Abstract
Microalgae are regarded as a promising source of biofuels, and the concept of a microalgae-based biorefinery has attracted increasing attention in recent years. From an economic perspective, however, the process remains far from competitive with fossil fuels. This is particularly true of lipid extraction, due in part to the energy-intensive drying step. As a result, wet extraction methods have been studied as an economic alternative. In the present study, a novel extraction approach which utilizes high shear stress mixing was adopted and demonstrated for simultaneous lipid extraction and cell disruption to enable the retrieval of lipids directly from concentrated wet biomass. When a high shear mixer (HSM) was used to extract lipid from a dense biomass (> 350 g/L) of the oleaginous algae Aurantiochytrium sp., it exhibited a yield of esterifiable lipids which exceeded 80% in 10 min at 15,000 rpm with various solvent types. The HSM was found to improve the lipid yields substantially with solvents less miscible with either lipids or water, such that the range of Hansen solubility parameters for the usable solvents became 3.3 times wider (14.9–26.5 MPa1/2). The HSM, which appeared effectively to loosen the water barrier that prevents solvent molecules from penetrating through the cell envelope, was found to be more efficient with hexane, hexane/isopropanol, and ethanol, all of which showed nearly identical lipid yields compared to the dry extraction process. The HSM can, indeed, offer a powerful mechanical means of lipid extraction with non-polar and less toxic solvents from wet biomass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The microalgae-based biorefinery, though still in its early stages, has grown continuously in recent years, and it is projected to continue to do so in the future. Some currently available microalgae-based commodities include biodiesel, food supplements and additives, animal and fish feed, natural pigments, cosmetics, and pharmaceuticals. Heterotrophic cultivation is uniquely advantageous in terms of biomass and oil productivity, far surpassing photoautotrophic cultivation (and of course land crops) [1]. When thraustochytrids are used, omega-3 oils, xanthophyll, and squalene, which are pharmaceutically active lipids, can be produced in a commercially viable manner [2, 3].
The commercialization of these products, however, has several difficult obstacles to overcome, with the most challenging of all being the high energy consumption especially associated with lipid extraction. The current lipid extraction process is typically done with dry biomass, which includes an energy-intensive drying step prior to solvent extraction [4, 5]. An alternative route is wet extraction, which skips the drying step and proceeds directly to solvent extraction, thereby reducing the amount of energy used. This seemingly promising approach, however, is associated with considerable inefficiency, as water molecules act as a barrier that prevents the effective mass transfer of an organic non-polar solvent from interacting with lipids [6]. To increase the accessibility of the solvents to intracellular lipids, therefore, various cell-disruption methods, such as high-pressure homogenization, bead milling, microwaves, and sonication, have been applied before the solvent extraction step [7,8,9]. This two-step approach, however, introduces different issues, such as a longer process time and a rather nominal advantage in terms of energy consumption overall, in addition to relatively low lipid extraction yields compared to dry extraction.
As an effort to resolve this, a one-step simultaneous process has been actively studied; and in relation to this, the aforementioned mechanical means have been utilized as well [10,11,12]. This one-step process, which undertakes cell disruption and lipid extraction concurrently, is assumed to be superior to the conventional two-step process, and this is particularly the case with regard to the extraction yield because the extraction solvent can remain in better contact with lipid bodies otherwise attached to cell debris [13]. This seemingly ideal approach, however, can only be realized with the proper choice of physical means; one promising option is a high shear mixer (HSM), as it is easy to handle, scalable, requires low energy levels, and can be used with high biomass concentrations.
The HSM, also known as a high-speed homogenizer, is fundamentally a mixing device that consists of a rotating rotor and a steady stator with a small gap (100–3000 μm), which induces high shear rates (20,000–100,000 s−1) with high rotor tip speeds (10–50 m/s) [14]. Such uniquely strong shear stress has been utilized not only for the mixing of viscous materials and biphasic systems, which are very difficult with the conventional static mixer, but also for other purposes, such as grinding, dissolving, and cell disruption [15]. Despite the fact that the HSM has a very high potential for cell disruption and lipid extraction, only limited research has been conducted for these purposes to date, apart from a few studies that only mentioned its potential in relation to dry microalgae [16, 17].
