Abstract
The effects of all-trans retinoic acid (RA) and sodium butyrate (NaBu) on growth, viability and antibody production of two types of transfected Chinese hamster ovary cell lines (CHO-K1 and CHO-S) were investigated using a batch mode cell culture. By adding 0.5 mM NaBu in the CHO-K1 cell culture, the cell specific productivity (Qp) and antibody concentration increased by five- and threefold, respectively. The optimal concentration of RA was 100 nM which resulted in twofold increase in antibody production. In a combination model, RA applied at early growth phase of CHO-K1 cells followed by addition of NaBu with lowering culture temperature at the end of stationary phase resulted in two- and threefold increase in Qp and final antibody concentration, respectively. The latter strategy was also applied on suspended CHO-S cells with enhanced Qp and antibody concentration, but to a lesser extent than the CHO-K1 cells. In conclusion, our results demonstrate that the addition of RA and NaBu along with lowering the culture temperature can increase cell culture period as well as Qp and the final concentration of recombinant monoclonal antibody in both CHO-K1 and CHO-S cells without any significant change in binding affinity of the mAb.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing protein expression can significantly reduce the cost of therapeutic protein production. Monoclonal antibodies (mAbs) are currently the best-selling biologics category [1], but their production is more expensive than low molecular weight drugs. Therefore, minimizing their production costs with efficient methods is currently the main active area of research [2]. To meet the growing demands for mAbs, several approaches such as engineering the host cells [3] and expression vectors [4], optimization of culture media [5] and improvement of feeding strategies [6] have been explored to improve mAb production. These strategies have been used to enhance both the integrated viable cell density (IVCD) over the time span of the cell culture and cell specific productivity (Qp). Most of these cell culture strategies have applied conditions which maximize cell growth and viable cell density. Alternatively, by purposely maintaining a small population of high producing cells in a specific phase of the cell cycle, similar or greater productivity can be achieved [7]. Cell cycle inhibition approaches have been widely used to increase Qp in cell cultures [8, 9], including nutrient limitation, culturing the cells at low temperatures [10, 11], addition of small molecule enhancers (SMEs) like sodium butyrate (NaBu) [9, 12] and cell engineering by overexpression of endogenous cyclin-dependent kinase inhibitors (CKIs) [13].
CHO cells have an established history of regulatory approval for recombinant protein expression. Many recombinant protein-producing CHO cell lines were derived from the CHO-K1, CHO-S and DG44 lineages. CHO cells, first isolated in 1957, are the preferred production host for many therapeutic proteins. The anchorage-dependent CHO-K1 cell strain was generated from the original CHO cell line by single-cell cloning in late 1960s. The CHO-S cells were first adapted to grow in suspension cultures in 1960s [14]. CHO-K1 cells are anchoraged-dependent, whereas CHO-S cells grow in suspension and reach higher viable cell density than CHO-K1 cells at the same volume.
NaBu is a member of chemical agents known as aliphatic acid-based histone deacetylase inhibitors which have been widely used to increase Qp in recombinant CHO cells producing a variety of recombinant therapeutic proteins such as erythropoietin [15], tissue plasminogen activator [16] and mAbs [17,18,19,20,21,22]. NaBu has an inhibitory role on cell growth and various strategies have been used to moderate the cytotoxic effect of NaBu [19, 23,24,25,26,27]. Low temperature shift of the cell culture is an alternative method to alleviate NaBu negative effects [19].
All-trans retinoic acid (RA), the main active form of vitamin A, modulates the differentiation and proliferation of a wide variety of mammalian cell types. It was also reported to enhance transcription and secretion of antibody as well as Qp in human hybridoma cells [28, 29].
Given the potential of NaBu and RA for enhancement of mAb production in recombinant CHO cells, in the present study, we investigated for the first time the combination effect of these two additives on humanized anti-HER2 mAb production by both adherent (CHO-K1) and non-adherent (CHO-S) stable transfected CHO cells.
