemical and biofuel. Ann N Y Acad Sci 1125:353–362. https://doi.org/10.1196/annals.1419.009
Chong M-L, Abdul Rahman NA, Rahim RA et al (2009) Optimization of biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent using response surface methodology. Int J Hydrogen Energy 34:7475–7482. https://doi.org/10.1016/j.ijhydene.2009.05.088
Chong M-L, Rahim RA, Shirai Y, Hassan MA (2009) Biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent. Int J Hydrogen Energy 34:764–771. https://doi.org/10.1016/j.ijhydene.2008.10.095
Liu H, Huang D, Wen J (2016) Integrated intracellular metabolic profiling and pathway analysis approaches reveal complex metabolic regulation by Clostridium acetobutylicum. Microb Cell Fact 15:1–14. https://doi.org/10.1186/s12934-016-0436-4
Yang X, Tu M, Xie R et al (2013) A comparison of three pH control methods for revealing effects of undissociated butyric acid on specific butanol production rate in batch fermentation of Clostridium acetobutylicum. AMB Express 3:3. https://doi.org/10.1186/2191-0855-3-3
Hung HC, Awg-Adeni DS, Johnny Q, Vincent M (2018) Production of bioethanol from sago hampas via simultaneous saccharification and fermentation (SSF). Nusant Biosci 10:240–245. https://doi.org/10.13057/nusbiosci/n100407
Sindhu R, Binod P, Pandey A (2016) α-Amylases. Curr Dev Biotechnol Bioeng Prod Isol Purif Ind Prod. https://doi.org/10.1016/B978-0-444-63662-1.00001-4
Wang X, Jin B, Mulcahy D (2008) Impact of carbon and nitrogen sources on hydrogen production by a newly isolated Clostridium butyricum W5. Int J Hydrogen Energy 33:4998–5005. https://doi.org/10.1016/j.ijhydene.2008.07.078
Chang HN, Kim NJ, Kang J et al (2011) Multi-stage high cell continuous fermentation for high productivity and titer. Bioprocess Biosyst Eng 34:419–431. https://doi.org/10.1007/s00449-010-0485-8
Desai RP, Harris LM, Welker NE, Papoutsakis ET (1999) Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab Eng 1:206–213
Buehler EA, Mesbah A (2016) Kinetic study of acetone-butanol-ethanol fermentation in continuous culture. PLoS ONE 11:1–21. https://doi.org/10.1371/journal.pone.0158243
Razak MNA, Ibrahim MF, Yee PL et al (2013) Statistical optimization of biobutanol production from oil palm decanter cake hydrolysate by Clostridium acetobutylicum ATCC 824. BioResources 8:1758–1770
Khani M, Bahrami A, Chegeni A et al (2016) Optimization of carbon and nitrogen sources for extracellular polymeric substances production by Chryseobacterium indologenes MUT2. Iran J Biotechnol 14:13–18. https://doi.org/10.15171/ijb.1266
Ehara H, Toyoda Y, Johnson DV (2018) Sago palm: multiple contributions to food security and sustainable livelihoods. Springer, Singapore
Fernández-Naveira Á, Veiga MC, Kennes C (2017) H-B-E (hexanol-butanol-ethanol) fermentation for the production of higher alcohols from syngas/waste gas. J Chem Technol Biotechnol 92:712–731. https://doi.org/10.1002/jctb.5194
Shi X, Lin J, Zuo J et al (2017) Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J Environ Sci (China) 55:49–57. https://doi.org/10.1016/j.jes.2016.07.006
Jiang J, Zhang Y, Li K et al (2013) Volatile fatty acids production from food waste: effects of pH, temperature, and organic loading rate. Bioresour Technol 143:525–530. https://doi.org/10.1016/j.biortech.2013.06.025
Zhang JN, Li YH, Zheng HQ et al (2015) Direct degradation of cellulosic biomass to bio-hydrogen from a newly isolated strain Clostridium sartagoforme FZ11. Bioresour Technol 192:60–67. https://doi.org/10.1016/j.biortech.2015.05.034
Shanmugam S, Sun C, Zeng X, Wu YR (2018) High-efficient production of biobutanol by a novel Clostridium sp. strain WST with uncontrolled pH strategy. Bioresour Technol 256:543–547. https://doi.org/10.1016/j.biortech.2018.02.077
Vilanova M, Ugliano M, Varela C et al (2007) Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl Microbiol Biotechnol 77:145–157. https://doi.org/10.1007/s00253-007-1145-z
Torrea D, Varela C, Ugliano M et al (2011) Comparison of inorganic and organic nitrogen supplementation of grape juice—effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem 127:1072–1083. https://doi.org/10.1016/j.foodchem.2011.01.092
Galadima AI, Deba AA, Mienda S, Wante SP (2015) Optimum utilization of Clostridia species towards biofuel production. Int J Curr Sci 14:82–90
Tran HTM, Cheirsilp B, Umsakul K, Bourtoom T (2011) Response surface optimisation for acetone-butanol-ethanol production from cassava starch by co-culture of clostridium butylicum and bacillus subtilis. Maejo Int J Sci Technol 5:374–389
Finch AS, Mackie TD, Sund CJ, Sumner JJ (2011) Metabolite analysis of Clostridium acetobutylicum: fermentation in a microbial fuel cell. Bioresour Technol 102:312–315. https://doi.org/10.1016/j.biortech.2010.06.149
Poincare H, Nancy I (1976) Carbon and electron flow in Clostridium butyricum grown in chemostat culture on glycerol and on glucose. Microbiology 142:1149–1158. https://doi.org/10.1099/13500872-142-5-1149
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39:658–662. https://doi.org/10.1021/es048927c
Chae KJ, Choi MJ, Lee JW et al (2009) Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol 100:3518–3525. https://doi.org/10.1016/j.biortech.2009.02.065
Li X, Zhang R, Qian Y et al (2017) The impact of anode acclimation strategy on microbial electrolysis cell treating hydrogen fermentation effluent. Bioresour Technol 236:37–43. https://doi.org/10.1016/j.biortech.2017.03.160
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686. https://doi.org/10.1016/j.watres.2005.02.002
Esteve-Núñez A, Sosnik J, Visconti P, Lovley DR (2008) Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ Microbiol 10:497–505. https://doi.org/10.1111/j.1462-2920.2007.01470.x
Shi L, Richardson DJ, Wang Z et al (2009) The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer. Environ Microbiol Rep 1:220–227. https://doi.org/10.1111/j.1758-2229.2009.00035.x
Krige A, Sjöblom M, Ramser K et al (2019) On-line Raman spectroscopic study of cytochromes’ redox state of biofilms in microbial fuel cells. Molecules 24:646. https://doi.org/10.3390/molecules24030646
Li XM, Cheng KY, Selvam A, Wong JWC (2013) Bioelectricity production from acidic food waste leachate using microbial fuel cells: effect of microbial inocula. Process Biochem 48:283–288. https://doi.org/10.1016/j.procbio.2012.10.001
Acknowledgements
The authors gratefully acknowledge the MyBrain 15 from Malaysian Higher Education Ministry for the financial support. We would like to share our greatest attitude to all the members of the Environmental Biotechnology Research Group, Universiti Putra Malaysia for their help and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jenol, M.A., Ibrahim, M.F., Kamal Bahrin, E. et al. Enhanced volatile fatty acid production from sago hampas by Clostridium beijerinckii SR1 for bioelectricity generation using microbial fuel cells. Bioprocess Biosyst Eng 43, 2027–2038 (2020). https://doi.org/10.1007/s00449-020-02391-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02391-9