Abstract
An anaerobic/aerobic moving-bed biofilm (A/O-MBBR) reactor system was constructed, and the treatment efficiency of aqueous antibiotics in wastewater was investigated. The effects of antibiotics on the microbial communities in the A/O-MBBR were also investigated. Under the optimized reaction conditions, removal of tetracycline antibiotics (TCs) was studied in a series of experiments. When a low concentration of tetracycline (TC) was added to the reactor system, high removal efficiency of conventional pollutants (TC concentration decreased from 10 turn to 2.8 μg L−1) was achieved. When mixed TCs (50 μg L−1) were added to the system, the removal efficiencies of chlortetracycline (CTC), TC and oxytetracycline (OTC) reached 52.03, 41.79, and 38.42%, respectively. TC degradation was decreased to 21.16% when the antibiotic concentration was 500 μg L−1; exposure to this TC concentration destroyed the community structure of the activated sludge bacteria in the reactor. The products of the biodegradation analysis revealed the possible degradation pathways functioning in the experimental A/O-MBBRs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Instruction
Increasing attention has been paid to surface water contamination and distribution of pharmaceuticals and personal care products (PPCPs) in recent years [1, 2]. PPCPs are discharged continuously into wastewater treatment systems and have received increasing attention in recent years as environmental contaminants [3, 4].
Among PPCPs, antibiotics in particular have increasingly been detected in wastewater, groundwater and surface water supply systems around the world in recent years [5]. Moreover, the negative effects of antibiotics on natural ecosystems and the development of pathogen resistance to several antibiotics and their intermediates have been reported [6]. Therefore, any wastewater containing antibiotics is a serious problem that should be treated before discharging it into the environment.
Tetracycline antibiotics (TCs) have a broad activity spectrum against a variety of Gram-positive and Gram-negative bacteria, so they have been broadly applied in livestock production and aquaculture despite their potential environmental risks [7]. TCs may be incompletely metabolized or eliminated in the body; as a result, TC residues have been detected in soil and sewage water, with typical concentrations ranging from 1 ng L−1 to 1 μg L−1 [8, 9]. There are no special treatment units for such drugs in the conventional water and wastewater treatment plants. TCs accumulate in surface water because of insufficient sewage treatment and continuous emission of TCs from treated organisms. Therefore, describing the behavior of these contaminants in wastewater and discovering potential removal mechanisms have become popular research topics.
Various physicochemical processes, including electrochemical adsorption [10], advanced oxidation [11], and photocatalysis [12], have been effectively used to treat wastewater containing high concentrations of antibiotics. However, these methods have several limitations, including high cost, high energy consumption, and generation of toxic by-products that are difficult to remove from treated water. In addition, physicochemical methods of removing antibiotics from wastewater are generally deemed unsuitable for treating large amounts of sewage with low antibiotic concentrations, which is a common situation, encountered in wastewater and water treatment facilities.
Biological methods are environmentally and friendly and cost-competitive alternatives to the physicochemical degradation processes that are commonly used for sewage degradation in urban environments [13]. Several biological treatment technologies have been used to achieve removal and degradation of TCs in wastewater. Huang [14] conducted research to assess the potential of A/A/O technology for antibiotic removal, which revealed that the removal efficiency of tetracycline (TC), oxytetracycline (OTC), and chlortetracycline (CTC) generally reached 60%. However, a mass balance analysis showed that sludge adsorption was responsible for removing 29, 38, and 39% of TC, OTC, and CTC, respectively, while biodegradation was responsible for removing only 21, 22 and 47% of these compounds, respectively. Xia et al. [15] used an anoxic–aerobic membrane bioreactor (A/O-MBR) to analyze TC degradation; the rate of antibiotic removal was as high as 92% after membrane filtration with a longer SRT (above 30 days). Shi et al. [16] performed an examination of the simultaneous TC sorption and biodegradation performance of nitrifying granular sludge, which revealed that, although TCs were rapidly adsorbed onto the granular sludge, the subsequent process of biodegradation was relatively slow. The technologies described above generally involve transferring antibiotics to a suitable environment rather than fundamentally degrading antibiotics into harmless products [17]. Therefore, more studies should be conducted to assess antibiotic degradation with new treatment technologies to achieve complete biodegradation or mineralization of TC antibiotics in effluent water following water treatment. Some studies [18,19,20] have shown that membrane bioreactors are an effective option for treating water containing low concentrations of antibiotics and high concentrations of other toxic compounds.