Materials and methods
Cell lines and culture media
Stable mAb-producing CHO-K1 and CHO-S cell lines expressing a recombinant anti-HER2 mAb [30] were used in this study. CHO-K1 cells were initially grown in Roswell Park Memorial Institute (RPMI) 1640 culture medium (Gibco, NY, USA) supplemented with 10% fetal bovine serum (Gibco). CHO-S cells were cultured in a 125 mL shake flask containing 20 mL of EX-CELL® CD CHO serum-free medium (Sigma–Aldrich, Saint Louis, USA) and incubated at 37 °C in a humidified atmosphere of 5% CO2 in an orbital shaker platform (19 mm diameter orbit) rotating at 120–140 rpm. Both media were supplemented with 100 µg/ml streptomycin and 100 U/ ml penicillin (Gibco).
Cell culture
For the dosage and time course experiments, CHO-K1 cells were cultured in 24-well plate (Corning, NY,USA) with 1 ml working volume and 3 × 104 cells/mL seeding density per well for 8–10 days. RPMI1640 with 10% fetal bovine serum was used as initial culture medium and after 24 h was replaced with fresh serum-free medium(CD OptiCHO medium, Gibco). Depending on additive types and experiments, the adding time of NaBu and RA into the medium was different. CHO-S cells were cultured in 100 mL baffled flask with 20 mL working volume and 4 × 105 cells/mL seeding density for 10 days in EX-CELL® CD CHO serum-free medium.
Sodium butyrate and RA treatment
A 0.3M stock solution of NaBu (Sigma) was prepared in CD OptiCHO medium and then sterilized with a 0.22-µm filter (Gelman, MI, USA), and stored at − 20 °C. The concentration ranging from 0 to 8 mM of NaBu was evaluated in all experiments.
A 0.1M stock solution of RA (Sigma) was prepared in ethanol and a concentration range from 0 to 1600 nM of RA was evaluated at the beginning of the experiments with CHO-S cells. RA was also added in CHO-K1 cell culture medium at the time of medium change from RPMI to CD OptiCHO. The amount of ethanol at the highest concentration of RA (1600 nM) was used as a vehicle control and all experiments were repeated three times.
Cell density and viability analysis
Cell viability was determined by counting a cell suspension diluted 1:1 v/v with 0.2% trypan blue (Merck, Hohenbrunn, Germany) using a Neubauer haemocytometer.
Concentration of mAb and cell productivity analysis
The mAb concentration was determined using an indirect ELISA method [31, 32]. In brief, a 96-well ELISA plate (Maxisorp, Roskilde, Denmark) was coated with 2 µg/ml of the recombinant HER2 extracellular domain (recombinant HER2-ECD) (prepared in our laboratory) in phosphate buffered saline (PBS), incubated for 1.5 h at 37 °C and blocked with PBS supplemented with 0.05% Tween 20® (Sigma) and 3% non-fat skim milk (Merck). 50 µl of supernatants from recombinant CHO-K1 and CHO-S cells were added at 37 °C for 1.5 h. Trastuzumab (Hoffmann-La Roche, Basel, Switzerland), a humanized anti-HER2 mAb, was used as a positive control. After washing with PBS-Tween, horseradish peroxidase (HRP)-conjugated rabbit anti-human immunoglobulin (Sina Biotec, Tehran, Iran) was added and plate was incubated for 1 h at 37 °C. After further washing, the reaction was revealed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (PishtazTeb, Tehran, Iran). Hydrochloric acid was added to stop the reaction and the optical density (OD) was measured by a multiscan ELISA reader (Biotek, Vermont, USA) at 450 nm. Cell specific productivity (Qp) was determined by dividing the total mAb content to integral of viable cells (IVC). IVC was calculated using the following equation in which X is the viable cell density (VCD), V is the volume of culture medium and t is the time of culture: IVC =\(\int_{0}^{t} {XV{\text{d}}t}\) [8].
