Abstract
N-Acetylglucosamine-2-epimerase (AGE) and N-acetylneuraminic acid lyase (NAL) were immobilized for synthesis of N-acetylneuraminic acid (Neu5Ac) on three resins: Amberzyme oxirane resin (AOR), poly (styrene-co-DVB)-Br resin (PBR) and amino resin (AR). The loading capacity and immobilized enzyme activity showed that AR was the best carrier. Three methods of glutaraldehyde cross-linking were tested and simultaneous cross-linking and immobilization was demonstrated to be the best method. The functional properties of immobilized AGE and NAL were studied and compared to those of the free enzyme. The highest enzyme activities of free and immobilized AGE were obtained in 0.1 M potassium phosphate buffer at pH 7.5 and a temperature of 37 °C. Comparatively, the highest NAL activities were at pH 8.5. Meanwhile, an increase in K m (from 1.14 to 1.31 mg·mL−1 for AGE and from 1.05 to 1.25 mg·mL−1 for NAL) and a decrease in V max (from 177.53 to 106.37 µg·min−1 mL−1 for AGE and from 126.41 to 95.96 µg·min−1 mL−1 for NAL) were recorded after immobilization. The AR–glutaraldehyde–enzyme system exhibited better thermal stability than the free enzyme, and retained 72% of its initial activity even after eight repeated runs. The apparent activation energy (E a) of the free and immobilized AGE (NAL) was 117.14 kJ·mol−1 (124.21 kJ·mol−1) and 78.45 kJ·mol−1 (66.64 kJ·mol−1), respectively, implying that the catalytic efficiency of the immobilized enzyme was restricted by mass-transfer rather than kinetic limit. Subsequently, Neu5Ac production from GlcNAc using immobilized enzymes in one reactor was carried out resulting 101.45 g·L−1 of Neu5Ac and the highest conversion ratio of 82%. This method of enzyme immobilization may have a promising future for Neu5Ac production in industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that natural biomolecules often play an important role in the maintenance of numerous physiological processes. Sialic acids (Neu5Ac), derivatives of the nine-carbon monosaccharide neuraminic acid, are distributed widely in animal tissues and, to a lesser extent, in other species ranging from plants and fungi to yeasts and bacteria. More than 40 forms of molecular species involving N- and O-substituted derivatives of neuraminic acid have been discovered in a variety of organisms [1]. Among them, N-acetyl-d-neuraminic acid (Neu5Ac) is the most ubiquitous from the bacteria to mammals and the almost only form found in humans. Due to its multifunctionality, Neu5Ac were expected to be utilized extensively as pharmaceuticals in the treatment of virus infection [2, 3], as diagnostic reagents in the detection of malignant and inflammatory diseases [4, 5], and synthesized in vitro for biologically functional sialyloligosaccharides as starting material [6]. Since Neu5Ac have attracted interest and increasing amounts of Neu5Ac are required, there is clearly a need for the development of an economic and efficient process for the large-scale manufacture of Neu5Ac.
Recently, AGE from porcine kidney [7], Synechocystis sp. PCC6803 [8], and Anabaena sp. CH1 [9] has been identified and used in the two-step enzymatic production of Neu5Ac coupled with Escherichia coli-expressed NAL in the soluble form [10, 11] or as whole cells [12] as biocatalyst, as shown in Fig. 1. Kragle et al. [10] developed a membrane reactor to produce Neu5Ac with a conversion rate of 18%. Maru et al. [11] employed recombinant enzyme for large-scale production of Neu5Ac from GlcNAc, after 240 h of reaction during which pyruvate was added, and 77% of GlcNAc was converted into Neu5Ac. Lee et al. [9] used a reaction with whole cells and obtained 33.3% reaction rate from GlcNAc within 12 h. One-step purification and immobilization of His-tagged protein via Ni2+-functionalized nanoparticles was also attempted [13], but its dispersion easiness and low reusability were unfavorable for industrial Neu5Ac production. All of these methods have some drawbacks, such as lengthy reaction time, low reaction rate and lack of stability. The objective of this research was to develop an efficient and recyclable process for synthesis of N-acetyl-d-neuraminic acid using immobilized NAL and AGE as biocatalysts.