Moving-bed biofilm reactors (MBBRs) integrate the major advantages of activated sludge and biofilm technologies. MBBRs have good treatment efficiency, strong anti-shock load capability, and the ability to operate at low temperature. MBBRs can improve the quality of discharged water from wastewater treatment processes; therefore, they are recognized as an effective way to upgrade and refurbish wastewater treatment plants [21]. In particular, MBBRs have been reported to be effective for aqueous contaminant oxidation [22]. Some aromatic compounds with high wastewater concentrations and resistance to biodegradation have been treated using the MBBR process [23]. In addition, some aerobically recalcitrant pesticides can also be treated using this process [24]. These findings indicate that MBBRs can be applied as membrane bioreactors to achieve biodegradation of TCs in wastewater; however, this application of MBBRs has not been assessed in a controlled environment.
A proper pilot-scale model with a full set of “real” operational parameters can provide guidance for the application of MBBRs in operational wastewater treatment plants. In this study, a new A/O-MBBR was constructed and used to treat wastewater containing three typical tetracycline antibiotics. The effectiveness of the treatment method was evaluated by measuring the degradation and transformation of TCs in sewage. Due to the extreme importance of the adsorption and biodegradation processes in MBBRs, the requirements of MBBRs are much more stringent than those of conventional activated sludge technology. Therefore, determination of the optimal operational parameters of MBBRs is essential to achieve maximum treatment efficiency. In addition, the effects of TCs on microbial activity in the attached-growth biofilm system of the A/O-MBBR were also determined. TC degradation pathways and the effects of antibiotics on TC degradation were also assessed by analyzing the products of TC biodegradation via LC/MS. The goal of this study was to provide fundamental guidance for the application of MBBRs to treat wastewater containing TCs.
Materials and methods
Reagents and apparatus
Oxytetracycline hydrochloride, chlortetracycline hydrochloride, and tetracycline hydrochloride (≥ 98.0%) were purchased from J&K Chemical (Shanghai, China). Three stock standard solutions of antibiotics were prepared by dissolving 100 mg of each antibiotic in 100 mL of methanol. Working standard solutions were prepared in water/acetonitrile (9:1, v/v) by diluting the stock standard solution. The working standard solutions were used for fortification in the recovery experiments and for preparation of calibration standards. All other chemicals and solvents were of analytical grade and purchased from a commercial supplier (Shanghai Guoyao Regents Co., Shanghai, China).
The antibiotic degradation tests were performed using an Agilent 1200 High Performance Liquid Chromatograph (HPLC). HPLC-grade solvents were used for liquid chromatography. An Agilent Eclipse XDB C18 column was used. The mobile phase was a solution of methanol–acetonitrile (0.01 mol L−1) oxalic acid (Shanghai Qiangshun Chemicals Company, chromatography grade). The flow rate of the mobile phase was 1.0 mL min−1.
A/O-MBBR reactor system
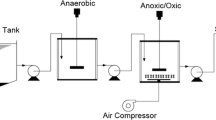
A schematic representation of the combined moving-bed biofilm reactor and secondary sedimentation system (A/O-MBBR) (designed and assembled with Plexiglas plates) used in this study is shown in Fig. 1. Each treatment system consisted of an anaerobic reaction section with an aerobic moving-bed biofilm section. The anaerobic reaction reactor had an effective volume (liquid and activated sludge) of 12 L, while the aerobic reaction reactor had an effective volume (liquid and suspended biomass; the total biomass volume was 30% of the working volume of each aerobic reactor) of 16 L. The water from the aerobic reactor entered the secondary sedimentation tank, and the final effluent was filtered by a flat membrane with a pore size of 0.1 μm, which prevented antibiotics from entering the sewage system. Reactor samples comprising effluent from the anaerobic, aerobic, and membrane sections were collected hourly from delivery ports. Aeration was supplied by an air flow meter at the bottom of the reactors to control the oxygen concentration in the mixed liquor. The continuous operation cycle of the anaerobic and aerobic reactors was controlled using a digital timer. The effluent flow rate in the MBBR reactor system was controlled by a flowmeter and a peristaltic pump at the bottom of the reactor outlet. The retention time was set by controlling the flow rate.