Determination and calculation of affinity constant of mAbs
Affinity constant was determined based on an ELISA procedure that was optimized by Hajighasemi et al. [33]. Wells of an ELISA microtiter polystyrene plate (Maxisorp, Nunc, Denmark) with reaction volume of 50 µl were coated with 0.075, 0.15, 0.3, and 0.6 µg/mL of rHER2 antigen. After blocking with 3% non-fat dry milk in PBST (PBS, 0.05% Tween 20), 0.075, 0.15, 0.3, and 0.6 µg/mL of cell culture supernatant were added to the wells and incubated for 1 h at 37 °C. After washing it three times, (HRP)-conjugated rabbit anti-human IgG was added and incubated for 1 h at 37 °C. TMB substrate was then added to each well after washing and the absorbance was measured at 450 nm.
The sigmoidal curve of ODs versus the logarithm of antibody concentration were plotted and the antibody concentration resulting in 50% of the maximum absorbance (OD50) value at a particular antigen coating concentration was selected for the affinity constant (Kaff) and calculated based on following formula:
[Ab] and [Ab′] represented antibody concentration at OD50 for wells coated with two concentrations of antigen [Ag] and [Ag′] [30, 34].
Results
Effect of NaBu and RA on CHO-K1 cells growth and antibody production
To investigate the effects of NaBu and RA on cell growth and antibody production, CHOK-1 cells were cultivated in CD OptiCHO medium containing different concentrations of NaBu and RA. The cultures were performed in batch cultivation mode as described in Materials and Methods, at three separate times.
For all experiments, according to the viability and density of the CHO-K1 cells, the days 1 and 5 were determined as the best time for adding RA and NaBu (data not shown) which are indicated by arrows in all figures.
The results showed that NaBu inhibited growth and viability of CHO-K1 cells in a dose-dependent manner and cell viability decreased substantially at 2 and 8 mM of NaBu (Fig. 1). Despite cell growth inhibition by NaBu, the highest antibody concentration (3.43 µg/mL) was obtained at 0.5 mM NaBu (Fig. 2a) which resulted in fivefold increase in Qp (Table 1).
Dose–response effects of NaBu and RA on cell density and viability of CHO-K1 cells after treatment with NaBu (a) and RA (b). Arrows indicate the timing of NaBu and RA addition. The error bars represent the standard deviations calculated based on the data obtained from three independent experiments. Solid lines and dot lines represent viable cell density and percent of cell viability, respectively
Effect of NaBu and RA addition on antibody production in CHO-K1 cells. NaBu (a) and RA (b) were added at the end of fifth and first day of the cell culture, respectively. Antibody concentration was examined every day after day 5. The error bars represent the standard deviations calculated based on the data obtained from three independent experiments
By adding RA, the concentration of antibody simultaneously increased together with IVCD and therefore, Qp of the cell line increased less than NaBu. The results showed that the highest concentration of antibody (2.83 µg/ml) was achieved after treatment with 100 nM RA which was approximately twofold higher than untreated cells (Fig. 2b) and Qp was increased 72% (Table 2).
Combination effect of RA, NaBu and low culture temperature on growth and antibody production of CHO-K1 cells
Two-step treatments were performed to examine the combined effects of NaBu and RA along with shift of culture temperature on antibody production of recombinant CHO-K1 cells. In the first experiment, 100 nM concentration of RA was added at the first day and subsequently different concentrations of NaBu were added at day 5 and culture temperature was simultaneously reduced to 30 °C. Cell growth and viability profile of the cell cultures subjected to simultaneous application of RA and NaBu and temperature reduction are shown in Fig. 3a, b.
