Abstract
Light and bacteria can be used in combination to enhance secondary metabolite production during fermentation. Red yeast rice powder (RYRP) was inoculated with Bacillus subtilis (B2) isolated from freshwater seafood and incubated under light-emitting diodes (LEDs) of different colors (blue, green, red, white), fluorescent white light, and in darkness. Blue LED-mediated fermentation with B2 significantly enhanced production of phenolic compounds (68.4 ± 1 mg GAE/g DW) and flavonoids (51.7 ± 1 mg QE/g DW) compared to white light and darkness. Total antioxidant activity of RYRP extract after fermentation with B2 was > 77%; hydroxyl radical and superoxide scavenging were > 66%. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) and ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) radical scavenging activities were 51% and > 67%, respectively. Reducing power was approximately twice that of extract from RYRP without B2. FTIR analysis showed a high content of hydroxyl, nitrile and carboxylic groups in the extract. Derivatives of cinnamic, benzoic and phophinodithioic acid, and quinazolinone were identified by GC–MS. Findings show that fermenting RYRP with B. subtilis B2 under blue LEDs enhances production of secondary metabolites, which should have applications in industrial fermentation processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Light can be a modulating factor in the production of secondary metabolites during microbial fermentation. Chemotrophic and non-phototrophic bacteria have photoreceptor and photo-physiological sensing genes that encode photoreceptor proteins [1]. Photoreceptors include blue light sensing using flavin adenine dinucleotide (BLUF) cryptochrome; light, oxygen, voltage (LOV); photoactive yellow protein (PYP); rhodopsin; and phytochrome [1, 2]. Davis et al. [3] discovered red light sensors and bacteriophytochromes in the non-phototrophic bacteria Deinococcus radiodurans and Pseudomonas aeruginosa. Subsequent research has shown that LOV photoreceptors are common in non-phototrophic bacteria [2]. For example, non-phototrophic Bacillus subtilis strains contain LOV and BLUF photoreceptors [1].
Light of different wavelengths (sunlight, white light and light-emitting diodes) has been shown to increase secondary metabolite production during fermentation [4]. Light-emitting diodes (LEDs) are particularly effective. LEDs emit a range of wavelengths, with color dependent on the wavelength and semiconductor material [5]. LEDs can have a major impact on the production of biotechnological and economically valuable substances, such as food additives, pharmaceuticals, beauty care products, and biofuels [4]. Ma et al. [6] reported increased fatty acid production by Nannochloropsis biomass under high-intensity LEDs, while low-intensity LEDs promoted carotenoid and eicosapentaenoic acid yields. Astaxanthin production by the green alga Haematococcus pluvialis was enhanced under blue light [7]. Cyanobacteria react to red, green, blue and ultraviolet light via phytochrome, rhodopsin and cryptochrome [8]. More phycobiliprotein and phycocyanin were produced under white and blue light [9]. Oh et al. [10] found that LEDs increased total phenol, flavonoid and reducing sugar production during fermentation of blueberry powder with Lactobacillus brevis and Bacillus amyloliquefaciens, and the phenolic metabolites exhibited effective antibacterial and antioxidant activities.
Bacillus subtilis is a rod-shaped, Gram-positive endospore-forming bacterium, capable of butanediol, glutamic acid, acetoin, sugar and vitamin production via fermentation; B. subtilis also can hydrolyze triglycerides and produce citrate permease and cytochrome C [11, 12]. B. subtilis is used to produce pharmaceutical products such as α-amylase, proinsulin, lipase A, streptavidin, endocellulase and endogulcanase [13]. Probiotics containing Bacillus species such as B. clausii, B. coagulans and B. subtilis have been shown to be highly effective in preventing gastrointestinal disorders [14]. B. subtilis has been widely studied at physiological and molecular levels and it is contained in probiotic products for human, veterinary, and aquaculture use [15]. Yellow soybean fermented with B. subtilis had higher free phenolic and amino acid contents and enhanced antioxidant properties [16]. Fermenting black soybeans with B. subtilis similarly increased phenolic and flavonoid compounds, as well as aglycone and vitamin K2 contents, and the products showed high antioxidant activity [17].