Reactor operation and antibiotic removal
Sludge from the anaerobic zone of reactors at a municipal wastewater treatment plant was cultivated and inoculated. After static settlement and supernatant removal, the sludge was added to the reactor and supplemented with artificial prepared domestic sewage to reach the sludge concentration in the reactor necessary for testing (4000 mg L−1). In the aerobic zone, the intake water temperature range of the carrier biofilm was 20–25 °C. The HRT was maintained at less than 8 h. The dissolved oxygen concentration (DO) was 3–7 mg L−1. The COD concentration of the intake water was 300–400 mg L−1. The ammonia nitrogen concentration was 30–40 mg L−1. The reactor was run continuously to cultivate and naturalize the microbial populations. The microorganisms attached to the carrier gradually diversified as the biofilm thickened, while some of the biofilm aged and was lost. After 30 days of operation, the removal rates of organics and ammonia nitrogen gradually stabilized at 85%. Stable reactor operation was considered to be an indicator that the biological community within the A/O-MBBR system was stable. The operating conditions for the reactors were as follows: temperature, 20–25 °C; inlet water COD concentration, 300–400 mg L−1; dosing rate of suspended filler in the aerobic zone, 30%; pH, 6.5–7.8; HRT, 8 h; reflux ratio, 100%. Finally, TC (1 μg L−1) was added to the influent to acclimatize the sludge in the reactor; after 10 days, the system was stable again.
TCs were added to the influent of the A/O-MBBR system at different concentrations to study the degradation efficiency of the system. The experiment was divided into four stages according to the concentration of antibiotics: 50, 100, 150, and 200 μg L−1. Each phase of the trial lasted for approximately 96 h.
Water sample collection and antibiotic detection
Water samples (50 μL) from the anaerobic, aerobic, and secondary sedimentation areas were collected separately, filtered using a microporous membrane, and placed into the HPLC system. The column temperature was set at 25 °C. The mobile phase was methanol, acetonitrile, and oxalic acid (0.01 mol L−1) (12:12:76, v/v). The flow rate of the mobile phase was 1.0 mL min−1. Antibiotic detection was performed at 268 nm. A standard curve was generated using gradient dilutions of the standard stock solutions. The calibration standards of each compound were made in water at concentrations of 10, 25, 50, 100, 200, 500, and 1000 μg L−1.
Raw wastewater and the MBBR biofilm
In the main experiments, artificial domestic sewage was prepared every 2 days with tap water, cane sugar (245.0 mg L−1), CH3COONa (285.6 mg L−1), NH4Cl (229.3 mg L−1), KH2PO4 (40 mg L−1), and NaHCO3 (354.3 mg L−1) as the main nutrient sources for microorganisms. Mineral components and a trace element solution of MgSO4·7H2O (20 mg L−1), CaCl2 (20 mg L−1), FeCl3·6H2O (0.60 mg L−1), Na2MoO4·2H2O (0.26 mg L−1), CuSO4·5H2O (0.06 mg L−1), and MnCl2·4H2O (0.13 mg L−1) were also added to the artificial sewage [25].
Sludge from the anaerobic zone of reactors at a municipal wastewater treatment plant was cultivated and inoculated. After static settlement and supernatant removal, the sludge was added to the reactor and supplemented with artificial prepared domestic sewage to reach the sludge concentration in the reactor necessary for testing (4000 mg L−1). The microorganisms attached to the carrier gradually diversified as the biofilm thickened, while some of the biofilm aged and was lost. After 30 days of operation, the removal rates of organics and ammonia nitrogen gradually stabilized at 85%. Stable reactor operation was considered to be an indicator that the biological community within the A/O-MBBR system was stable.