Enzyme immobilization is very important in industrial applications and has considerable advantages such as the possibility of continuous processing, enzyme reuse and a reduction in autodigestion [14]. The immobilization of biologically active species on a large variety of supports has given rise to a great number of studies due to both its academic and practical uses [15, 16]. Therefore, the development of cost-effective and efficient strategies for enzyme immobilization for use as industrial biocatalysts has attracted significant attention in scientific and engineering communities [17]. However, the selection of an immobilization strategy should be based on the effectiveness of immobilized enzyme biocatalysis, overall enzyme activity, deactivation and regeneration characteristics, cost of the immobilization procedure and toxicity of the immobilization [18, 19]. In this study, N-acetylglucosamine-2-epimerase (AGE) and N-acetylneuraminic acid lyase (NAL) (NAL gene from E. coli K12 and AGE from Synechocystis sp. PCC 6803) were immobilized, respectively, for synthesis of Neu5Ac on three resins: Amberzyme oxirane resin (AOR), poly (styrene-co-DVB)-Br resin (PBR) and amino resin (AR). Amino resin is a highly porous solid support material and has many important advantages for enzyme immobilization: (1) the ability to react rapidly by removing the enzyme from the reaction solution, (2) the production is not contaminated by the enzyme, (3) easy separation of the enzyme from substrates and products, (4) its potential reuse and application using a continuous packed-bed reactor. Glutaraldehyde (GA), as a protein cross-linking agent, has undoubtedly found a wide application in enzyme immobilization technology [20, 21].
To get the best performance, a method which coupled immobilization and cross-linking simultaneously was carried out, and the immobilized enzyme was characterized. Furthermore, the two-step bioconversion of GlcNAc to Neu5Ac was carried out in a single reactor. And a batch feed strategy for the continuous production of Neu5Ac was investigated.
Materials and methods
Materials
Neu5Ac, GlcNac and ManNAc were purchased from Sigma Chemical Co. The poly (styrene-co-DVB)-Br resin (PBR) and the Amberzyme oxirane resin were obtained from Mitsubishi Chemical Co. and Rohm and Haas Co., Ltd, respectively. Amino resin (AR) used throughout the study was made and stored in our laboratory. AGE and NAL were made as our previous studies [22]. The strains carrying the recombinant plasmids, pET-28a-SD-NAL and pET-28a-AGE, were incubated on LB agar (solid medium) containing 10 µg/mL kanamycin and 34 µg/mL chloromycetin at 37 °C. A single colony was transferred into 5 mL of LB medium (50 mL flask) with the same concentrations of kanamycin and chloromycetin used above. The cultures were incubated about 12 h at 37 °C, 200 rpm. One milliliter of the resulting culture was transferred to 100 mL of LB medium in a 1-L flask (containing 10 µg/mL kanamycin and 34 µg/mL chloromycetin) overnight at 37 °C, 220 rpm. When the optical density (OD600) of the cultures were up to 0.6–0.8, 0.5 mM of β-d-1-thiogalactopyranoside (IPTG) were supplemented for inducement, and the conditions were 30 °C, 200 rpm and 10 h. Strains were harvested by centrifugation at 6000 g for 10 min at 4 °C (Eppendorf, Germany) and washed twice using 100 mM Tris–HCl buffer (pH 7.5) before sonication. Glutaraldehyde (25% aqueous solution) was purchased from Sinopharm Chemical Reagent Co., Ltd. All other chemicals were of analytical grade and used without further purification.
Purification of AGE and NAL
The E. coli Rosetta cells were washed twice with a fivefold volume (volume against wet cell weight of recombinant cells) of chilled potassium phosphate buffer (0.1 M, pH 7.5) and suspended in a threefold volume of the same buffer containing 5% (v/v) glycerol. The E. coli cell pellet was disrupted in a French press (Aminco Co.) operated at a pressure of 2500 psi (1 psi ≈ 6.9 kPa). The cell supernatant was obtained by centrifugation at 12,000×g for 1 h. To purify the AGE and NAL, the crude extract of AGE or NAL was loaded onto an affinity support column (FPIDA-Co, Mitsubishi Chemical Co.), which was prepared according to the manufacturer’s instructions [23]. The affinity support was initially washed with 50 column volumes of buffer A (10 mM Tris–HCl, pH 7.5, 5% glycerol, 250 mM NaCl, and 5 mM imidazole), and AGE or NAL was eluted with buffer B (10 mM Tris–HCl, pH 7.5, 5% glycerol, 250 mM NaCl, and 500 mM imidazole).