Red yeast rice (RYR) is a nutrition supplement prepared from fermented rice via the mold Monascus purpureus; RYR is consumed to ameliorate indigestion and hyperlipidemia and red yeast rice powder (RYRP) is accepted as a food ingredient by the Korea Food and Drug Administration (KFDA) [18]. RYR secondary metabolites include ankaflavin, rubropunctatin, monascorburin, rubropunctamine, and monascorburamine, xanthomonasin, monacolin K, γ-aminobutyric acid, citrinin and amino acids [19]. Juan and Chou [17] reported that B. subtilis-fermented products enhance antioxidant activity associated with a reduced risk of disease. B. subtilis is used to produce fermented soybean products such as Indian kinema and Japanese natto, Korean Chungkookjang, and is an efficient producer of secondary metabolites [20, 21]. RYR fermentation with B. subtilis produced lovastatin, an effective cholesterol synthesis inhibitor, and enhanced antioxidant activity [18]. Importantly, RYR has the potential to be a superior substrate for solid-state fermentation [22]. Solid-state fermentation is carried out in the absence of added water; the substrate holds sufficient humidity for microbial growth and metabolic activity. The bacteria utilize carbon and obtain macro and minor nutrients from solid substrate. The products of solid-state RYR fermentation have desirable biological properties, antioxidant activity, and pigment intensity [16, 23].
The objective of the present research was to compare secondary metabolite production under light-mediated solid-state fermentation of red yeast rice powder inoculated with a B. subtilis isolate under LEDs of different colors (blue, green, red and white) to production under fluorescent white light and in the dark. Phenolic and flavonoid metabolites were identified and their antioxidant activity was assayed.
Materials and methods
Materials
Red yeast rice powder (RYRP) was obtained from Mfoods. LTD (Gyeongsangbuk-do, Gyeongju, Republic of Korea); 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), gallic acid, quercetin, ascorbic acid and pyrogallol were purchased from Sigma (St. Louis, MO, USA). Luria Bertani (LB) medium was purchased from Difco laboratories (Becton, Dickson and Co., USA) and other chemicals and solvents were purchased from DAEJUNG Reagents and Chemicals Co. Ltd (Korea). LEDs were purchased from HM Trade Co. (Korea).
Bacteria isolation, DNA extraction and amplification
The bacterial strain B. subtilis B2 was isolated from freshwater shrimp salted seafood using LB medium containing 1% tryptone, 0.5% yeast and 0.5% NaCl. Microbiological pour plates were used and isolated colonies were streaked to obtain a pure culture. An overnight pure bacterial liquid culture (2 mL) was added to 50% glycerol (2 mL) in a 5-mL screw cap tube, the tubes were stored at − 20 °C for maintenance of the stock culture. The stock culture was subcultured and used for all experiments.
The procedure of Rajasekar et al. [24] was used for molecular identification of the B2 isolate. Bacterial DNA was extracted using a genomic DNA extraction kit (Bio-Rad, USA). The purity of the genomic DNA was checked using a NanoDropTM 2000 spectrophotometer. The genomic DNA was amplified by polymerase chain reaction (PCR) using primers 27F (forward primer) 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R (reverse primer) 5′-ACGGCTACCTTGTTACGACTT-3′. PCR was performed with a 50-µL reaction mixture containing 2 µL (10 ng) of DNA as the template, each primer at a concentration of 0.5 µM, 1.5 mM MgCl2, and each dNTP at a concentration of 50 µM, as well as 1 µL of Taq DNA polymerase and buffer, as recommended by the manufacturer (MBI Fermentas Kit). PCR was carried out with a Mastercycler Personal thermal cycler (Eppendorf) with the following procedure: initial denaturation at 95 °C for 1 min; 40 cycles of denaturation (3 min at 95 °C); annealing (1 min at 55 °C), and extension (2 min at 72 °C); followed by a final extension (5 min at 72 °C). Unincorporated PCR primers and dNTPs were eliminated from PCR products with a Montage PCR Clean up kit (Millipore).
Gene sequencing, similarity, and phylogenetic analysis
The amplified DNA was partially sequenced by 16S rRNA gene sequencing using the Big Dye terminator cycle sequencing kit (Applied BioSystems, USA). Sequencing products were resolved on an Applied Biosystems model 3730XL automated DNA sequencing system. Relatedness of the gene sequence of strain B2 (1182 base pairs) to reference sequences available in the Nucleotide Database of the National Center for Biotechnology Information (NCBI) was determined using the basic local alignment search tool (BLASTn). Reference sequences of 16S rDNA genes from Bacillus spp. were downloaded and multiple nucleotide alignments of the selected sequences were made using ClustalX. A phylogenetic tree was constructed (neighbor-joining method) using MEGA version 3.1 and 1,000 bootstrap replications were carried out to validate internal branches. The B2 gene sequence was submitted to NCBI to obtain an accession number.