Reactor operation and antibiotic removal
The operating conditions for the reactors were as follows: temperature, 20–25 °C; inlet water COD concentration, 300–400 mg L−1; COD:N:P ratio, 100:5:1. The HRT was less than 8 h. The intake water dissolved oxygen concentration in the aerobic zone was 3–7 mg L−1. The dosing rate of suspended filler in the aerobic zone was 30%. The pH of the synthetic wastewater was maintained at 6.5–7.8 ± 0.2 by the addition of NaHCO3. The ammonia nitrogen concentration was 30–40 mg L−1. The reactor was run continuously to cultivate and naturalize the microbial populations. The reflux ratio was 100%. Antibiotics (1 μg L−1) were added to the influent to acclimatize the sludge in the reactor; after 10 days, the system was stable again. Raw wastewater and MBBR permeate were collected and analyzed every day. The reported values are the average values across 3 days.
TCs were added to the influent of the A/O-MBBR system at different concentrations to study the degradation efficiency of the system. The experiment was divided into four stages according to the concentration of antibiotics: 50, 100, 150, and 200 μg L−1. Each phase of the trial lasted for approximately 96 h.
Water sample collection and antibiotic detection
Water samples (50 μL) from the anaerobic, aerobic, and secondary sedimentation areas were collected separately, filtered using a microporous membrane, and placed into the HPLC system. The column temperature was set at 25 °C. The mobile phase was methanol, acetonitrile, and oxalic acid (0.01 mol L−1) (12:12:76, v/v). The flow rate of the mobile phase was 1.0 mL min−1. Antibiotic detection was performed at 268 nm. A standard curve was generated using gradient dilutions of the standard stock solutions. The calibration standards of each compound were made in water at concentrations of 10, 25, 50, 100, 200, 500, and 1000 μg L−1.
Results and discussion
Optimization of reactor operating parameters
Biological removal of antibiotics by MBBRs is typically influenced by biochemical oxygen demand (BOD5), hydraulic retention time (HRT), mixed liquor-suspended solids (MLSS), pH, temperature, and other factors [26].
In A/O-MBBRs, the MLSS is an adsorption anaerobic pool that may enhance adsorption, biodegradation, and other functions. In addition, the MLSS is an indicator of the density of microorganisms in the reactor. Three MLSS concentrations were tested. An MLSS concentration of 3500 mg L−1 achieved the best performance. The main reason for the phenomenon described above is that the suspended packing offers ample space for microbial growth under nutrient-rich conditions, so the activated sludge and biofilm mass provide an A/O-MBBR that is able to withstand a greater load of pollutants. The synergistic activities of microorganisms at different positions in the reactor maintain steady reactor operation when the concentration of pollutants in the influent changes.
The influence of pH and DO was also tested. Among the tested pH values (6.5, 7.2, 7.8 and 8.4), 7.8 ± 0.2 was determined to be the optimal value. Optimal reactor performance was achieved when DO was maintained in the range of 3.5–6.8 ± 0.2 mg L−1 in the aerobic pool.
Solids retention time (SRT) is a key factor influencing bioreactor performance, because the microbial community and population may change in the inoculation sludge when the SRT is lengthy [27]. To closely model an MBBR at an operational wastewater treatment plant, the HRT was set at 8 h.
The organic inlet provides nutrients to support microbial growth. Different COD concentrations (200–300, 300–400, and 400–500 mg L−1) were tested for periods of 15 days, while the other conditions were kept constant (ammonia nitrogen concentration, 30 mg L−1; HRT, 8 h; control MLSS, 3000 mg L−1). The results of these experiments are shown in Fig. 2.
Influence of COD removal rate on the process performance of the AO-MBBR. Three batch experiments were conducted to assess the influence of changes in the influent and effluent COD concentrations on the removal rate of the reactor. The error bars represent the standard deviation of three parallel measurements
As shown in Fig. 2, when the inlet COD concentration was increased, the COD removal rate of the MBBR reactor remained stable. The COD removal efficiency was not obviously influenced when the inlet COD concentration was increased from 200 to 500 mg L−1.
However, NH4–N removal efficiency showed a slight upward trend as the inlet COD was increased, as shown in Fig. 3. The COD and ammonia nitrogen degradation rates changed when the temperature was changed. The ammonia nitrogen removal rate did not show instability when the temperature was decreased from 20 to 15 °C in a deammonification moving-bed biofilm reactor [28]. Another study showed that a high maximum total nitrogen removal rate was achieved at a relatively low temperature of 20 °C in a moving-bed biofilm reactor utilizing an anaerobic ammonium oxidation (anammox) process [29]. These results were also similar to those previously reported for municipal activated sludge systems [19].
