Abstract
Pigments produced by species of Monascus have been used to coloring rice, meat, sauces, wines and beers in East Asian countries. Monascus can produce orange (precursor), yellow and red pigments. Orange pigments have low solubility in culture media and when react with amino groups they become red and largely soluble. The orange pigments are an alternative to industrial pigment production because the low solubility facilitates the downstream operations. The aim of this work was to study the kinetic on the production of orange pigments by Monascus ruber CCT 3802. The shaking frequency of 300 rpm was favorable to production, whereas higher shaking frequencies showed negative effect. Pigment production was partially associated with cell growth, the critical dissolved oxygen concentration was between 0.894 and 1.388 mgO2 L−1 at 30 °C, and limiting conditions of dissolved oxygen decreased the production of orange pigments. The maintenance coefficient (mo) and the conversion factor of oxygen in biomass (Yo) were 18.603 mgO2 g −1x h−1 and 3.133 gx gO −12 and the consideration of these parameters in the oxygen balance to estimate the biomass concentration provided good fits to the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Color plays an important role in our enjoyment of food stuffs and is appreciated both for its aesthetic and as a basis for the assessment of quality. In the latter respect, color gives visual clues to flavor identification and taste thresholds [1]. Since the number of permitted synthetic colorants has decreased because of undesirable toxic effects including mutagenicity and potential carcinogenicity, interest focuses on the development of food pigments from natural sources [2, 3]. The production of pigments by microorganisms capable of producing natural dyes, including seaweed, algae, yeasts and filamentous fungi, is away of increasing the production of natural compounds as an alternative to the use of synthetic ones [4].
The genus Monascus involves three main species (M. pilosus, M. purpureus and M. ruber) belonging to the family Monascaceae and class Ascomyceta, whose main characteristic is the ability of producing secondary metabolites, such as pigments, monacolins, γ-aminobutyric acid, dimerumic acid, and many others biomolecules [5–9]. Pigments produced by species of Monascus have been used to coloring rice, meat, sauces, wines and beers in East Asian countries, and besides these mentioned applications, they have been used as a fabric dye and in hair pigmentation and even in ink jet printers [10–14].
Monascus can produce yellow, orange and red pigments. Medium chain fatty acids are synthesized by the fatty acid pathway and bind to the structure of the chromophore through a trans-etherification reaction, generating the precursor orange pigment monascorubrin—C23H26O5 and/or rubropunctatin—C21H22O5 by trans-etherification of the octanoic acid. The reduction of the orange pigment monascorubrin forms the yellow pigment ankaflavin—C23H30O5, or monascin—C21H26O5 for rubropunctatin, whereas the amination of orange pigments with NH3 or NH2 units (amino group of amino acids, peptides, proteins and nucleic acids) gives rise to red pigments monascorubramine—C23H27NO4 and rubropunctamine C21H23NO4 [15, 16].
Monascorubrin and rubropunctatin are produced in the cell-bound state; insoluble in water and synthesized in the cytosol from ACoA by multi-enzyme complex of poliketide synthase I and possess aminophiles structure responsible for the color [17, 18]. Low pH values inhibit the transformation of orange into red pigment, consequently, orange pigment production becomes attractive because of the low solubility is favorable to downstream operations [19].
Research focused on red pigments production, however, the orange pigment is an alternative for obtaining Monascus pigments. Accurate and precise measurements of the kinetic of biomass and pigments production are important to process development. Aiming to contribute for the development of an efficient technology for large scale production of orange pigments the objective of the present work was to study the kinetics of monascorubrin and rubropunctatin production from Monascus ruber.
Materials and methods
Microorganism
Monascus ruber CCT 3802 was obtained from the Tropical Culture Collection André Tosello (Campinas-SP, Brazil). The strain was frozen at −20 °C after adding 100 μL glycerol mL−1 spore suspension as a cryoprotector [20]. The culture was maintained on potato dextrose agar (Biolife Italiana, Milan, Italy) sterilized at 121 °C for 15 min, incubated at 30 °C for 7 days and subsequently stored at 4 °C.