Bacterial growth under light
B2 cells (100 µL) from the stock culture were inoculated into 150 mL of autoclaved LB broth in 250-mL Erlenmeyer flasks. The flasks were incubated in a box on a rotatory shaker at 37 °C and 150 rpm overnight under light conditions (light intensity 150 µmol/m2/s): fluorescent white light (400 nm), darkness (360 nm), blue LEDs (460–490 nm), red LEDs (620–645 nm), green LEDs (520–550 nm), and white LEDs (380–780 nm). The box contained LED chambers and a temperature controller with a 12 V power supply. Bacterial growth rate was measured by optical density at 600 nm at 4, 8, 12 and 16 h.
Solid-state fermentation
12 g of RYRP was suspended (1:8 w/v) in 150 mL sterilized distilled water containing 1% tryptone and 0.5% NaCl in a 250-mL Erlenmeyer flask. The pH was adjusted to 7.0 using HCl and NaOH and the substrate was autoclaved at 121 °C for 30 min to eradicate native microbiota and spore-forming microbes. Sterilized RYRP was inoculated with 10% (w/v) B2 culture from pre-cultured broth inoculum and the flasks were incubated under fluorescent white light, darkness and LEDs of different colors (blue, green, red, white). Controls consisting of flasks containing RYRP without B2 inoculum were used as controls. The flasks were sealed and incubated at 37 °C for 7 d in a static mode under strict aseptic conditions and 80% relative humidity. All experiments were conducted in triplicate.
Extraction of secondary metabolites
After incubation, 100 mL of 70% ethanol solution was added to the fermented RYRP and shaken at 100 rpm for 3 h at 37 °C [22]. The mixture was removed and centrifuged at 3500 rpm for 15 min; the supernatant was freeze-dried in a lyophilizer and stored in darkness at − 20 °C until analysis. Phenolic and flavonoid metabolites were quantified, major compounds identified, and antioxidant activity was assayed.
Total phenolic content (TPC) and total flavonoid content (TFC)
For TPC and TFC measurements, 0.1 g of lyophilized powder was dissolved in 10 mL of methanol/water solution (70:30 v/v), sonicated for 30 min, and centrifuged at 10,000 rpm for 10 min. TPC in the supernatant was determined by the Folin–Ciocalteu [25] method with minor modification. A 96-well microplate was used for this and all assays. Briefly, a 50 µL extract was mixed with 50 µL of the Folin–Ciocalteu reagent and 50 µL of a sodium carbonate (Na2CO3) solution (7.5%, w/v) was added. The mixture was stirred in the dark for 30 min at 25 °C and absorbance measured at 765 nm (UV–Vis absorption spectrometer, Agilent 8453, USA). The reference blank contained only water and reagents. Measurements are reported as mg of gallic acid equivalents per g of dry weight (mg GAE/g DW). TFC was determined by adapting the AlCl3 method [25] with minor modification. Briefly, 50 µL of the supernatant was added to 20 µL of NaNO2 (5%). After 5 min at 25 °C, 20 µL of AlCl3 (10%) was added, the mixture was allowed to stand for 5 min, and 130 µL of 1 mM NaOH was added. The absorbance of the solution mixture was determined at 510 nm against a water blank. Measurements are reported as mg quercetin equivalents per g dry weight of freeze-dried powder (mg QE/g DW).
FTIR and GC–MS analyses
FTIR analysis (Perkin Elmer FTIR spectrophotometer, Norwalk, CT, USA) was conducted to determine the major functional groups in extracts from fermented RYRP. Freeze-dried extract powder was analyzed in KBr pellets using the diffuse reflectance mode at a resolution of 4 cm− 1, as described by Oh et al. [10].