In this study, therefore, an HSM was utilized for a simultaneous process of cell disruption and lipid extraction from highly concentrated wet biomass (> 350 g/L). To this end, the oleaginous microalgae Aurantiochytrium sp. KRS101 was used as a model species of microalgae, as it is well suited for the concept of a microalgae-based biorefinery [18, 19]. The capabilities of the HSM with regard to cell disruption and lipid extraction were investigated by measuring the particle-size distribution and lipid extraction yield so as to assess and improve the wet extraction process of microalgae. Various factors such as the solvent polarity (Hansen solubility parameters) and the use of both dry and wet biomass samples were examined.
Materials and methods
Materials
The Aurantiochytrium sp. KRS101 biomass used in this study was provided by the Korea Research Institute of Bioscience and Biotechnology (KRIBB), and it was stored in a deep freezer (− 70 °C) until use. The water content of the harvested biomass was 61.27 ± 1.04 wt%. For wet extraction, the biomass was thawed immediately before the experiment, whereas it was freeze-dried for dry extraction. Chloroform, hexane, methanol, ethanol, and isopropanol (HPLC grade, Merck) were used as solvents for lipid extraction. Sulfuric acid (98%, Sigma-Aldrich) and nonadecanoic acid (≥ 99.5%, Sigma-Aldrich) were used as an acid catalyst and an internal standard, respectively, for the quantification of esterifiable lipids.
Particle-size analysis and microscopic observation
For sample preparation, amounts of 20 g/L of cell culture underwent mixing in the HSM at 15,000 rpm for 5, 10, 15, 20, and 30 min. The particle-size distribution of the KRS101 before and after HSM treatment was examined with a Coulter counter, model LS 230 (Beckman Coulter, USA). Optical microscopic images of the disrupted cells were obtained with a DM2500 microscope equipped with a DFC425C camera (Leica Microsystems, Germany).
Lipid extraction and quantification
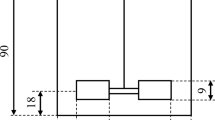
Lipid extraction from both wet and dry biomass was carried out in duplicate either with a shaker (Lab Companion CBS-350 Heating Shaker, Korea) or the HSM (DAIHAN Wisd HT1010, Korea), the geometry of which is shown in Fig. 1. First, an amount of 0.2 g of dry cells or 0.5 g of wet cells was put into a 50 ml glass tube with 10 ml of solvent to adjust the final biomass concentration to 20 g/L of solvent. The following six organic solvents or solvent mixtures were used in this experiment: chloroform, a chloroform/methanol mixture (2:1 v/v), hexane, a hexane/isopropanol mixture (3:2 v/v), methanol, and ethanol. Simple shaking was then carried out at 900 rpm for 10 min, while, for the HSM-based method, this was conducted at 15,000 rpm for 10 min. A cover was placed between the HSM and the glass vial to minimize evaporation of the solvents; to confirm the intended negligible solvent loss, the total volume of solvents was also checked before and after experiment. Each vial was centrifuged at 3,500 rpm for 10 min, and the supernatant-containing lipid was filtered using a 0.22 μm polytetrafluoroethylene (PTFE) filter.
The extracted lipid content was measured using the conventional gravimetric method. To do this, 5 ml of filtrate was decanted into a pre-weighed aluminum dish and evaporated under a fume hood. The extracted lipid content was calculated by the following equation:
where, Wl, Wd, and Wb represent the weights of the aluminum dish with the lipids, the aluminum dish, and the sample biomass weight, respectively, and Vt and Vd denote the total volume and the decanted volume of the solvent, respectively.