Enzyme assay
NAL activity was assayed by measuring its ability to condense ManNAc and pyruvate into Neu5Ac. The reaction mixture was composed of 0.1 ml free NAL or 0.1 g immobilized NAL, 0.1 M pyruvate, 0.1 M ManNAc and 0.1 M Tris–HCl pH 7.5. AGE activity was assayed by measuring its ability to transform GlcNAc into ManNAc. The reaction mixture contained 0.1 ml free AGE or 0.1 g immobilized AGE, 0.1 M GlcNAc, 5 mM ATP and 0.1 M Tris–HCl pH 7.5. Reactions were terminated by boiling the mixture for 5 min. After centrifugation at 12,000g for 10 min and filtration through 0.22-µm membrane, the concentrations of the substrate and the product were analyzed by high-performance liquid chromatography (HPLC). All tests were performed in triplicate and 1 unit enzyme activity was defined as the amount of enzyme needed to produce 1 μmol of product per min. Concentrations of GlcNAc, ManNAc, Neu5Ac, and pyruvate were analyzed on an Agilent 1200 system, equipped with a Bio-Rad Aminex HPX-87H column (300 × 7.8 mm) using a refractive index detector. The mobile phase consisted of 10 mM H2SO4 at 0.4 mL·min−1, 55 °C. The protein concentration was determined by the Bradford method using bovine serum albumin as standard.
Immobilization of AGE and NAL
Resins were washed with a tenfold volume of 1 M potassium phosphate buffer, pH 8.0, and suspended in the same buffer. During the immobilization procedure, immobilization was performed in the following manners. For the first type of immobilized AGE or NAL (SCI, simultaneous cross-linking immobilization): 1 g wet carrier and 1.25 mL glutaraldehyde were added to 25 mL of enzyme solution. The mixture was incubated at 20 °C and 120 rpm in a rotary shaker (HYG-A, Taicang, China) for 12 h. For the second type of immobilized enzyme (GCE, glutaraldehyde–carrier–enzyme): 1 g of wet carrier was added to 25 mL of 5% (v/v) glutaraldehyde solution and was activated at 20 °C and 120 rpm in a rotary shaker for 6 h. The activated carriers were added to 25 mL enzyme solution immobilizing for 12 h after they were collected and washed thoroughly with deionized water. For the third type of immobilized enzyme (GEC, glutaraldehyde–enzyme–carrier): 1.25 mL glutaraldehyde and 25 mL enzyme solution were mixed at 20 °C and 120 rpm in a rotary shaker for 12 h. Then, 1 g of wet carriers was added in the mixture for another 12 h. After immobilization, the AR–glutaraldehyde–enzyme (AR–GA–E) was collected and washed thoroughly with deionized water to remove the weak immobilized enzyme and unbound glutaraldehyde. The eluent was tested for enzymatic activity by HPLC, and glutaraldehyde concentration was determined at 245 nm [14]. After the last washing, enzyme activity and glutaraldehyde were undetected. The immobilized enzyme was washed with 100 mM sodium phosphate buffer, pH 7.5 and stored in the sealed flask at 4 °C until use. The immobilization yield (IY, %) was the ratio of the total activity recovered on the carrier to the total activity offered for immobilization.
Determination of protein concentration
Protein concentration was determined as described by Bradford [24] using bovine serum albumin as a reference. Coomassie brilliant blue solution was used as a dye reagent. The amount of immobilized protein was calculated by subtracting the initial amount of protein from residual protein in the solution after immobilization and the leak protein during rinsing. The loading capacity was calculated by the following equation:
Reusability stability
The reusability of immobilized enzyme was tested by repeated batch experiments using the method for activity determination. At the end of each batch, the immobilized enzyme was filtered out using a gauze screen (pore diameter: 0.2 mm) from the reaction medium, washed with deionized water and dried at room temperature. Then, the immobilized enzyme was used for another reaction cycle using fresh substrates.
Determination of kinetic parameters
The free or immobilized enzyme was reacted with various concentrations of substrates at 37 °C for different time periods. The activity of free and immobilized enzyme at different reaction times was measured as outlined in “Enzyme assay”. The Michaelis–Menten constant (K m) and maximum initial reaction rate (V max) of free and immobilized enzyme were determined by non-linear analysis using the Michaelis–Menten equation.
Characterization of immobilized enzyme
The validity of the amino resin for immobilization of AGE or NAL with glutaraldehyde cross-linking for use in industry was determined by the following experiments.