Inoculum and growth media
Monascus ruber was initially grown on potato dextrose agar in a Roux bottle incubated at 30 °C for 7 days and subsequently stored at 4 °C. Fungal mycelia were obtained by germination of the spores suspended in a 1 L baffled flask containing 0.4 L of inoculum culture medium incubated at 30 °C on a rotary shaker at 120 rpm for 60 h (exponential growth phase). The inoculum and culture medium were prepared according to Vendruscolo et al. [20] containing: 20 g L−1 glucose, 5 g L−1 glycine, 2.5 g L−1 KH2PO4, 2.5 g L−1 K2HPO4, 0.5 g L−1 MgSO4·7H2O, 0.1 g L−1 FeSO4·7H2O, 0.1 g L−1 CaCl2, 0.03 g L−1 MnSO4 and 0.01 g L−1 ZnSO4. After sterilization, the initial pH was adjusted with 1 M HCl to 3.0.
Submerged fermentation
Fermentation was carried out in a 6 L (working volume of 4 L) batch bioreactor Bioflo III from New Brunswick Scientific Co. (New Jersey, USA) with an internal diameter of 0.170 m, a rounded bottom and a height/diameter ratio of 1.4. Agitation was provided by two six-blade Rushton turbines with a d/t ratio of 0.38 and a w/d ratio of 0.18. The culture medium (3.6 L) was inoculated with 0.4 L of the inoculum culture (10 % v/v; ≈0.5 g L−1 dry cells), the temperature was maintained at 30 °C and the pH was controlled at 3.0 with 1 M HCl. The cultivations were performed into two steps: (1) kinetic determination of growth at different stirring frequencies and (2) cultivation under different dissolved oxygen levels in the liquid medium.
Biomass, substrate and pigments
Biomass was quantified by dry weight by filtration of 10 mL samples through a Whatman # 1 filter paper and the retained material was submitted to drying in a microwave (Consul, Brazil) for 15 min at 180 W. After cooled in a desiccator for 15 min, it was weighed in order to determine the dry biomass retained in the filter paper. The filtered was submitted to glucose residual estimation by 3,5-dinitrosalicylic acid [21] and orange pigment (monascorubrin and rubropunctatin) concentration was estimated by scanning in spectrophotometer (Spectronic Unicam Genesys 10 vis) where in our experimental conditions 1.0 absorbance unit at 470 nm corresponds to 44 mg of orange pigments per liter [19].
Oxygen transfer
A polarographic oxygen sensor InPro 6800 (Mettler-Toledo, Switzerland), which is connected to a DO analyzer of the computer-controlled fermentor system, was used. The DO saturation used was 6.772 mgO2 L−1 at 30 °C, as determined by Vendruscolo et al. [22]. The response time of the DO sensors was estimated by applying sudden changes in DO concentration, according to Eq. (1), consisting to subjecting the electrode previously polarized and calibrated in an inert atmosphere containing only nitrogen gas (\(p_{{{\text{O}}_{2} }}\) ≈ 0 atm). When the value reported by the electrode is below to 1 % of the saturation concentration the electrode was immediately placed in atmospheric air (\(p_{{{\text{O}}_{2} }}\) ≈ 0.209 atm) and the values of the percentage of oxygen saturation were collected by the data acquisition software with 1 s interval.
where C P is the measured DO concentration (% or mgO2 L−1), C S is the DO saturation concentration (% or mgO2 L−1), k P is the time constant and t is the time (s). C P = 0 for t = 0 and C P = Cs for t = ∞. All DO concentrations measured were corrected by k P. The critical dissolved oxygen concentration (C Crit) in the liquid for the M. ruber was determined by the dynamic method [23]. The C Crit may be determined experimentally by observing the decrease in the dissolved oxygen concentration with time until all oxygen is consumed. At the critical concentration the rate of oxygen consumption starts to decrease gradually, resulting in a subsequent decrease in the slope of the dO2 /dt.