Quantification and characterization of the esterifiable lipids in the extracted lipids
The portion of esterifiable lipids in the extracted lipids was analyzed by converting the lipids into fatty acid methyl esters (FAMEs) via transesterification [20]. The extracted lipids were treated with chloroform containing nonadecanoic acid as an internal standard, methanol, and sulfuric acid at 100 °C for 20 min. After the samples were cooled to room temperature, distilled water was added for phase separation. The FAMEs in the organic phase were analyzed using a gas chromatograph (HP6890, Agilent, USA) with a flame ionized detector and an HP-INNOWAX column (30 m × 0.32 mm × 0.5 μm, Agilent, USA). The fatty acid composition was identified and quantified by a comparison of the retention times and peak areas with FAME standards.
Determination of the total esterifiable lipids in cells
Concentrated cells were lyophilized at − 80 °C for 2 days and lipids were extracted from 10 mg of freeze-dried cells with chloroform–methanol (2:1, v/v) following the modified Folch method [20]. For the transesterification of the extracted lipids, the aforementioned procedure was used. The total esterifiable lipids were found to be 379.7 ± 15.7 mg FAME/g dry biomass. The FAME yield for each experiment was calculated by the following equation:
Calculation of the Hansen solubility parameter
The Hansen solubility parameter (δ) of each organic solvent was calculated according to the following equation,
where δmix and δi are the Hansen solubility parameters of the mixture and component i, respectively, and X i is the volumetric fraction of component i in the solvent mixture. The δ i values of the solvents used in this study are summarized in Table 1.
Results and discussion
Cell-disruption effect of the HSM
To investigate the cell-disruption effect of the HSM on the KRS101, the particle-size distribution was measured (Fig. 2). The HSM treatment yielded three new major peaks at 1.8, 10, and 33 μm from an initial broad peak ranging from 17 to 30 μm. As the treatment time was increased, the peaks at 1.8 and 33 μm grew, while the peak at 10 μm decreased. This indicated that the cells were detached from the thick floc at an early time point, followed by the high shear-causing cell rupture and concomitant cell aggregation. A previous study indicated that lipids released from ruptured cells act as an adhesive and cause cells to form thick clusters [21]. Microscopic images supported such morphological changes (Supplement Fig. 1). The untreated cells, which existed as thick flocs and were filled with intracellular matter, were distinctly different from the treated, disintegrated, single cells that exhibited hollow, disrupted cell structures.
Lipid extraction of wet biomass using the HSM with various solvents
The effectiveness of the HSM for wet extraction was investigated together with various organic solvents and compared to that of a conventional shaker (Fig. 3). The extracted lipids were represented as esterifiable lipids (FAME) and non-esterifiable lipids (extracted lipids–esterifiable lipids). The lipid extraction performance of the shaker was strongly affected by the solvent type. Chloroform and chloroform/methanol led to high extracted lipid contents of about 462 and 341 mg/g dry biomass, respectively, while the other solvents ended in poor results. Chloroform and chloroform/methanol also yielded esterifiable lipids at a rate of more than 300 mg/g dry biomass, while the other solvents resulted in yields of less than 150 mg/g dry biomass. Although chloroform has repeatedly been reported to be superb for wet extraction, its industrial applicability is limited mainly due to its toxicity [22, 23]. For this reason, mild solvents such as hexane or alcohols, though less efficient, are used. It is, therefore, of necessity that a powerful mechanical means such as the HSM, instead of simple shaking, be employed, so that these less efficient yet safer solvents become equally effective.
Extracted lipid yields including esterifiable lipids (EL, diagonal line) and non-esterifiable lipids (NEL, no line) of conventional shaking (unfilled square) and high shear mixing (filled square) from wet biomass with various solvents. CL chloroform, CL/MeOH chloroform/methanol (2:1 v/v), Hex hexane, Hex/IPA hexane/isopropanol (3:2 v/v), MeOH methanol, EtOH ethanol
The HSM yielded far better overall results with all the tested solvents in comparison to the shaker in terms of both esterifiable and non-esterifiable lipids. The HSM produced esterifiable lipids at a rate higher than 300 mg/g dry biomass with all of the solvents except for methanol. Moreover, the HSM showed a higher non-esterifiable lipid yield than the shaker with all solvents, with a yield higher than 100 mg/g dry biomass. Notably, the HSM led to a higher lipid yield with hexane by fivefold compared to the shaker. This result is interesting and potentially useful, because hexane, a solvent of choice in the biomass-based industry due to its high selectivity for lipids and low latent heat for solvent recycling [24,25,26], has shown low effectiveness for wet extraction even when cells were completely disrupted (lipid recovery yield of up to 70%) [27]. This problem of suboptimal performance has not been completely resolved even with completely disrupted cells, because the mixability of wet biomass and hexane is very low [28]. With the HSM, however, hexane did not pose as much of a problem; and simultaneous cell disruption and lipid extraction were achieved.