Optimum pH measurement
To determine the optimum pH of the immobilized and free enzyme, the pH of the reaction buffer was varied from pH 5.5–9.0 using 0.1 M potassium phosphate buffer and the reaction temperature was 37 °C. Relative activities were calculated as the ratio of the activity of the enzyme measured at different pH to the maximal activity of the enzyme. The pH was determined using a digital pH-meter (Shanghai, China).
Optimum temperature and temperature stability
The optimum temperature of the free and immobilized enzyme and the effect of temperature on the relative activity of the free and immobilized enzyme were investigated within the temperature range 25–40 °C at pH 7.5. The data were normalized to 100% activity. The relative activity at each temperature was expressed as a percentage of 100% activity. In addition, below the inactivation temperature, temperature dependence on the rate constant can be described by the Arrhenius equation [25]:
where k is the rate constant, A is the pre-exponential factor, Ea is the apparent activation energy, R is the gas constant (8.31 J·mol−1 K−1), and T is the absolute temperature. The apparent activation energy (E a) of catalysis was determined by the slope of the Arrhenius plot using the following equation:
To compare the stability of the immobilized enzyme to that of the free enzyme, a thermal stability assay was conducted by incubating the immobilized or free enzyme at the optimum reaction temperature (37 °C) for 15, 30, 45, 60, and 90 h. The initial enzyme activity of immobilized or free enzyme was considered as 100% activity.
Production of Neu5Ac from GlcNAc in one reactor using immobilized AGE and NAL
As NAL is the key enzyme for Neu5Ac synthesis, pH 8.5 was selected in the fed-batch production of Neu5Ac. The conversion reaction was started by adding 100 U of immobilized AGE and 400 U immobilized NAL to 50 ml of reaction solution (0.4 M GlcNAc, 0.8 M pyruvate, 2.5 mM ATP, and 7.5 mM MgCl2 in 0.1 M potassium phosphate buffer at pH 8.5). The conversion reaction was initiated at 37 °C with an agitation speed of 200 rpm after immobilized AGE and NAL were added into the reactor. Samples (100 μl) were taken every hour and immediately assayed by HPLC. An appropriate amount of pyruvate was added to the reactor once the molar ratio of pyruvate/Neu5Ac was <4.0. After the conversion was completed, the reaction was stopped by separating the immobilized enzymes from the reaction solution by vacuum.
Experimental results were analyzed using three parallel measurements. All statistical analyses were conducted using Origin 8.5. Otherwise, Fig. 1 was formed using ChemDraw 2014.
Results
Selection of carriers
To obtain a satisfactory resin for the immobilization of enzymes of AGE and NAL, three different resins, Amberzyme oxirane resin (AOR), poly (styrene-co-DVB)-Br resin (PBR) and amino resin (AR) were investigated using the method of SCI. As reported in Table 1, the loading capacity without GA cross-linking was higher than that with GA cross-linking which indicated that the loading capacity was affected by GA. This could be explained that the enzyme binding sites occupied by GA on the surface of carriers were more than that supplied by GA. AR showed marked advantages both in terms of immobilized enzyme activity and loading capacity. Also, further research revealed that AGE and NAL immobilized on AOR or PBR exhibited less thermostability or reusability than that immobilized on AR. So, in the following experiments, AR was selected as the support for AGE and NAL immobilization.
Methods for enzyme immobilization
From the perspective of industrialization, enzyme reuse is the foremost advantage of biocatalyst immobilization. Reusability of the immobilized AGE and NAL using three different methods was assessed under optimal assay conditions, i.e. at 37 °C. As shown in Fig. 2, the results illustrated that enzyme reusability using the SCI method was better than that of GEC and GCE. In addition, the maximal enzyme activities with SCI, GCE and GEC were about (AGE/NAL) 536/183, 244/68 and 272/75 U·g−1 immobilized enzyme, respectively. In aqueous solution, the polymerized glutaraldehyde (GA) can occur spontaneously at room temperature. Tetramer, pentamer and polymer forms containing approximately one free aldehyde group were proposed to be formed through the intramolecular–intermolecular interaction [26].
Calculation of kinetic parameters
One of the most important characteristics of an enzyme is its affinity for substrate, usually assessed by the Michaelis–Menten constant (K m) and maximum effective velocity (V max) [27]. The relation between enzymatic reaction rate and substrate concentration is described by the Michaelis–Menten equation.