k La and \(Q_{{{\text{O}}_{2} }}\) estimation
The k L a and the specific oxygen uptake rates (\(Q_{{{\text{O}}_{2} }}\)) were determined by combined analysis of oxygen mass balance described by Eq. (2).
where C S is the DO saturation concentration (mgO2 L−1), C is the DO concentration measured (mgO2 L−1), \(Q_{{{\text{O}}_{2} }}\) is the specific oxygen uptake rate (mgO2 g −1x h−1), X is the biomass (g L−1), t is the time (h) and k L a is the volumetric oxygen transfer coefficient (h−1). The product \(Q_{{{\text{O}}_{2} }} \times X\) being the oxygen uptake rate (OUR) (mgO2 L−1 h−1).
Kinetic parameters
The specific growth rate (μ X), specific rate of glucose consumption (μ S), specific rate of pigment production (μ P) and biomass yield (Y X/S) were estimated. The dependency of μ P as a function of μ X was determined by Luedeking and Piret [24] model (Eq. 3), the O2 maintenance coefficient of the cells (mo) and the O2 conversion factor in cells (YO) was estimated by the Eq. (4) [25].
where μ X is the specific growth rate (h−1), μ P is the specific rate of pigment production (gP g −1x h−1), θ is the empirical constant that describes the dependence of pigment production associated with cell growth, β is the empirical constant that describes the non-dependence of pigment production associated with cell growth, mo is the maintenance coefficient for oxygen (mgO2 g −1x h−1) and YO is the true cell yield from oxygen consumption (gx gO −12 ). The mo and YO parameters were used to estimate the cell concentration by the mathematical model developed by Zabriskie and Humphrey [26] (Eq. 5).
where X n and X n−1 are the biomass (g L−1) at cultivation times t n and t n−1 (h), Δt is the time interval from t n−1 to t n and \(Q_{{{\text{O}}_{2} }} \times X_{n}\) and \(Q_{{{\text{O}}_{2} }} \times X_{n - 1}\) are the oxygen uptake rates at cultivation times t n and t n−1. The first value of X n−1 must be estimated from the biomass concentration of the inoculum and \(Q_{{{\text{O}}_{2} }} \times X\)values obtained from the Eq. (2) should be in mmolO2 L−1 h−1.
Results and discussion
The delay constant of the electrode ranged from 393.48 to 415.44 h−1 and corrected k L a values considering k p showed variations lower than 1 % of their original (data not shown). Therefore, the electrode dynamic response was characterized experimentally and found to be a first-order response with time delay. Even with small variations, all results were corrected by considering k p.
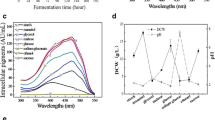
Figure 1 shows the results of the cultivation of M. ruber at different agitation frequencies. M. ruber showed shorter lag phase (~10 h), reaching maximum cell concentration of 4.5 g L−1 and maximum pigment production of 3.5 UA470nm (Fig. 1a). The maximum specific growth rate and maximum specific respiration rates were 0.05 h−1 and 12 mgO2 g −1x h−1, respectively, and the Y X/S was 0.37 gx g −1s . The cultivations carried out under stirring frequencies of 600 and 900 rpm (Fig. 1b, c) showed similar behavior on the evolution of the biomass and pigments concentrations, as well as in the calculated kinetic parameters. The cellular concentration (1.8 and 1.7 g L−1) and the pigment production (0.150 UA470nm 0.170 UA470nm) were far below the results obtained under shaking frequency of 300 rpm. Similarly, the maximum specific rates 0.04 and 0.036 h−1 and the conversion factor of 0.20 and 0.29 g −1x g −1s were below the experiment under 300 rpm, however, the respiration rate was higher in the cultures under 600 and 900 rpm, 28.7 mgO2 g −1x h−1 and 22.9 mgO2 g−1 h−1, respectively. The cultivation carried out under the shaking frequency of 300 rpm had k L a values between 7.3 and 46 h−1, whereas the cultures shaken at 600 and 900 rpm showed variations of 130–160 and 120–169 h−1, respectively.