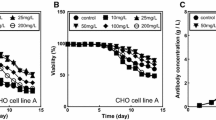
Combination effect of RA, NaBu and low culture temperature on cell density and viability of CHO-K1 cells. a Individual and combined effects of RA and NaBu at 37 °C. (b) Effect of reduced culture temperature. (c) Combination effects of RA and NaBu along with reducing culture temperature. Viable cell density and percent of cell viability were examined every day after day 5. The error bars represent the standard deviations calculated based on the data obtained from three independent experiments. Solid and dot lines represent viable cell density and percent of cell viability, respectively
According to the results, although cell viability was reduced after 48 h treatment with 0.5 mM of NaBu at 37 °C, it remained around 90% at the end of the day 8 at 30 °C (Fig. 3b). Moreover, VCD, viability and antibody concentration in CHO-K1 cells treated with combination of RA and NaBu, at 30 °C were higher than those obtained at 37 °C (Figs. 3c, 4).
Eventually, by applying our combined strategy, as shown in Fig. 4 and Table 3, the final concentration of antibody was enhanced from 1.25 to 5.77 µg/ml and Qp increased by more than twofold.
Combination effect of RA, NaBu and low culture temperature on cell growth, affinity constant and antibody production of CHO-S cells
To investigate whether the enhancement of Qp and antibody production by this combined strategy is a general phenomenon, we applied it on suspended recombinant CHO-S cell line. As shown in Fig. 5 and summarized in Table 4, the Qp of the cell line and final antibody concentration were approximately increased by 120 and 118%, respectively. So, the results demonstrate enhancement of both parameters in CHO-S cells, but to a lesser extent than in CHO-K1 cells.
Combination effect of RA, NaBu and low culture temperature on viable cell densities and percent of cell viability (a) and antibody production (b) of CHO-S cells. The error bars represent the standard deviations calculated based on the data obtained from three independent experiments. Solid and dot lines represent viable cell density and percent of cell viability, respectively
In addition, we determined the affinity constant of the recombinant antibody in culture supernatants of CHO-S cells collected in presence or absence of RA and NaBu. Representative antibody titration curve obtained at three serial antigen (recombinant HER2-ECD) concentrations which are employed to determine the affinity constant of the recombinant anti-HER2 mAb are illustrated in Fig. 6. The results are summarized in Table 4. No substantial variation was observed in the level of binding affinity of the antibody at different culture conditions.
Discussion
Cell cycle consists of a series of checkpoints which are regulated by a complex network of kinases, inhibitors and some other signaling molecules that control proliferation in mammalian cells. Inhibiting the functions of the primary regulatory kinase enzymes is the key element for controlling cell proliferation which allow the transition from the G1 to S phase. These enzymes, a family of cyclin-dependent kinases (CDKs), are controlled through either positive or negative regulation and require the presence of the protein cyclin, or inactivated by members of the family of cyclin-dependent kinase inhibitors (CKI). Retinoblastoma protein (Rb) is the downstream target of CDK regulation and the unphosphorylated forms of Rb inhibit cell proliferation. Controlled proliferation strategies prevent phosphorylation of Rb by activation of upstream CKIs [8, 35]. Retinoic acid (RA) reduces Rb expression and enhances Rb phosphorylation by a mechanism that involves down-regulation of the CKIs. This mechanism induces cell proliferation and increase viable cell density. On the other hand, NaBu induces cell cycle arrest through dephosphorylation of Rb as well as upregulation of CKIs [8].
With regards to NaBu, our results demonstrated that although Qp was increased after addition of NaBu at 37 °C (Table 1), cell viability declined at all concentrations of NaBu in comparison with untreated cells due to negative effects of NaBu on cell growth and viability (Figs. 1a, 2a). This phenomenon has previously been observed in many CHO cell cultures [19,20,21,22,23,24,25,26,27]. Cell cycle arrest by NaBu, results in restricted consumption of the cell energy for production of antibody and increase of Qp rather than cell proliferation.