Major secondary metabolites were identified by GC–MS following the procedure of Gum et al. [18]. Freeze-dried powder was dissolved in methanol for analysis using an Agilent 7890A gas chromatograph with a 5975C mass-selective detector (Agilent Technologies, Santa Clara, CA, USA). Separation was performed on a 30 mm × 0.25 mm i.d. fused silica capillary column with 0.25 µm film thickness. The carrier gas was ultrapure helium at 1 mL/min and the splitless injector temperature was 280 °C. Initial column oven temperature was held at 70 °C for 4 min, then increased by 2 °C/min to 100 °C (held 2 min), increased by 5 °C/min to 200 °C (held 20 min), and increased by 10 °C/min to 280 °C (held 5 min), for a total run time of 74 min. Mass spectra were identified using the NIST05 library.
Antioxidant activity
Total antioxidant activity (TAA)
Total antioxidant activity was determined according to the method of Huang et al. [22] with minor modifications. 20 µL samples (RYRP and RYRP with B2 freeze-dried extract dissolved in 1:1 (water and methanol)) were mixed with 300 µL of a 1:1:1 solution of 0.6 M sulfuric acid, 4 mM ammonium molybdate, and 28 mM sodium phosphate. The reaction mixture was incubated for 90 min in a 95 °C water bath, cooled to room temperature, and absorbance was measured at 695 nm. TAA was calculated as µg of ascorbic acid equivalents (AAE) per mg fermented extract:
where Acontrol is the absorbance of the control (1:1:1 solution mixture only) and Asample is the absorbance of the solution mixture containing the extract sample.
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity (HRSA) was determined as described by Huang et al. [22], with a minor change. 50 µL of ferrous sulphate (1.5 mM), 50 µL of hydrogen peroxide (10 mM), and 50 µL of sodium salicylate (20 mM) were added to 50 µL samples (freeze-dried extracts of RYRP and RYRP with B2 dissolved in 1:1 water and methanol). The mixture was incubated at 37 °C for 1 h and absorbance was measured at 562 nm. Hydroxy radical scavenging activity was calculated:
where A1 is the absorbance of sample with sodium salicylate, A2 is the absorbance of sample without sodium salicylate, and A0 is the absorbance of sodium salicylate without a sample.
DPPH radical scavenging activity
DPPH activity was determined according to Oh et al. [10] with slight modification. This method is based on conversion of the colored DPPH free radical to the non-radical form (decreasing color intensity). 10 mg of freeze-dried extract was suspended in DMSO and diluted to obtain concentrations of 400, 200, 100, 50, 25, 12.5 µg/mL. 50 µL samples were mixed with 200 µL of freshly prepared 0.10 mM DPPH-DMSO mixture and kept in the dark at 37 °C for 30 min. Absorbance was measured at 517 nm. Ascorbic acid standard was prepared in DMSO (10 mg/mL). Results were expressed as a percentage of free-radical DPPH inhibition:
where Acontrol is the absorbance of the control (0.10 mM DPPH solution), and Asample is the absorbance of the 0.10 mM DPPH solution with sample.
ABTS radical scavenging capacity
Following the method of Zhou et al. [25], ABTS (9.5 mL, 7 mM) was mixed with potassium persulfate (245 µL, 100 mM), and the volume was adjusted to 10 mL with distilled water. The mixture was kept in the dark at room temperature for 18 h, then diluted with potassium phosphate buffer (0.1 M, pH 7.4), and absorbance was measured at 734 nm. 50 µL of sample (freeze-dried extracts of RYRP and RYRP dissolved in 1:1 water and methanol) was mixed with 200 µL ABTS radical solutions, allowed to react for 30 min in the dark, and absorbance was measured at 734 nm. ABTS radical scavenging capacity was calculated in a manner similar to the DPPH scavenging percentage.
Superoxide radical scavenging activity
Superoxide radical scavenging activity (SRCA) was determined as described by Sanjukta et al. [16] with minor modification. 200 µL of 50 mM phosphate buffer (pH 8.2) and 10 µL of newly prepared 3 mM pyrogallol (dissolved in 10 mM HCl) were added to 20 µL of the sample (freeze-dried extracts of RYRP and RYRP with B2 dissolved in 1:1 water and methanol). Absorbance at 325 nm was measured at 0 and 10 min. Distilled water and phosphate buffer served as blanks. The capacity of the sample to scavenge the superoxide radical was calculated:
where S0 and S10 are the absorbance of sample and C0 and C10 are the absorbance of the control at 0 and 10 min, respectively.