This characteristic effectiveness of the HSM is related to cavitation. A previous study reported that the HSM, when operated at rotor speeds higher than 8000 rpm, gives rise to the cavitation phenomenon [15]. When cavitation occurs, microbubbles are formed and subsequently collapse, producing shock waves and resulting in momentary increases in the pressure (100–5000 atm) and temperature (500–15,000 K) [13]. This destructive force enables the solvent to overcome the water barrier surrounding the cells and to come into contact with lipid droplets inside the cell membrane, thereby resulting in effective lipid extraction [25]. In addition, the HSM can reduce the particle size of the dispersed phase (wet biomass) from 0.5 to 100 μm [14], which increases the contact area between the cell and the solvent, and, consequently, the mass transfer rate and lipid extractability [29]. This effect can be more effectively realized on a large scale even with a low operating rpm (1000–3000), because the values of the centrifugal force and shear stress are proportional to rotor diameter. For example, a large-scale batch HSM with a 24-inch diameter operating at 1000 rpm can produce shear rates which are five times higher than those of the HSM used in this study.
Effect of the HSM and solvent on the fatty acid composition
The quality of the lipids extracted by the HSM was analyzed in terms of the fatty acid composition and the findings were compared with those obtained from dry biomass using the modified Folch method (Table 2). All of the solvents, though different in terms of their extraction performance, produced nearly identical lipids containing significant amounts of palmitic acid (36.5–38.7%) and DHA (42.9–44.0%); the only exception was methanol. This rather consistent composition was somewhat contradictory to the findings of a previous study which showed that when a high-pressure homogenizer was employed as a mechanical means, the fatty acid composition varied substantially depending on the solvent type (heptane and chloroform/methanol 2:1) [30]. This was due to the fact that the lipid recovery step did not provide enough mixing to overcome the water barrier and therefore failed to disperse the biomass into the solvent medium effectively. The HSM-based treatment, which offers concomitant cell disruption and lipid extraction, appears to maintain the lipid composition (and DHAs) more effectively as compared to separate treatments owing to the high mass transfer rate between the lipid and the solvent facilitated by the strong mixing effect of the HSM. Methanol led to a lower fraction of saturated fatty acids and a higher fraction of unsaturated fatty acids than the other solvents, indicating that the highly polar property of the solvent, indeed, affected both the extractability and quality of the extracted lipids.
Effect of the Hansen solubility parameter of the solvent on the FAME yield
To investigate why different solvents exhibited different levels of efficiency on wet biomass, the Hansen solubility parameter (δ) of each solvent was calculated. The δ value is a convenient tool with which to determine the miscibility of a solute and a solvent [31]. Polar solvents such as water, methanol, and isopropanol have high δ values, while non-polar solvents such as hexane and chloroform have low δ values. Mixing takes place when the δ values of a solute and a solvent are sufficiently close. It was our working hypothesis that the δ values of the lipids, the water on the surface of the biomass, and the solvent would all affect lipid extractability in a rather complex manner.
The correlation between the δ value and the FAME yield was found and plotted in a graph (Fig. 4). In the case of the shaker, the FAME yield followed a quadratic equation with the δ value, and the highest value was obtained between 18.9 (chloroform) and 22.4 MPa1/2 (chloroform/methanol). At a relatively low δ value, the FAME yield decreased due to the low miscibility between water and the solvent, which inhibited the mass transfer of the solvent through the cell membrane. At a relatively high δ value, the FAME yield also decreased due to the low miscibility between the solvent and the crude lipids. It appears that it is very important to select a solvent with high miscibility with both water and crude lipids to attain a high lipid yield, and this is particularly true with simple shaking [32].