The relationship between the initial enzymatic reaction rate and the substrate concentration fitted the Michaelis–Menten curves for the free enzymes and immobilized enzymes (Fig. 3). The model equation fitted the experimental observations satisfactorily, with a correlation coefficient (R 2) of 0.998 and 0.986 for free and immobilized AGE, 0.998 and 0.988 for free and immobilized NAL, respectively. The values of K m and V max for the free and immobilized enzymes are shown in Table 2. An increase in K m (from 1.14 to 1.31 mg·mL−1 for AGE and from 1.05 to 1.25 mg·mL−1 for NAL) and a decrease in V max (from 177.53 to 106.37 µg·min−1·mL−1 for AGE and from 126.41 to 95.96 µg·min−1·mL−1 for NAL) were recorded after immobilization. Usually, the K m of a free enzyme is higher than the immobilized enzyme [28, 29]. However, in organic polymers and inorganic supports, this is a very rarely observed phenomenon. Only a few researchers have observed a lower K m for the immobilized enzyme than for the free enzyme on mediated silica [17] and egg shell membrane [30].
Characterization of immobilized AGE and NAL
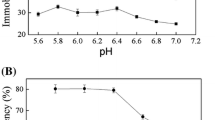
Effect of pH on enzyme activity
The effect of pH on immobilized enzyme activity relies on the free enzyme. Figure 4 shows the activity of free and immobilized AGE and NAL at different pH values ranging from 5.5 to 9.0 at 37 °C. Enzyme immobilization did not cause a shift in the optimal pH for the activity; in fact, the highest AGE (NAL) activity was obtained for both the free and immobilized enzymes at pH 7.5 (8.5). AGE and NAL immobilized on amino resin (AR) endured a broader acidic pH than the free enzyme. Beyond pH 7.5, a loss of free and immobilized AGE activity was noted. Generally, the optimum pH of many immobilized enzymes moves into the acidic range when the enzymes are immobilized on cationic or positive carriers, while immobilization on anionic or negative carriers causes a shift to alkaline values [16, 19]. Also, immobilized enzymes can endure a broader pH range than free enzymes [31, 32].
Thermal characteristics of free and immobilized enzyme
The effect of temperature on the activity of free and immobilized AGE (NAL) was examined at pH 7.5 (8.5) which was maintained at the optimum level over a temperature range of 25–40 °C. As shown in Fig. 5, the reaction expressed maximum activity for both the free and immobilized enzymes at 37 °C. Also, the behavior of the immobilized enzyme was similar to that of the free enzyme. This may be explained that the enzyme was immobilized on the carrier through the flexible linking of GA “arms”. The optimum temperature for the free enzyme and the immobilized enzyme was the same, similar to the research by Kharrat et al. [17] and Karra-Chaabouni et al. [33].
The apparent activation energy (E a) of catalysis for the free and immobilized AGE was evaluated using the Arrhenius plot. The regression equations for the Arrhenius plot of free and immobilized AGE were y = −6.2601x + 18.522 and y = −4.2410x + 11.125, for NAL were y = −5.2351x + 12.315 and y = −4.1204x + 10.053, respectively. The correlation coefficients (R 2) of the model for the free and immobilized AGE were 0.976 and 0.985, for NAL were 0.988 and 0.974, respectively, which indicated that the experimental data fitted the model. The E a of the free and immobilized AGE (NAL) was 117.14 kJ·mol−1 (124.21 kJ·mol−1) and 78.45 kJ·mol−1 (66.64 kJ·mol−1), respectively, implying that the immobilized enzyme had a higher catalytic efficiency than the free enzyme. Also, the decreased E a confirmed that the catalytic efficiency of the immobilized enzyme was restricted by mass-transfer rather than kinetic limit as postulated by Ortega et al. [25].
Immobilized enzymes and the free enzymes were heated for different time periods at the optimum reaction temperature (37 °C) to measure thermal stability, and the results of residual activity are shown in Fig. 6. Approximately 50% of the free enzyme activity was lost after 60 h, while the immobilized enzyme retained almost all of its initial activity after 90 h. These results indicated that immobilized AGE showed significant resistance to thermal inactivation.
Influence of the amount of support on immobilization
Figure 7 shows that the amount of support affected the immobilized enzyme activity and loading capacity of the amino resin with GA cross-linking. When the amount of support was lower than 1 g, the resin reached maximal loading capacity. Also, due to the change in spatial conformation induced by the immobilization or due to the substrates diffusing into the active sites of the immobilized enzyme, enzyme activity decreased even though a high proportion of the enzyme was immobilized. This may be the main reason why the immobilization yield (IY) was not satisfactory. By increasing the amount of support, the immobilized AGE and NAL activity decreased with the loading capacity, which demonstrated that the enzyme could not saturate the attachment sites offered by GA and AR.