Furthermore, treatment with 100 nM of RA improved VCD and viability of the cells and the final concentration of antibody was approximately twofold higher in comparison with untreated cells (Fig. 2a, b) and also the Qp was increased by 72% (Table 2). Similar results have been reported by Inoue et al. who have shown that IgG secreted by BD9 hybridoma is enhanced about eightfold by treatment with 100 nM of RA for 4 days [28]. There are some possible mechanisms for this observation. RA by down-regulation of the CKIs through reducing Rb expression and enhancing Rb phosphorylation increases cell proliferation and VCD leading to higher cell number and production of higher antibody titer. Alternatively, RA binds to its receptor (RAR) and forms a complex which interacts with DNA at specific promoter sequences, called RA response elements and as a result regulates gene expression [28, 29].
In this study, we decided to add RA 1 day and NaBu 5 days after culture initiation to study their combination effect on antibody production. This method was used because in our pilot study we found that when RA and NaBu were added separately to the cell culture on the first day, RA increased proliferation of the cells until day 8, while NaBu arrested cell proliferation on day 4 and cell viability declined (data not presented). Meanwhile, the stability of RA in a serum-free medium is reduced in comparison to the serum supplemented media [37]. Therefore, to use the enhancement proliferation advantage of RA and the enhancement Qp advantage of NaBu. we decided to add RA at the first day of cell culture and NaBu at day 5. Maximum antibody concentration in CHO-K1 cell culture treated with combination of RA and NaBu, was higher than cells treated with single RA or NaBu (Table 3).
In addition to the timing of RA and NaBu supplementation, we also assessed temperature shift from 37 to 30 °C on antibody productivity of the CHO-K1 cells. It has been reported that the cell culture under mild hypothermic conditions (30–35 °C) induces an actively controlled growth reduction in cells and increased expression of recombinant proteins. Lowering temperature of culture has more advantages such as extension of culture duration, reducing overall nutrient uptake and waste production [19], decreasing O2 demand, reducing intermolecular product aggregation, increasing sensitivity to deviations in culture pH and decreasing sensitivity to pro-apoptotic agents [8]. In addition, lowering culture temperature has been shown to reduce adverse effects of NaBu on VCD and viability leading to enhanced Qp and final concentration of mAb at the end of the cell culture period [19]. This is in agreement with our results showing reduced cell viability after 48 h treatment with 0.5 mM of NaBu at 37 °C, but higher viability (~ 90%) at the end of day 8 at 30 °C (Fig. 3c). Similarly, lowering culture temperature in treatment with combination of RA and NaBu, resulted in increased viability and enhanced maximum antibody concentration (Fig. 4; Table 3).
We also applied this culture protocol to CHO-S cells which are routinely employed in industrial manufacturing of recombinant proteins and mAbs. Due to suspension culture, scale-up of these cells at significantly higher cell density in stirred tank bioreactors is rather simple and much more cost-effective. By applying our new strategy on this cell line, Qp and maximum antibody concentration were approximately increased by 120 and 118%, respectively (Table 4) compared to untreated cells, implying adaptation of this protocol to industrial process of mAb production.
The binding affinity of an antibody to its antigen is a crucial parameter which could affect its biological activity and therapeutic performance [33].The affinity of a mAb might be influenced by post-translation modifications and particularly glycosylation of the antibody [38]. Since some residues within the VH and/or VL region might be glycosylated and are prone to shift in glycosylation pattern due to addition of some additives which could influence the binding affinity of the mAb, we determined the affinity constant of our mAb in supernatant samples of CHO-S cells at different culture conditions to be able to explore such effects. Our results revealed that applying combination strategy of NaBu and RA together with lowering culture temperature has no significant effect on the binding affinity (Table 4). This finding implies that no substantial variation in glycosylation pattern of the VH/VL regions of our mAb is expected though direct inspection of the glycosylation pattern and charge variation of the whole molecule, particularly the Fc fragment is required to draw a firm conclusion. Unfortunately, we could not perform the glycosylation and charge variation experiments due to technical limitations.