Reducing power
The method of Liu et al. [26], with minor modification, was used to determine reducing power. Briefly, 100 µL of sample (freeze-dried extracts of RYRP and RYRP dissolved in 1:1 water and methanol) was mixed with 100 µL of potassium ferric cyanide (1% w/v) in a phosphate buffer (0.2 M, pH 6.6), maintained at 50 °C for 20 min and then added to 50 µL of trichloroacetic acid (10%, w/v). This was followed by centrifugation at 3500×g for 10 min. Supernatant (90 µL) was mixed with 50 µL of distilled water and 10 µL of ferric chloride (0.1%, w/v), and absorbance was measured at 700 nm. The control consisted of all reagents without sample extract. Reducing power was calculated:
where Acontrol contains all reagents without sample extract and Asample contains all reagents with sample extract. An increase in absorbance indicates greater reducing power.
Results
Identification of the B. subtilis isolate
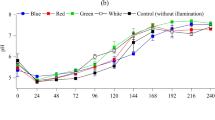
A bacterial strain (B2) was isolated from freshwater shrimp salted seafood and cultivated at optimum temperature (37 °C) and pH (7.0). The partial 16 s rRNA gene sequence (1182 base pairs) was submitted to a BLAST search to retrieve the corresponding bacterial families. Phylogenetic relationships were confirmed by investigating correlated strains recognized by the taxonomic and classification of the NCBI Taxonomy Homepage. A neighbor-joining phylogenetic tree was constructed (Fig. 1). B2 exhibited high nucleotide sequence similarity (100%) with Bacillus subtilis and accession number MH027419 was obtained from NCBI. B. subtilis B2 growth under the various light conditions was indicated by turbidity, which was measured as OD600 = 1.2–1.6 at 16 h (data not shown). Maximum growth (OD600 = 1.6 at 16 h) was observed under blue LEDs. This suggests that light does not adversely affect B. subtilis B2 growth, which was greatest under blue LEDs.
TPC and TFC yields
Table 1 shows the amounts of TPC (mg GAE/g DW) and TFC (mg QE/g DW) in ethanol extract of RYRP from the control (RYRP) and RYRP after fermentation with B2 under white light, LEDs of different colors (blue, green, red, white), and in the dark. In the absence of B2, TPC and TFC were low with no differences among light sources. Yields were greater with B2 under all LED lights compared to white fluorescent light or darkness, but were greatest under blue LEDs. Therefore, only the ethanol extract of RYRP powder fermented under blue LEDs was further characterized and assayed for antioxidant activity.
FTIR and GC–MS analyses
FTIR spectra (Fig. 2) contained new peaks and peaks of greater intensity in the extract of RYRP fermented with B2 bacteria under blue LEDs than under the other conditions. New peaks were at 2900–3000 cm− 1 (=CH3) aliphatic, 2500–2600 cm− 1 (CHO) aldehydes, 2100–2200 cm− 1 (C≡N) nitrites, 2000–2100 cm− 1 (N≡C) isonitriles, 1500–1600 cm− 1 (NO2) aromatic nitro compounds, and 1300–1400 cm− 1 (COO−) carboxylic acids, (C=N) oximes, (N=N–O) azoxy, (C–O–C) esters and lactones, (CH2–O–H) cyclic alcohols, (CH=CH2) vinyl, (Si–CH3) silanes, (C–S) sulfonyl chloride and (C–C–CN) nitriles were identified in both fermentation system but were more intense in RYRP fermented with B2 under blue LEDs. These findings are consistent with a higher content of secondary metabolites.
The results of GC–MS analysis are shown in Figs. 3 and 4. Aside from the general prominence of phenolic compounds, among the secondary metabolites produced in B2-fermented RYRP under blue LEDs identified antioxidant compounds include (1) cinnamic acid, m-methoxy-, trimethylsilyl ester; (2) benzoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy; (3) 1,2-benzenedicarboxylic acid, 4-methyl-5-(1-methylethyl)-, dimethyl ester; (4) phosphinodithioic acid, diphenyl; (5) 4(3H)-quinazolinone, 2-amino-3-(2-pyridinylmethyl); and (6) cinnamic acid, p-(trimethylsiloxy)-, methyl ester (Fig. 4).