Neu5Ac production from GlcNAc using immobilized enzymes in one reactor
A two-step enzymatic system involving immobilized AGE and NAL was used for the conversion of GlcNAc to NeuAc in one reactor as described in “Materials and methods”. We tested different activity ratios of immobilized AGE and NAL in the reactor that catalyzed GlcNAc and pyruvate to produce Neu5Ac. We found that the optimum ratio was 2 U·mL−1 of AGE and 8 U·mL−1 of NAL. Because ATP was the vital factor in this reaction, we used different concentrations of ATP (7.5, 5, 2.5, 1, and 0.25 mM) and found that the reaction requires ATP at the concentration of not less than 2.5 mM.
The conversion reaction was initiated at 37 °C at an agitation speed of 200 rpm after immobilized AGE and NAL were added to the reactor. The production of Neu5Ac with GlcNAc and pyruvate as substrates was achieved using 100 U of immobilized AGE and 400 U of immobilized NAL in 50 mL of reaction solution. The initial concentration of pyruvate was 0.8 M, and during the reaction, the pyruvate/Neu5Ac molar ratio was assayed by HPLC, and 4.2 g and 2.4 g pyruvate was added at 12 and 18 h after initiation of the reaction (Fig. 8). The conversion rate from GlcNAc was about 82% within 24 h, with not more than 0.1 M GlcNAc remaining at the end of reaction. After the reaction, the immobilized AGE and NAL were used for the next conversion, and five cycles of the coupled reaction could be completed within 24 h, with a final conversion rate of >75%. The reaction solution and the washing solution from the five cycles of the reaction were combined. Finally, 101.45 g·L−1 of Neu5Ac was obtained. Furthermore, AR-GA−Es retained about 75% of its initial activity even after five repeated runs.
Discussion
The method for enzyme immobilization was extremely important. When using the GCE method, GA may be polymerized with each other and then absorbed onto the surface of AR or polymerization with each other on the surface of AR. Thus, the amount of free aldehyde groups was greatly less than that when the GEC and SCI methods were used. If the enzyme was first cross-linked with GA, a large amount of the enzyme was fixed by the reaction between the amino groups and free aldehyde groups. However, the GA–enzyme polymers were just absorbed on the surface of supports rather than penetrated into the center of AR. When GA cross-linking and enzyme immobilization occurred simultaneously in the same reactor viz. SCI, AR acted as a “core” absorbed GA on the surface or within the resins, and the enzyme which reacted with the free aldehyde or amino groups was immobilized on AR by the “arms”. Therefore, enzyme reusability with the SCI method was better than GEC and GCE. Also, the immobilized enzyme used with the SCI method was found to exhibit a better stability. Upon further research, only 5% of protein absorbed on resins was leaked from the carriers in deionized water with stirring at 37 °C for 12 h.
An increased K m of the immobilized enzyme suggests lower affinity for its substrate than the free enzyme. This may be caused by the steric hindrance of the active site by the support and GA, substrate diffusional limits or the loss of enzyme flexibility necessary for substrate binding [14, 16, 25]. Also, increased K m for the immobilized enzyme may be due to mass-transfer limitation caused by the enzyme activity being assayed with high–molecular-weight substrates like ManNAc. The V max for AGE and NAL immobilized on amino resin was lower than that for the free enzyme. This was probably due to considerable attenuation in V max for the immobilized enzyme resulting from lower transport of the substrate into the matrix or restricted mobility of the immobilized enzyme [17].
The higher stability of immobilized AGE could be due to the diminished protein aggregation and conformational change in the enzyme when fixed to the support. The restricted interaction of the immobilized enzyme in the AR–glutaraldehyde–enzyme system (AR–GA–Es) could play an important role in retaining enzyme activity. During the process of enzyme inactivation, unfolding of the protein structure is the major factor and has been reported by various authors [34, 35]. However, immobilization of the enzyme may reduce the unfolding rate of the secondary and tertiary structure of the enzyme, and the relative distance between the residues remains unaltered during any spatial conformational range induced by distorting agents (heat, organic solvents, etc.) [36].