Conclusion
Our results demonstrated for the first time that combination of RA and NaBu enhances recombinant mAb production in CHO-K1 and CHO-S cells. These findings might be of value for therapeutic mAb production at large-scale industrial level.
References
Walsh G (2014) Biopharmaceutical benchmarks 2014. Nat Biotechnol 32(10):992–1000. https://doi.org/10.1038/nbt.3040
Konno Y, Aoki M, Takagishi M, Sakai N, Koike M, Wakamatsu K, Hosoi S (2011) Enhancement of antibody production by the addition of Coenzyme-Q(10). Cytotechnology 63(2):163–170. https://doi.org/10.1007/s10616-010-9330-9
Choi SS, Rhee Wj Fau - Kim EJ, Kim Ej Fau -. Park TH, Park TH (2006) Enhancement of recombinant protein production in Chinese hamster ovary cells through anti-apoptosis engineering using 30Kc6 gene. Biotechnol Bioeng 95(3):459–467. https://doi.org/10.1002/bit.21023
Chen K, Liu Q, Xie L, Sharp PA, Wang DI (2001) Engineering of a mammalian cell line for reduction of lactate formation and high monoclonal antibody production. Biotechnol Bioeng 72 (1):55–61. https://doi.org/10.1002/1097-0290(20010105)72:1<55::AID-BIT8>3.0.CO;2-4
Kim DY, Lee JF, Chang HN, Chang Hn Fau - Oh DJ, Oh DJ (2005) Effects of supplementation of various medium components on chinese hamster ovary cell cultures producing recombinant antibody. In: Cytotechnology (0920–9069 (Print)):37–49. doi:D-NLM: PMC3449820 EDAT-2008/11/13 09:00 MHDA-2008/11/13 09:01 CRDT-2008/11/13 09:00 PHST-2005/03/31 [received] PHST-2005/07/29 [accepted] AID—https://doi.org/10.1007/s10616-005-3775-2. PST-publish
Sun Y-t, Zhao L, Ye Z, Fan L, Liu X-p, Tan W-S (2013) Development of a fed-batch cultivation for antibody-producing cells based on combined feeding strategy of glucose and galactose. Biochem Eng J 81:126–135. https://doi.org/10.1016/j.bej.2013.10.012
Kumar N, Gammell P, Clynes M (2007) Proliferation control strategies to improve productivity and survival during CHO based production culture: a summary of recent methods employed and the effects of proliferation control in product secreting CHO cell lines. Cytotechnology 53(1–3):33–46. https://doi.org/10.1007/s10616-007-9047-6
Sunley K, Butler M (2010) Strategies for the enhancement of recombinant protein production from mammalian cells by growth arrest. Biotechnol Adv 28(3):385–394. https://doi.org/10.1016/j.biotechadv.2010.02.003
Park JH, Noh SM, Woo JR, Kim JW, Lee GM (2016) Valeric acid induces cell cycle arrest at G1 phase in CHO cell cultures and improves recombinant antibody productivity. Biotechnol J 11(4):487–496. https://doi.org/10.1002/biot.201500327
Ahn WS, Jeon J-J, Jeong Y-R, Lee SJ, Yoon SK (2008) Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotechnol Bioeng 101(6):1234–1244. https://doi.org/10.1002/bit.22006
Chen Z-L, Wu B-C, Liu H, Liu X-M, Huang P-T (2004) Temperature shift as a process optimization step for the production of pro-urokinase by a recombinant Chinese hamster ovary cell line in high-density perfusion culture. J Biosci Bioeng 97(4):239–243. https://doi.org/10.1016/S1389-1723(04)70198-X
Allen MJ, Boyce JP, Trentalange MT, Treiber DL, Rasmussen B, Tillotson B, Davis R, Reddy P (2008) Identification of novel small molecule enhancers of protein production by cultured mammalian cells. Biotechnol Bioeng 100(6):1193–1204. https://doi.org/10.1002/bit.21839