In our experiment, when the temperature was adjusted from 10 to 35 °C, the removal efficiencies of COD and ammonia nitrogen initially increased as the temperature was increased, but they showed a downward trend when the temperature was increased above 25 °C. These results show that proper control of the inlet COD and reactor temperature is essential to ensure maximum reactor biomass and smooth operation. Therefore, the reactor system temperature was controlled in the range of 20–25 °C by adjusting the ambient temperature in the laboratory.
Monomer antibiotic degradation efficiency in the reactor
When the reactor was stable, solutions with different concentrations of TCs were added to the influent to investigate TC removal efficiency. Water samples were collected from different sections of the reactor at different time intervals over 3 consecutive days. The changes in the TC concentration in each section and among batches are shown in Fig. 4. The average TC removal efficiency was calculated as follows:
where C 0 is the initial TC concentration and C is the detected TC concentration in each reactor area during the operating period.
As shown in Fig. 4, TC antibiotic removal efficiency declined gradually as the TC concentration was increased. When the TC concentration was increased from 150 to 200 μg L−1, the antibiotic removal rate decreased from 37.65 to 33.19%.
During the process of fostering the sludge biofilm, we found that adding antibiotics at low concentrations (1–10 μg L−1) enhanced the drug resistance of bacteria and thus increased the removal rate of the reactor (when the inlet included 10 μg L−1 TC was added, the TC removal rate reached 72.06%, the TC concentration decreased from 10 turn to 2.8 μg L−1). The reflux ratio was 100%. The high rate of TC degradation at low concentrations was due to sludge backflow, which prolonged the time during which the TCs were exposed to microorganisms. This finding provides a basis for potential improvements to other wastewater treatment technologies, such as sequential batch reactors (SBRs). Adding antibiotics at low concentrations during the process of fostering the sludge biofilm enhanced the drug resistance of the bacteria in the reactor. These findings suggest that A/O-MBBR reactors can be applied in wastewater treatment plants to enhance antibiotic degradation in wastewater with low concentrations of antibiotics.
Removal of hybrid antibiotics
An antibiotic solution containing hydrochloride tetracycline (TC), CTC, and OTC was added to the reaction system. The antibiotic removal efficiencies of the system are shown in Table 1.
As shown in Table 1, the CTC degradation rate reached 46% in the A/O-MBBR system, whereas the COD removal rate was higher than 54%. As the total antibiotic concentration was increased, the efficiency of antibiotic degradation decreased gradually. A total antibiotic concentration as high as 200 μg L−1 did not inhibit antibiotic degradation. These findings indicate the concentration range within which the A/O-MBBR process can decompose antibiotics.
Overall antibiotic degradation performance of the A/O-MBBR
The results described above show that the A/O-MBBR effectively removed antibiotics at low concentrations. However, the degradation efficiency of the reactor significantly decreased as the antibiotic concentration was increased. Therefore, the antibiotic concentration was increased to 500 μg L−1 to further study the efficiency of the A/O-MBBR process for removing high-concentration antibiotics from wastewater. The results showed that only a small portion of each antibiotic was degraded when the antibiotic concentration was 500 μg L−1. The removal rates of OTC, TC, and CTC were 19.45, 21.16, and 22.32%, respectively. The removal rates of COD, NH4 +–N, and total-P reached 49.99, 48.40, and 35.54%, respectively. However, when the antibiotic concentration was 500 μg L−1, some of the sludge floated in the reactor and emitted a fishy smell, which indicated that the activity of the sludge microorganisms declined dramatically. Indeed, the observed antibiotic removal under these conditions was likely due to adsorption of the biofilm on the suspended fillers, not degradation.
The degradation efficiencies of the A/O-MBBR for monomer and hybrid antibiotics are shown in Fig. 5.
As shown in Fig. 5, when only TC was added to the influent, the degradation efficiency of the reactor decreased as TC concentration was increased, but the performance of the reactor remained relatively stable when controlling the TC concentration in a certain range, which showed that the microbial system in the pilot test system was best suited to TC. When a large number of sensitive bacteria slowed their metabolism or became dormant, bacteria with TCs resistance or other advantages gradually became predominant, and adsorption/metabolism by Zoogloea became the main process by which contaminants were removed from the system.