In conclusion, this work focused on AGE and NAL immobilization on amino resin (AR) with glutaraldehyde cross-linking. This support was highly stable both chemically and mechanically, and did not swell or shrink in aqueous solution. The immobilized enzyme was fairly stable. Based on the kinetics characterization and analysis of the enzyme, an increase in K m (from 1.14 to 1.31 mg·mL−1 for AGE and from 1.05 to 1.25 mg·mL−1 for NAL) and a decrease in V max (from 177.53 to 106.37 µg·min−1 mL−1 for AGE and from 126.41 to 95.96 µg·min−1 mL−1 for NAL) were recorded after immobilization. AR–GA–Es retained about 72% of its initial activity even after eight repeated runs. The apparent activation energy (E a) of the free and immobilized AGE (NAL) was 117.14 kJ·mol−1 (124.21 kJ·mol−1) and 78.45 kJ·mol−1 (66.64 kJ·mol−1), respectively, implying that the catalytic efficiency of the immobilized enzyme was restricted by mass-transfer rather than kinetic limit. Neu5Ac production from GlcNAc using immobilized AGE and NAL in one reactor was carried out subsequently resulting 101.45 g·L−1 of Neu5Ac and the highest conversion ratio of 82%. This method of enzyme immobilization may have a promising future for Neu5Ac production in industry.
References
Schauer R (2000) Achievements and challenges of sialic acid research. Glycoconj J 17:485–499
Vonitzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Phan TV, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR (1993) Rational design of potent sialidase-based inhibitors of influenza-virus replication. Nature 363:418–423
Maru I, Ohnishi J, Ohta Y, Tsukada Y (2002) Why is sialic acid attracting interest now? Complete enzymatic synthesis of sialic acid with N-acylglucosamine 2-epimerase. J Biosci Bioeng 93:258–265
Shamberger RJ (1984) Serum sialic acid in normals and in cancer patients. J Clin Chem Clin Biochem 22:647–651
Saez JJ, Senravarela A (1995) Evaluation of lipid-bound sialic acid (Lsa) as a tumor-marker. Int J Biol Marker 10:174–179
Nilsson KGI (1989) Enzymic-synthesis of di-saccharide and trisaccharide glycosides, using glycosidases and beta-d-galactoside 3-alpha-sialyl-transferase. Carbohydr Res 188:9–17
Maru I, Ohta Y, Murata K, Tsukada Y (1996) Molecular cloning and identification of N-acyl-d-glucosamine 2-epimerase from porcine kidney as a renin-binding protein. J Biol Chem 271:16294–16299
Tabata K, Koizumi S, Endo T, Ozaki A (2002) Production of N-acetyl-d-neuraminic acid by coupling bacteria expressing N-acetyl-d-glucosamine 2-epimerase and N-acetyl-d-neuraminic acid synthetase. Enzyme Microb Technol 30:327–333
Lee YC, Chien HC, Hsu WH (2007) Production of N-acetyl-d-neuraminic acid by recombinant whole cells expressing Anabaena sp. CH1 N-acetyl-d-glucosamine 2-epimerase and Escherichia coli N-acetyl-d-neuraminic acid lyase. J Biotechnol 129:453–460
Kragle U, Gygax D, Ghisalba O, Wandrey C (1991) Enzymatic two-step synthesis of N-acetyl-neuraminic acid in the enzyme membrane reactor. Angew Chem Int Ed Engl 30:827–828
Maru I, Ohnishi J, Ohta Y, Tsukada Y (1998) Simple and large-scale production of N-acetylneuraminic acid from N-acetyl-d-glucosamine and pyruvate using N-acyl-d-glucosamine 2-epimerase and N-acetylneuraminate lyase. Carbohydr Res 306:575–578
Lin BX, Zhang ZJ, Liu WF, Dong ZY, Tao Y (2013) Enhanced production of N-acetyl-d-neuraminic acid by multi-approach whole-cell biocatalyst. Appl Microbilo Biotechnol 97:4775–4784
Yang JB, Ni KF, Wei DZ, Ren YH (2015) One-step purification and immobilization of his-tagged protein via Ni2+-functionalized Fe3O4 @ polydopamine magnetic nanoparticles. Biotechnol Bioprocess Eng 20:901–907
Pal A, Khanum F (2011) Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of immobilized enzyme. Process Biochem 46:1315–1322
White CA, Kennedy JF (1980) Popular matrices for enzyme and other immobilizations. Enzyme Microb Technol 2:82–90
Ghiaci M, Aghaei H, Soleimanian S, Sedaghat ME (2009) Enzyme immobilization. Part 2. Immobilization of alkaline phosphatase on Na-bentonite and modified bentonite. Appl Clay Sci 43:308–316