GC–mass spectra of red yeast rice powder extract after fermenting with Bacillus subtilis B2 under blue LEDs. Notable identified antioxidant compounds: a cinnamic acid, m-methoxy-, trimethylsilyl ester; b benzoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy; c 1,2-benzenedicarboxylic acid, 4-methyl-5-(1-methylethyl)-, dimethyl ester; d phosphinodithioic acid, diphenyl; e 4(3H)-quinazolinone, 2-amino-3-(2-pyridinylmethyl); and f cinnamic acid, p-(trimethylsiloxy)-, methyl ester
Antioxidant activity
Total antioxidant and hydroxyl radical scavenging activities
The total antioxidant activity of extract powder from fermented RYRP (control) and RYRP with B2 under blue LEDs was evaluated based on the formation of the phosphomolybdenum complex. Antioxidant activity of RYRP fermented with B2 under blue LEDs was concentration dependent and exceeded 77% TAA at 400 µg/mL, compared to less than 10% TAA for the control (RYRP without B2) (Fig. 5a).
Hydroxyl radical scavenging activity is shown in Fig. 5b. At 400 µg/mL the hydroxyl radical scavenging activity of RYRP fermented with B2 under blue LEDs exceeded 67% inhibition efficiency, compared to less than 15% for the control.
DPPH and ABTS radical scavenging activity
The DPPH and ABTS assays are indicators of radical scavenging and cation radical scavenging antioxidant activity. The DPPH radical scavenging activities of RYRP fermented with and without B2 under blue LEDs are shown in Fig. 6a. At 400 µg/mL, the DPPH radical scavenging activity of extract from RYRP fermented with B2 under blue LEDs reached 51%, compared to less than 10% in the control. Similarly, ABTS cation radical scavenging exceeded 67% for extract from RYRP fermented with B2 under blue LEDs but was less than 15% in the control (Fig. 6b).
Superoxide scavenging activity
Superoxide radical anions are the precursors to dynamic free radicals. Superoxide scavenging was much higher in RYRP fermented with B2 under blue LEDs than in the control (Table 2). Activity at 400 µg/mL exceeded 66% but was less than 20% in the control.
Reducing power
The reducing power potential is based on reduction of the Fe3+/ferricyanide complex to Fe2+, indicated by a change in color from yellow to green and blue shades. The reducing power of the extract of RYRP fermented with B2 under blue LEDs was about twice that of the control (Fig. 7).
Discussion
RYRP contains an extraordinary amount of water-soluble sugars, particularly polysaccharides, phenolic compounds and γ-aminobutyric acid, which can be beneficial to gastrointestinal film development by enhancing abdominal functions [27]. RYRP naturally contains monacolin K; it helps strengthen the hypocholesterolemic effect, relieve hyperlipidemia and decrease body weight gain [18]. RYRP can increase assimilation of low-density lipoprotein in liver and reduce the hepatic production of very low density lipids [28]. In the present study, fermenting red yeast rice with B2 under blue LEDs increased production of secondary metabolites, which are important in developing optimally effective preventive and remedial dietary strategies [18].
RYRP fermented with B. subtilis B2 bacteria exhibited high antioxidant activity, including total antioxidant and hydroxyl radical scavenging; this includes DPPH radical scavenging and ABTS cation radical scavenging, superoxide radical scavenging, and reducing power. Free radicals can damage tissue by oxidizing organic macromolecules, including lipids, proteins and DNA. Antioxidant activity depends on antioxidant structure, oxidation conditions, and the nature of the substance being oxidized [28]. Extract powder from RYRP fermented with B2 contained hydroxyl, nitrile and carboxylic functional groups, all of which contribute to antioxidant activity [29].
Phenolic compounds are the main category of antioxidants, which act as free radical scavengers that delay or inhibit initiation of lipid oxidation [30, 31]. The antioxidant potential of phenolic compounds depends on the number and location of hydroxyl groups [29]. Simple phenolics such as hydroxycinnamic acid conjugates and flavonoids have been shown to exhibit a wide range of antioxidant activity in vitro [32]. Aniya et al. [33] and Kuo et al. [34] reported that purified phenolic compounds from red yeast rice had strong antioxidant properties via free radical scavenging. Hydroxybenzoic acid derivatives are often found in naturally occurring phenols and flavonoids; those metabolites have superoxide radical and hydroxy radical scavenging properties [32]. Quinazolinone and quinazoline derivatives are heterocycles with a wide range of biological properties, including anti-microbial, anti-cancer, anti-viral and anti-inflammatory activity [35]. Quinazolines function as DNA repair catalysts, epidermal development factor receptors, and promote the action of thymidylate synthase and tubulin polymerase; all of these properties can help inhibit cancer development [35].