The three tested types of antibiotics have similar structures and similar physicochemical properties, but their degradation efficiency in biological systems varies greatly. The experimental results indicate that, under the same reaction conditions, the reactor degraded CTC most effectively, followed by TC, while OTC was degraded least effectively.
As the dosage was increased, the degradation efficiencies of CTC, TC, and OTC were slightly reduced, while the total descend range increases. When the antibiotic concentrations were increased, degradation of each antibiotic was impaired. However, when the antibiotics were added simultaneously, TC was degraded preferentially when the dosage was 500 μg L−1, while CTC was degraded preferentially when the concentration was 200 μg L−1. The tested antibiotics had different degradation rates when they were present at the same concentration, because the adaptive capacity of the bacteria to each drug differed; TC was degraded most efficiently, because it was added at a low concentration at the first stage of the reactor, which induced more bacterial resistance. Further study of the mechanisms by which bacteria in wastewater treatment systems adapt to antibiotics and other drugs could lead to the development of strains with enhanced resistance mechanisms, thus improving the efficiency of drug removal from wastewater. The TC removal rate reported in this paper was lower than that reported in a previous study [15], in which the TC degradation efficiency of the A/O-MBR process was 93.6%, but this reactor system removed a large percentage of both COD and TOC.
Influence of the antibiotic concentration on the microbial population in the reactor
The microbial population provides a measure of overall microbial activity and consequently indicates whether stimulation or inhibition of the microbial communities is occurring. The microbial population was investigated during the process of antibiotic degradation. At first, the A/O-MBBR process achieved steady-state operation; the pollutant removal efficiency was stable, the sludge settling ratio was 15%, and the activated sludge appeared brown, which indicated that the microbial activity was good. When the concentration of antibiotics was increased, the color of the activated sludge became darker, while it exuded a malodorous, fishy smell. The activated sludge and biofilm in the reactor were sampled and examined by microscopy (Fig. 6).
Comparison of the early and last stages of the activated sludge (a–d) in reactor shows that the sessile ciliate protozoa population is gradually reduced or disappears with the TCs concentration increased. When the mixed antibiotic concentration was increased to 500 μg L−1, a large number of filamentous bacteria were observed, while the structure of the sludge became loose, and the sludge settling ratio reached 50–60%. The diversity and abundance of microorganisms were decreased, and the attached-growth biofilm on the suspended carrier was thin or absent.
Considering the decrease in the microbial population when high concentrations of antibiotics were present in the environment, as well as the change in the antibiotic removal efficiency during the treatment process (from 43 to 22%), it can be concluded that the removal of antibiotics from reactor was mainly due to abiotic adsorption rather than biodegradation.
Although the antibiotic concentration in the original discharged water was relatively high, the concentration of antibiotics in the inlet of the MBBR reactor was relatively low. Therefore, it can be concluded that this A/O-MBBR technology should be an effective treatment option for wastewater containing low concentrations of antibiotics.
Analysis of antibiotic degradation products and pathways
Although the antibiotic removal efficiency of wastewater treatment systems has been studied before, a few studies have analyzed the degradation pathways of antibiotics in such systems. Analysis of water samples from different positions in the reactor showed that the concentration of antibiotics was reduced dramatically in the anaerobic zone; however, the concentration of antibiotics in this part of the reactor tended to stabilize over time. Accordingly, in the reactors used in this study, antibiotics were absorbed by activated sludge, followed by hydrolysis and bacterial digestion within the activated sludge.
During the hydrolytic process, hydroxyl groups and enol structures on the tetraphenyl TC skeleton are vulnerable to attack and hydrolysis to form open-loop lipid compounds. These compounds undergo hydrolysis, in which the macromolecular carbon ring is used as the carbon source and degraded into CO2 and H2O, while the amino group is utilized for bacterial proteins via ADP ribosylation. As for the hydrolysis conditions of antibiotics and biodegradation products in water, Xuan et al. [30] showed that the speed of hydrolysis was maximal at high temperature and in neutral environmental conditions, as opposed to alkaline or acidic environments. Moreover, antibiotic degradation in aerobic environments is significantly more rapid than antibiotic degradation in anoxic environments [31].