Bi J-X, Shuttleworth J, Al-Rubeai M (2004) Uncoupling of cell growth and proliferation results in enhancement of productivity in p21CIP1-arrested CHO cells. Biotechnol Bioeng 85(7):741–749. https://doi.org/10.1002/bit.20025
Wurm MF (2013) CHO quasispecies—implications for manufacturing processes. Processes. https://doi.org/10.3390/pr1030296
Yoon SK, Hong JK, Lee GM (2004) Effect of simultaneous application of stressful culture conditions on specific productivity and heterogeneity of erythropoietin in chinese hamster ovary cells. Biotechnol Prog 20(4):1293–1296. https://doi.org/10.1021/bp034382z
Hendrick V, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G, Werenne J (2001) Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology 36(1–3):71–83. https://doi.org/10.1023/A:1014088919546
Hong JK, Lee SM, Kim K-Y, Lee GM (2014) Effect of sodium butyrate on the assembly, charge variants, and galactosylation of antibody produced in recombinant Chinese hamster ovary cells. Appl Microbiol Biotechnol 98(12):5417–5425. https://doi.org/10.1007/s00253-014-5596-8
Hong JK, Lee GM, Yoon SK (2011) Growth factor withdrawal in combination with sodium butyrate addition extends culture longevity and enhances antibody production in CHO cells. J Biotechnol 155(2):225–231. https://doi.org/10.1016/j.jbiotec.2011.06.020
Chen F, Kou T, Fan L, Zhou Y, Ye Z, Zhao L, Tan W-S (2011) The combined effect of sodium butyrate and low culture temperature on the production, sialylation, and biological activity of an antibody produced in CHO cells. Biotechnol Bioprocess Eng 16(6):1157–1165. https://doi.org/10.1007/s12257-011-0069-8
Jiang Z, Sharfstein ST (2008) Sodium butyrate stimulates monoclonal antibody over-expression in CHO cells by improving gene accessibility. Biotechnol Bioeng 100(1):189–194. https://doi.org/10.1002/bit.21726
Mimura Y, Lund J, Church S, Dong S, Li J, Goodall M, Jefferis R (2001) Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. J Immunol Methods 247(1–2):205–216. https://doi.org/10.1016/S0022-1759(00)00308-2
Cherlet M, Marc A (2000) Stimulation of monoclonal antibody production of hybridoma cells by butyrate: evaluation of a feeding strategy and characterization of cell behaviour. Cytotechnology 32(1):17–29. https://doi.org/10.1023/A:1008069523163
Sung YH, Lee JS, Park SH, Koo J, Lee GM (2007) Influence of co-down-regulation of caspase-3 and caspase-7 by siRNAs on sodium butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin. Metab Eng 9(5–6):452–464. https://doi.org/10.1016/j.ymben.2007.08.001
Oh HK, So MK, Yang J, Yoon HC, Ahn JS, Lee JM, Kim JT, Yoo JU, Byun TH (2005) Effect of N-acetylcystein on butyrate-treated chinese hamster ovary cells to improve the production of recombinant human interferon-β-1a. Biotechnol Prog 21(4):1154–1164. https://doi.org/10.1021/bp050057v
Kim NS, Lee GM (2002) Inhibition of sodium butyrate-induced apoptosis in recombinant Chinese hamster ovary cells by constitutively expressing antisense RNA of caspase-3. Biotechnol Bioeng 78(2):217–228. https://doi.org/10.1002/bit.10191
Kim NS, Lee GM (2000) Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnol Bioeng 71 (3):184–193. https://doi.org/10.1002/1097-0290(2000)71:3<184::AID-BIT1008>3.0.CO;2-W
Sung YH, Hwang SF - Lee GM, Lee GM Influence of down-regulation of caspase-3 by siRNAs on sodium-butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin (1096–7176 (Print))