On the other hand, the HSM caused a high FAME yield with a δ value ranging from 14.9 to 26.5 MPa1/2 which 3.3 times wider than that of a conventional shaker. This indicated that the HSM was able to expand the effective range of usable solvents for lipid extraction. The HSM facilitated the interaction between the solvent with a δ value lower than chloroform and esterifiable lipids by reducing the influence of water molecules, as mentioned earlier in “Lipid extraction of wet biomass using the HSM with various solvents”. In the case of ethanol, which is more miscible with water than esterifiable lipids, the HSM helped to increase the FAME yield through an increase in the mass transfer rate compared to shaking. In contrast, methanol provided a low FAME yield even when used with the HSM, indicating that esterifiable lipids are not soluble in a solvent if their δ values are higher than that of methanol. In fact, a previous study using another thraustochytrid strain reported that methanol exhibited only mediocre performance even with dry biomass [28].
It should be noted that chloroform and chloroform/methanol showed high FAME extraction performance when both the shaker and the HSM were used. Some synergistic effects between chloroform-based solvents and water have been reported, but the underlying mechanism has not been fully elucidated [33]. Halim et al. [6] speculated that existing water molecules act to swell cells and increase the mass transfer rate of an organic solvent. Chloroform is an organic solvent that has a high solubility for neutral lipids, and at the same time, it has polarity sufficient to interact with water, in contrast to hexane. This difference is seen in the water solubility rates: 0.8% for chloroform vs 0.0014% for hexane [25].
Dry vs wet extraction: lipid extractability
To determine the performance drop of wet extraction relative to dry extraction, lipid extraction efficiency values (in terms of FAME yield) of the shaker and the HSM were obtained with hexane, hexane/isopropanol, and ethanol (Fig. 5). The FAME yield was high with the shaking/dry extraction process, and it exceeded 50% in 10 min. The yield with the shaking/wet extraction process, however, dropped to just below half of that obtained with the dry biomass. This indicated that shaking-based lipid extraction was more difficult with wet biomass than with dry biomass due to the barrier effect of water, which hindered the mass transfer of the solvents [17, 34]. With the HSM-assisted extraction, there was only a marginal difference between the FAME yields obtained with wet and the dry biomass; they were almost equal for hexane, and there was only a 5% difference for hexane/isopropanol and ethanol. This further supported the contention that the HSM indeed effectively helps the solvent overcome the water barrier on the cell membrane.
Simple shaking was found to be ineffective in terms of lipid extraction even from dry biomass when mild solvents such as hexane or alcohols were used. This showed that proper mixing is the key to ensure a satisfactory level of lipid yields, and it is particularly important if the extraction is to be done in a short treatment time [29]. During dry extraction, the HSM exhibited 30–50% higher FAME yields than the shaker for the same treatment times for hexane, hexane/isopropanol, and ethanol, which again could be attributed to its improved mass transfer rate of the solvent.
Conclusion
High shear mixing was demonstrated to be an effective physical means to attain cell disruption and lipid extraction from Aurantiochytrium sp. in a simultaneous manner, even with wet biomass. At 15,000 rpm, 10 min of mixing was enough to retrieve nearly all esterifiable lipids with most of the tested extraction solvents. In particular, the HSM showed equivalent lipid extraction yields for wet and dry extraction with hexane, hexane/isopropanol, and ethanol, thus indicating that high shear stress and a strong cavitation effect helped the solvents to overcome the interference of water. This mechanical mixing device, which has not been actively explored for the application of microalgae, can offer a powerful option for lipid extraction, especially from wet biomass.