Light enhanced secondary metabolite production when RYRP was fermented with B2 under blue LEDs. Products included flavonoids and phenolic compounds with antioxidant properties. Liu et al. [26] found that blue LED light enhanced total phenolic compounds in pea sprouts, including gallic, chlorogenic, o-phthalic, p-hydroxybenzoic, caffeic, p-coumaric and ferulic acids, and total flavonoids, especially rutin, phloridzin, resveratrol, and kaempferol. The extract of RYRP fermented with B. subtilis B2 under blue LEDs exhibited greatly enhanced antioxidant activity. Some fermentation bacteria possess a light activator gene that regulates cellular rhythmicity and metabolic activity through photoreactive pathways [1]. Enhanced production of secondary metabolites during RYRP fermentation with B. subtilis under blue LED light can be attributed to BLUF photoreceptors. Future studies should focus on molecular mechanisms and optimization of conditions for commercial-scale production of secondary metabolites.
References
Gomelsky M, Hoff WD (2011) Light helps bacteria make important lifestyle decisions. Trends Microbiol 19:441–448. https://doi.org/10.1016/j.tim.2011.05.002
van der Horst MA, Key J, Hellingwerf KJ (2007) Photosensing in chemotrophic, non-phototrophic bacteria: let there be light sensing too. Trends Microbiol 15:554–562. https://doi.org/10.1016/j.tim.2007.09.009
Davis SJ, Vener AV, Vierstra RD (1999) Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science 286:2517–2520. https://doi.org/10.1126/science.286.5449.2517
Darko E, Heydarizadeh P, Schoefs B, Sabzalian MR (2014) Photosynthesis under artificial light: the shift in primary and secondary metabolism. Philos Trans R Soc B Biol Sci 369:20130243–20130243. https://doi.org/10.1098/rstb.2013.0243
Margit Olle AV (2013) The effect of light-emitting diode lighting on greenhouse plant growth and quality. Agric Food Sci 22:223–234. https://doi.org/10.1016/j.envexpbot.2009.06.011
Ma R, Thomas-Hall SR, Chua ET et al (2018) LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Bioresour Technol 252:118–126. https://doi.org/10.1016/j.biortech.2017.12.096
Katsuda T, Lababpour A, Shimahara K, Katoh S (2004) Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme Microb Technol 35:81–86. https://doi.org/10.1016/j.enzmictec.2004.03.016
Mullineaux CW (2001) How do cyanobacteria sense and respond to light? Mol Microbiol 41:965–971. https://doi.org/10.1046/j.1365-2958.2001.02569.x
Khatoon H, Kok Leong L, Abdu Rahman N et al (2018) Effects of different light source and media on growth and production of phycobiliprotein from freshwater cyanobacteria. Bioresour Technol 249:652–658. https://doi.org/10.1016/j.biortech.2017.10.052
Oh BT, Jeong SY, Velmurugan P et al (2017) Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J Biosci Bioeng 124:542–550. https://doi.org/10.1016/j.jbiosc.2017.05.011
Gomaa EZ (2016) Cryoprotection of probiotic bacteria with poly-γ-glutamic acid produced by Bacillus subtilis and Bacillus licheniformis. J Genet Eng Biotechnol 14:269–279. https://doi.org/10.1016/j.jgeb.2016.10.001
Tian Y, Fan Y, Liu J et al (2016) Effect of nitrogen, carbon sources and agitation speed on acetoin production of Bacillus subtilis SF4-3. Electron J Biotechnol 19:41–49. https://doi.org/10.1016/j.ejbt.2015.11.005
Westers L, Westers H, Quax WJ (2004) Bacillus subtilis as cell factory for pharmaceutical proteins: A biotechnological approach to optimize the host organism. Biochim Biophys Acta Mol Cell Res 1694:299–310. https://doi.org/10.1016/j.bbamcr.2004.02.011
Hong HA, Le HD, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835. https://doi.org/10.1016/j.femsre.2004.12.001
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220. https://doi.org/10.1016/j.fm.2010.03.007
Sanjukta S, Rai AK, Muhammed A et al (2015) Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J Funct Foods 14:650–658. https://doi.org/10.1016/j.jff.2015.02.033
Juan MY, Chou CC (2010) Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol 27:586–591. https://doi.org/10.1016/j.fm.2009.11.002