Lai JK, Chuang TH, Jan JS, Wang SSS (2010) Efficient and stable enzyme immobilization in a block copolypeptide vesicle-templated biomimetic silica support. Colloids Surf B 80:51–58
Panzavolta F, Soro S, Amato RD, Palocci C, Cernia E, Russo MV (2005) Acetylenic polymers as new immobilization matrices for lipolytic enzymes. J Mol Catal B Enzym 32:67–76
Kharrat N, Ali YB, Marzouk S, Gargouri YT, Karra-Chaabouni M (2011) Immobilization of Rhizopus oryzae lipase on silica aerogels by absorption: comparison with the free enzyme. Process Biochem 46:1083–1089
Olson AC, Stanley WL (1973) Lactase and other enzymes bound to a phenolformaldehyde resin with glutaraldehyde. J Agric Food Chem 21:440–445
Zhang Y, Zhang Y, Jiang J, Li L, Yu C, Hei T (2011) Surface derivatization with spacer molecules on glutaraldehyde-activated amino-microplates for covalent immobilization of β-glucosidase. Appl Surf Sci 257:2712–2716
Sun WJ, Ji WY, Li N, Tong P, Cheng J (2013) Construction and expression of a polycistronic plasmid encoding N-acetylglucosamine 2-epimerase and N-acetylneuraminic acid lyase simultaneously for production of N-acetylneuraminic acid. Bioresour Technol 130:23–29
Hu SY, Chen J, Yang ZY, Shao LJ, Bai H, Luo JL, Jiang WH, Yang YL (2010) Coupled bioconversion for preparation of N-acetyl-d-neuraminic acid using immobilized N-acetyl-d-glucosamine-2-epimerase and N-acetyl-d-neuraminic acid lyase. Appl Microbiol Biotechnol 85:1383–1391
Bradford A (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Ortega N, Perez-Mateos M, Pilar MC, Busto MD (2009) Neutrase immobilization on alginate-glutaraldehyde beads by covalent attachment. J Agric Food Chem 57:109–115
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–802
Wei L, Zhang W, Lu H, Yang P (2010) Immobilization of enzyme on detonation nanodiamond for highly efficient proteolysis. Talanta 80:1298–1304
Heitmann T, Wenzig E, Mersmann A (1997) Characterization of three different potato starches and kinetics of their enzymatic hydrolysis by an alpha-amylase. Enzyme Microb Technol 20:259–267
Shi LE, Tang ZX, Yi Y, Chen JS, Wang H, Xiong WY (2010) Study of immobilization of nuclease P1 on paper cellulose. Biotechnol Biotechnol Equip 24:1997–2003
Pundir CS, Bhambi M, Chauhan NS (2009) Chemical activation of egg shell membrane for covalent immobilization of enzymes and its evaluation as inert support in urinary oxalate determination. Talanta 77:1688–1693
Karout A, Chopard C, Pierre AC (2007) Immobilization of a lipoxygenase in silica gels for application in aqueous media. J Mol Catal B Enzym 44:117–127
Ye P, Wang RB, Wang XP (2009) Quantitative enzyme immobilization: control of the carboxyl group density on support surface. J Mol Catal B Enzym 61:296–302
Karra-Chaabouni M, Bouaziz I, Boufi S, Rego AMB, Gargouri Y (2008) Physical immobilization of Rhizopus oryzae lipase onto cellulose substrate: activity and stability studies. Colloids Surf B 66:168–177
Mozhaev VV, Martinek K (1990) Structure–stability relationships in proteins: a guide to approaches to stabilizing enzymes. Adv Drug Delivery Rev 4:387–419
Mozhaev VV (1993) Mechanism-based strategies for protein thermostabilization. Trends Biotechnol 11:88–95
Mateo C, Grazu V, Palomo JM, Lopez-Gallego F, Fernandez-Lafuente R, Guisan JM (2007) Immobilization of enzymes on heterofunctional epoxy supports. Nat Protoc 2:1022–1033
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2013CB733602), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Major Research Plan of the National Natural Science Foundation of China (21390204), Program for Changjiang Scholars and Innovative Research Team in University (Grant No.: IRT_14R28), and Jiangsu National Synergistic Innovation Center for Advanced Materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheng, J., Zhuang, W., Tang, C. et al. Efficient immobilization of AGE and NAL enzymes onto functional amino resin as recyclable and high-performance biocatalyst. Bioprocess Biosyst Eng 40, 331–340 (2017). https://doi.org/10.1007/s00449-016-1700-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1700-z