References
Liang YN, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31(7):1043–1049. https://doi.org/10.1007/s10529-009-9975-7
Li Q, Chen GQ, Fan KW, Lu FP, Aki T, Jiang Y (2009) Screening and characterization of squalene-producing thraustochytrids from Hong Kong Mangroves. J Agr Food Chem 57(10):4267–4272. https://doi.org/10.1021/Jf9003972
Suen YL, Tang H, Huang J, Chen F (2014) Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of vitreoscilla hemoglobin. J Agric Food Chem 62(51):12392–12398. https://doi.org/10.1021/jf5048578
Lardon L, Helias A, Sialve B, Steyer JP, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43(17):6475–6481. https://doi.org/10.1021/es900705j
Kim J, Yoo G, Lee H, Lim J, Kim K, Kim CW, Park MS, Yang JW (2013) Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol Adv 31(6):862–876. https://doi.org/10.1016/j.biotechadv.2013.04.006
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30(3):709–732. https://doi.org/10.1016/j.biotechadv.2012.01.001
Samarasinghe N, Fernando S, Lacey R, Faulkner WB (2012) Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew Energ 48:300–308. https://doi.org/10.1016/j.renene.2012.04.039
Ma YA, Cheng YM, Huang JW, Jen JF, Huang YS, Yu CC (2014) Effects of ultrasonic and microwave pretreatments on lipid extraction of microalgae. Bioproc Biosyst Eng 37(8):1543–1549. https://doi.org/10.1007/s00449-014-1126-4
Byreddy AR, Barrow CJ, Puri M (2016) Bead milling for lipid recovery from thraustochytrid cells and selective hydrolysis of Schizochytrium DT3 oil using lipase. Bioresour Technol 200:464–469. https://doi.org/10.1016/j.biortech.2015.10.019
Lohman EJ, Gardner RD, Halverson L, Macur RE, Peyton BM, Gerlach R (2013) An efficient and scalable extraction and quantification method for algal derived biofuel. J Microbiol Meth 94(3):235–244. https://doi.org/10.1016/j.mimet.2013.06.007
Kim YH, Park S, Kim MH, Choi YK, Yang YH, Kim HJ, Kim H, Kim HS, Song KG, Lee SH (2013) Ultrasound-assisted extraction of lipids from Chlorella vulgaris using [Bmim][MeSO4]. Biomass Bioenerg 56:99–103. https://doi.org/10.1016/j.biombioe.2013.04.022
Piasecka A, Krzemiñska I, Tys J (2014) Physical methods of microalgal biomass pretreatment. Int Agrophys 28(3):341
Lee I, Han JI (2015) Simultaneous treatment (cell disruption and lipid extraction) of wet microalgae using hydrodynamic cavitation for enhancing the lipid yield. Bioresour Technol 186:246–251. doi:https://doi.org/10.1016/j.biortech.2015.03.045
Zhang JL, Xu SQ, Li W (2012) High shear mixers: a review of typical applications and studies on power draw, flow pattern, energy dissipation and transfer properties. Chem Eng Process 57–58:25–41. https://doi.org/10.1016/j.cep.2012.04.004
Shirgaonkar IZ, Lothe RR, Pandit AB (1998) Comments on the mechanism of microbial cell disruption in high-pressure and high-speed devices. Biotechnol Progr 14(4):657–660. doi:https://doi.org/10.1021/bp980052g
Lee AK, Lewis DM, Ashman PJ (2012) Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioenerg 46:89–101. doi:https://doi.org/10.1016/j.biombioe.2012.06.034
Balasubramanian RK, Doan TTY, Obbard JP (2013) Factors affecting cellular lipid extraction from marine microalgae. Chem Eng J 215:929–936. doi:https://doi.org/10.1016/j.cej.2012.11.063
Hong WK, Rairakhwada D, Seo PS, Park SY, Hur BK, Kim CH, Seo JW (2011) Production of lipids containing high levels of docosahexaenoic acid by a newly isolated microalga, Aurantiochytrium sp KRS101. Appl Biochem Biotech 164(8):1468–1480. doi:https://doi.org/10.1007/s12010-011-9227-x
Choi SA, Jung JY, Kim K, Kwon JH, Lee JS, Kim SW, Park JY, Yang JW (2014) Effects of molten-salt/ionic-liquid mixture on extraction of docosahexaenoic acid (DHA)-rich lipids from Aurantiochytrium sp KRS101. Bioproc Biosyst Eng 37(11):2199–2204. https://doi.org/10.1007/s00449-014-1197-2