Inoue Y, Fujisawa M, Shoji M, Hashizume S, Katakura Y, Shirahata S (2000) Enhanced antibody production of human–human hybridomas by retinoic acid. Cytotechnology 33(1–3):83–88. https://doi.org/10.1023/A:1008155609072
Inoue Y, Fujisawa M, Kawamoto S, Shoji M, Hashizume S, Fujii M, Katakura Y, Shirahata S (1999) Effectiveness of vitamin A acetate for enhancing the production of lung cancer specific monoclonal antibodies. Cytotechnology 31(1–2):77–83. https://doi.org/10.1023/A:1008016020785
Amiri MM, Jeddi-Tehrani M, Kazemi T, Bahadori M, Maddah M, Hojjat-Farsangi M, Khoshnoodi J, Rabbani H, Shokri F (2013) Construction and characterization of a new chimeric antibody against HER2. Immunotherapy 5(7):703–715. https://doi.org/10.2217/imt.13.67
Tahmasebi F, Kazemi T, Amiri MM, Khoshnoodi J, Mahmoudian J, Bayat AA, Jeddi-Tehrani M, Rabbani H, Shokri F (2014) In vitro assessment of the effects of anti-HER2 monoclonal antibodies on proliferation of HER2-overexpressing breast cancer cells. Immunotherapy 6(1):(1750–7448 (Electronic)):43–49. https://doi.org/10.2217/imt.13.156
Kazemi T, Tahmasebi F, Bayat AA, Mohajer N, Khoshnoodi J, Jeddi-Tehrani M, Rabbani H, Shokri F (2011) Characterization of novel murine monoclonal antibodies directed against the extracellular domain of human HER2 tyrosine kinase receptor. Hybridoma 30(4):347–353. https://doi.org/10.1089/hyb.2011.0023
Hajighasemi FS-YA., Shokri F (2004) Measurement of affinity constant of anti-human IgG monoclonal antibodies by an ELISA-based method. Irn J Immunol 1(3):154–161
Golsaz Shirazi F, Mohammadi H, Fau-Amiri MM, Singethan K, Xia Y, Bayat AA, Bahadori M, Rabbani H, Jeddi-Tehrani M, Protzer U, Shokri F (2014) Monoclonal antibodies to various epitopes of hepatitis B surface antigen inhibit hepatitis B virus infection. J Gastroenterol Hepatol 29(5):1083–1091. https://doi.org/10.1111/jgh.12483
Du Z, Treiber D, McCarter JD, Fomina-Yadlin D, Saleem RA, McCoy RE, Zhang Y, Tharmalingam T, Leith M, Follstad BD, Dell B, Grisim B, Zupke C, Heath C, Morris AE, Reddy P (2015) Use of a small molecule cell cycle inhibitor to control cell growth and improve specific productivity and product quality of recombinant proteins in CHO cell cultures. Biotechnol Bioeng 112(1):141–155. https://doi.org/10.1002/bit.25332
Rahimpour A, Ahani R, Najaei A, Adeli A, Barkhordari F, Mahboudi F (2016) Development of genetically modified chinese hamster ovary host cells for the enhancement of recombinant tissue plasminogen activator expression. The Malaysian Journal of Medical Sciences: MJMS 23(2):6–13
Sharow KA, Temkin B, Asson-Batres MA (2012) Retinoic acid stability in stem cell cultures. Int J Dev Biol 56(4):273–278. https://doi.org/10.1387/ijdb.113378ks
Jefferis R (2016) Posttranslational Modifications and the Immunogenicity of Biotherapeutics. J Immunol Res 2016:5358272. https://doi.org/10.1155/2016/5358272
Acknowledgements
This work was supported partially by grants from Tarbiat Modares University and Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Rahimi-Zarchi, M., Shojaosadati, S.A., Amiri, M.M. et al. All-trans retinoic acid in combination with sodium butyrate enhances specific monoclonal antibody productivity in recombinant CHO cell line. Bioprocess Biosyst Eng 41, 961–971 (2018). https://doi.org/10.1007/s00449-018-1927-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1927-y