Gum S, Nguyen PA, Lee JR et al (2017) The physico-chemical alteration of lovastatin and enhanced antioxidant effect of Bacillus subtilis fermented-red yeast rice product. Food Chem 232:203–209. https://doi.org/10.1016/j.foodchem.2017.04.023
Sun H, Wu Y, Wang X et al (2015) Effects of dietary supplementation with red yeast rice on laying performance, egg quality and serum traits of laying hens. Ital J Anim Sci 14:532–537. https://doi.org/10.4081/ijas.2015.4059
Yin LJ, Lin HH, Jiang ST (2010) Bioproperties of potent nattokinase from Bacillus subtilis YJ1. J Agric Food Chem 58:5737–5742. https://doi.org/10.1021/jf100290h
Tamang JP (2015) Naturally fermented ethnic soybean foods of India. J Ethn Foods 2:8–17. https://doi.org/10.1016/j.jef.2015.02.003
Huang Q, Zhang H, Xue D (2017) Enhancement of antioxidant activity of Radix Puerariae and red yeast rice by mixed fermentation with Monascus purpureus. Food Chem 226:89–94. https://doi.org/10.1016/j.foodchem.2017.01.021
Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochem Eng J 81:146–161. https://doi.org/10.1016/j.bej.2013.10.013
Rajasekar A, Anandkumar B, Maruthamuthu S et al (2010) Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol 85:1175–1188. https://doi.org/10.1007/s00253-009-2289-9
Zhou J, Zheng X, Yang Q et al (2013) Optimization of ultrasonic-assisted extraction and radical-scavenging capacity phenols and flavonoids from Clerodendrum cyrtophyllum Turcz leaves. PLoS One 8:1–8. https://doi.org/10.1371/journal.pone.0068392
Liu HK, Chen YY, Hu TT et al (2016) The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J Funct Foods 25:459–465. https://doi.org/10.1016/j.jff.2016.06.028
Zhao L, Zhang X, Cao F et al (2013) Effect of dietary supplementation with fermented Ginkgo-leaves on performance, egg quality, lipid metabolism and egg-yolk fatty acids composition in laying hens. Livest Sci 155:77–85. https://doi.org/10.1016/j.livsci.2013.03.024
Shahidi F, Naczk M (2006) Phenolics in food and nutraceuticals. CRC Press, Washington
Shahidi F, Ambigaipalan P (2015) Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—a review. J Funct Foods 18:820–897. https://doi.org/10.1016/j.jff.2015.06.018
Alamed J, Chaiyasit W, Mcclements DJ, Decker E (2009) Relationships between free radical scavenging and antioxidant activity in foods. J Agric Food Chem 2969–2976. https://doi.org/10.1021/jf803436c
Kiokias S, Varzakas T, Oreopoulou V (2008) In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit Rev Food Sci Nutr 48:78–93. https://doi.org/10.1080/10408390601079975
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956. https://doi.org/10.1016/0891-5849(95)02227-9
Aniya Y, Yokomakura T, Yonamine M et al (1999) Screening of antioxidant action of various molds and protection of Monascus anka against experimentally induced liver injuries of rats. Gen Pharmacol 32:225–231. https://doi.org/10.1016/S0306-3623(98)00183-9
Kuo C-F, Chyau C-C, Wang T-S et al (2009) Enhanced antioxidant and anti-inflammatory activities of Monascus pilosus fermented products by addition of turmeric to the medium. J Agric Food Chem 57:11397–11405. https://doi.org/10.1021/jf9027798
Jafari E, Khajouei MR, Hassanzadeh F et al (2016) Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res Pharm Sci 11:1–14
Acknowledgements
This work was supported by the BK21 plus program through the National Research Foundation (NRF) funded by the Ministry of Education of Korea. This work was supported by Korea Environment Industry & Technology Institute (KEITI) through Public Technology Program based on Environmental Policy, funded by Korea Ministry of Environment (MOE) (2018000700001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Elumalai, P., Park, YJ., Cho, M. et al. Red yeast rice fermentation with Bacillus subtilis B2 under blue light-emitting diodes increases antioxidant secondary products (Manuscript ID: BPBSE-18-0387). Bioprocess Biosyst Eng 42, 529–539 (2019). https://doi.org/10.1007/s00449-018-2056-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-2056-3