The LC/MS analysis showed that TC hydrolysis rarely occurred in the anaerobic zone, but TC degradation was obvious in the aerobic zone. Among the tested antibiotics, the degradation efficiency of CTC was much higher than that of OTC. After examining the molecular structures of OTC, TC, and CTC, we surmised that the difference in biodegradation efficiency among these compounds might be due to the hydroxyl group on the third benzene of OTC, which easily forms a hydrogen bond with the ortho amino group, making it relatively difficult to hydrolyze. In addition, the chlorine atom on the first benzene ring of CTC is particularly susceptible to electrophilic substitution, which breaks the benzene ring down into smaller molecular groups.
Conclusions
In this study, an anaerobic/aerobic MBBR reactor model was constructed, and the effect of operation factors on reactor performance and the microbial community composition of the reactor were investigated. This work assessed the removal performance of an MBBR for treating wastewater containing low concentrations of TCs. The reactor achieved good TC removal efficiency when three TCs were added at a concentration of 50 μg L−1; at this concentration, the TC degradation rate was greater than 42% with an HRT of 8 h. Adding TCs at low concentrations (1–10 μg L−1) during the initial stage of sludge development may increase the TC removal efficiency of the reactor. The bacterial community analyses suggest that the bacteria in the reactor were affected by changes in the concentrations of TCs when the drugs were added individually and simultaneously. The removal efficiencies of CTC, TC, and OTC seemed to be inversely related to the complexity of their molecular structures.
References
Yang Y, Okb YS, Kimc KH, Kwond E, Tsanga YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ 596(15):303–320
Bui XT, Vo TPT, Ngo HH, Guo WS, NguyenT T (2016) Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Sci Total Environ 563–564:1050–1067
Cazes MD, Abejon R, Belleville MP, Sanchez-Marcano J (2014) Membrane bioprocesses for pharmaceutical micropollutant removal from waters. Membranes 4:692–729
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446
Prabhasankar VP, Joshua DI, Balakrishna K (2016) Removal rates of antibiotics in four sewage treatment plants in South India. Environ Sci Pollut Res 23(9):8679–8685
Clellan KM, Halden RU (2010) Pharmaceuticals and personal care products in archived US biosolids from the 2001 EPA national sewage sludge survey. Water Res 44(2):658–668
Matsuia Y, Ozu T, Inoue T, Matsushia T (2008) Occurrence of a veterinary antibiotic in streams in a small catchment area with livestock farms. Desalination 226(1–3):216–221
Vieno NM, Hurkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination in a pilot scale drinking water treatment plant. Environ Sci Technol 41(14):5077–5084
Verma B, Headley JV, Robarts RD (2007) Behaviour and fate of tetracycline in river and wetland waters on the Canadian Northern Great Plains. J Environ Sci Health Part A 42(2):109–117
Yang SF, Lin CF, Wu CJ, Ng KK, Lin AY (2012) Fate of sulfonamide antibiotics in contact with activated sludge-sorption and biodegradation. Water Res 46(4):1301–1308
Khan MH, Bae H, Jungb JY (2010) Tetracycline degradation by ozonation in the aqueous phase: proposed degradation intermediates and pathway. J Hazard Mater 181(1–3):659–665
Aba-Guevara CG, Medina-Ramírez IE, Hernández-Ramírez A, Jáuregui-Rincón J, Lozano-Álvarez JA (2017) Comparison of two synthesis methods on the preparation of Fe, N–Co-doped TiO2 materials for degradation of pharmaceutical compounds under visible light. Ceram Int 43(6):5068–5079
Zekker I, Rikmann E, Kroon K, Mandel A, Mihkelson J, Tenno T, Tenno T (2017) Ameliorating nitrite inhibition in a low-temperature nitritation–anammox MBBR using bacterial intermediate nitric oxide. Int J Environ Sci Techol 15:1–15