Kwak M, Park W-K, Shin S-E, Koh H-G, Lee B, Jeong B-r, Chang YK (2017) Improvement of biomass and lipid yield under stress conditions by using diploid strains of Chlamydomonas reinhardtii. Algal Res 26:180–189
Yoo G, Yoo Y, Kwon JH, Darpito C, Mishra SK, Pak K, Park MS, Im SG, Yang JW (2014) An effective, cost-efficient extraction method of biomass from wet microalgae with a functional polymeric membrane. Green Chem 16(1):312–319. https://doi.org/10.1039/c3gc41695j
Li Y, Naghdi FG, Garg S, Adarme-Vega TC, Thurecht KJ, Ghafor WA, Tannock S, Schenk PM (2014) A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb Cell Fact 13:14. https://doi.org/10.1186/1475-2859-13-14
Willis RM, McCurdy AT, Ogborn MK, Wahlen BD, Quinn JC, Pease LF, Seefeldt LC (2014) Improving energetics of triacylglyceride extraction from wet oleaginous microbes. Bioresource Technol 167:416–424. https://doi.org/10.1016/j.biortech.2014.06.013
Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90(1):420–426
Dong T, Knoshaug EP, Pienkos PT, Laurens LM (2016) Lipid recovery from wet oleaginous microbial biomass for biofuel production: a critical review. Appl Energ 177:879–895
Moradi-Kheibari N, Ahmadzadeh H, Hosseini M (2017) Use of solvent mixtures for total lipid extraction of Chlorella vulgaris and gas chromatography FAME analysis. Bioproc Biosyst Eng 40(9):1363–1373. https://doi.org/10.1007/s00449-017-1794-y
Olmstead ILD, Kentish SE, Scales PJ, Martin GJO (2013) Low solvent, low temperature method for extracting biodiesel lipids from concentrated microalgal biomass. Bioresource Technol 148:615–619. https://doi.org/10.1016/j.biortech.2013.09.022
Byreddy AR, Gupta A, Barrow CJ, Puri M (2015) Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar Drugs 13(8):5111–5127. https://doi.org/10.3390/md13085111
Ranjan A, Patil C, Moholkar VS (2010) Mechanistic assessment of microalgal lipid extraction. Ind Eng Chem Res 49(6):2979–2985. https://doi.org/10.1021/ie9016557
Angles E, Jaouen P, Pruvost J, Marchal L (2017) Wet lipid extraction from the microalga Nannochloropsis sp.: disruption, physiological effects and solvent screening. Algal Res 21:27–34
Grima EM, González MJI, Giménez AG (2013) Solvent extraction for microalgae lipids. In: Borowitzka M, Moheimani N (eds) Algae for biofuels and energy. Developments in applied phycology, vol 5. Springer, Dordrecht, pp 187–205
Ramluckan K, Moodley KG, Bux F (2014) An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 116:103–108. https://doi.org/10.1016/j.fuel.2013.07.118
Im H, Lee H, Park MS, Yang JW, Lee JW (2014) Concurrent extraction and reaction for the production of biodiesel from wet microalgae. Bioresour Technol 152:534–537. https://doi.org/10.1016/j.biortech.2013.11.023
Halim R, Rupasinghe TWT, Tull DL, Webley PA (2014) Modelling the kinetics of lipid extraction from wet microalgal concentrate: a novel perspective on a classical process. Chem Eng J 242:234–253. https://doi.org/10.1016/j.cej.2013.12.070
Acknowledgements
This work was supported by the Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Science and ICT (ABC-2010-0029728). We thank Dr. Chul-Ho Kim at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) for providing Aurantiochytrium sp. KRS101.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwak, M., Kang, S.G., Hong, WK. et al. Simultaneous cell disruption and lipid extraction of wet aurantiochytrium sp. KRS101 using a high shear mixer. Bioprocess Biosyst Eng 41, 671–678 (2018). https://doi.org/10.1007/s00449-018-1901-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1901-8