Huang MH, Zhang W, Liu C, Hu HY (2015) Fate of trace tetracycline with resistant bacteria and resistance genes in an improved AAO wastewater treatment plant. Process Saf Environ Protect 93:68–74
Xia SQ, Jia RY, Feng F, Xie K, Li HX, Jing DF, Xu XT (2012) Eefect of solids retention time on antibiotics removal performance and microbial communities in an A/O-MBR process. Biores Technol 106(2):36–43
Shi YJ, Wang XH, Qi M, Diao H, Gao MM, Xing SF (2011) Sorption and biodegradation of tetracycline by nitrifying granules and the toxicity of tetracycline on granules. J Hazard Mater 191:103–109
Chiarello M, Minetto L, Giustina SVD, Beal LL, Moura S (2016) Popular pharmaceutical residues in hospital wastewater: quantification and qualification of degradation products by mass spectroscopy after treatment with membrane bioreactor [J]. Environ Sci Pollut Res 16:1–11
Song XY, Liu R, Chen LJ, Kawagishi T (2017) Comparative experiment on treating digested piggery wastewater with a biofilm MBR and conventional MBR: simultaneous removal of nitrogen and antibiotics. Front Environ Sci Eng 11(2):11–18
Aydin E, Şahin M, Taşkan E, Hasar H, Erdem M (2016) Chlortetracycline removal by using hydrogen based membrane biofilm reactor. J Hazard Mater 320:88–95
Tran NH, Chen H, Reinhard M, Mao F, Gin YH (2016) Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res 104:461–472
Hosseini KE, Alavi Moghaddam MR, Hashemi SH (2011) Post-treatment of anaerobically degraded azo dye Acid Red 18 using aerobic moving bed biofilm process: enhanced removal of aromatic amines. J Hazard Mater 195(1):147–154
Plósz BG, Vogelsang C, Macrae K, Heiaas HH, Lopez A, Liltved H, Langford KH (2010) The BIOZO process—a biofilm system combined with ozonation: occurrence of xenobiotic organic micro-pollutants in and removal of polycyclic aromatic hydrocarbons and nitrogen from landfill leachate. Water Sci Technol 61(12):3188–3197
Kawan JA, Hasan HA, Suja F, Jaafar O, Abd-Rahman R (2016) A review on sewage treatment and polishing using moving bed bioreactor (MBBR). J Eng Sci Technol 11(8):1098–1120
Chen S, Sun D, Jong-Shik C (2007) Treatment of pesticide wastewater by moving-bed biofilm reactor combined with Fenton-coagulation pretreatment. J Hazard Mater 144:577–584
Jia RY, Jing DF, Li HX, Xie K, Huang TS, Zhang ZQ, Xu XT, Xia SQ (2011) Membrane bioreactor process for removing selected antibiotics from wastewater at different solids retention times. Fresenius Environ Bull 20(3a):754–763
Kovalova L, Siegrist H, Singer H, Wittmer A, McArdell CS (2012) Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ Sci Technol 46:1536–1545
Sombatsompop K (2011) A comparative study of sequencing batch rector and moving bed sequencing batch reactor for piggery wastewater treatment. Maejo Int J Sci Technol 5(02):191–203
Zekker I, Tenno T, Tenno T (2016) Step-Wise temperature decreasing cultivates a biofilm with high nitrogen removal rates at 9 °C in short-term anammox biofilm tests. Environ Technol 37(15):1933–1946
Daija L, Selberg A, Rikmann E, Zekker I, Tenno T, Tenno T (2016) The influence of lower temperature, influent fluctuations and long retention time on the performance of an upflow mode laboratory-scale septic tank. Desalin Water Treat 57(40):1–9
Xuan R, Arisi L, Wang Q, Yates SR, Biswas KC (2010) Hydrolysis and photolysis of oxytetracycline in aqueous solution. J Environ Sci Health Part B 45(1):73–78
Yang YD, Chen DH, Chen L (2010) Studies on characteristics of adsorption and biodegradation of typical PPCPs oxytetracycline in the activated sludge treatment system [J]. Technol Water Treat 36(5):66–69 (in Chinese)
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (Project no. 51008216) and the research fund for the Doctoral Program of Higher Education of China (31400347) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, HY., Liu, YD. & Dong, B. Biodegradation of tetracycline antibiotics in A/O moving-bed biofilm reactor systems. Bioprocess Biosyst Eng 41, 47–56 (2018). https://doi.org/10.1007/s00449-017-1842-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1842-7