Monascus ruber consumed all of the glucose from the culture medium, reaching maximum cell concentration of 6.3 g L−1 and 0.596 UA470nm (Fig. 2) and the biomass yield was 0.49 gx g −1s . The orange pigment production was partially associated to the cell growth due to dependency of μ P vs. μ X (Fig. 3), verified by the constants θ = 0.0805 and β = 0.0002. θ × μ X is the production of pigments associated with cell growth and β is independent of the cell growth. The maintenance coefficient (mo) and oxygen-biomass conversion factor (YO) were 18.603 mgO2 g −1x h−1 and 3.133 gx gO −12 , respectively (Fig. 4). These values were used in the cultivations under different O2 concentrations to estimate biomass production using the data from the oxygen balance.
In the bioprocess industry, synthetic and/or complex culture media are used. Gas solubility data are required to establish mass balance, calculate yields coefficients, volumetric mass transfer coefficients by gas balance techniques, and design and scale up bioreactors in gas-limiting conditions. Because oxygen partial pressure in the gas phase and presence of dissolved material in the liquid are major factors affecting oxygen solubility, the values of oxygen solubility in water cannot be applied directly to bioprocess systems [22].
The critical concentration of dissolved oxygen ranged from 0.894 to 1.388 mgO2 L−1 at 30 °C (Fig. 5), justified by large pellets are mainly physiologically active around the edge of the pellet, with oxygen and nutrient depletion in the centre. The minimum concentration of dissolved oxygen in the bulk mixed liquor that is required to avoid oxygen becoming a limiting factor inside the pellet is called the critical dissolved oxygen concentration. Its numerical value depends on several factors, of which the oxygen uptake rate value and the stirring intensity are the most important. Oxygen is not especially soluble in growth media but can be consumed in large amounts by rapidly growing microbial cultures. For that reason is important to know the DO2 level in the vessel and control it to the required set point.
For many fermentations especially where filamentous fungi and actinomycetes are involved, or the broth is viscous, it is necessary to agitate the medium with the aid of impellers. In large-scale operations, where aeration is maintained by agitator-created swarms of tiny air bubbles floating through the medium, the cost is very high and for this reason careful aeration is done based on mathematical calculations conducted by chemical engineers [27].
In the cultivation carried out with the saturation concentration of dissolved oxygen of 50 % (Fig. 6a), the production of pigments showed a gradual increase as a function of the cultivation time, following the biomass production. The maximum concentrations of biomass (6.30 g L1) and pigments (0.330 UA470 nm) were reached within 29 h of cultivation. On the other hand, the cultivation carried out in the range of 30 ± 3 % O2, (Fig. 6b) showed similar behavior with the maximum cell concentration of 5.30 g L−1, although the production of pigments was 0.170 UA470nm. Despite this reduction, the data differed mainly in the production of pigments compared with the experiment under O2 limitation (Fig. 6c). Even with higher cell concentration, pigment production has not been verified.
From the results of maintenance coefficient (mo) and oxygen-biomass conversion factor (Yo), the evolution of biomass concentration of cultivations under different concentrations of dissolved oxygen was estimated using the Eq. (4). The data of oxygen balance represented the behavior of the biomass evolution as a function of cultivation time (Fig. 7) evidencing the importance of knowing mo and Yo. The results show that the respiratory mathematical model of Zabriskie and Humphrey [26] could be used to estimate the biomass concentration of Monascus ruber CCT 3802 in spite of the morphological complexity observed during the growth of this microorganism. The knowledge of the kinetics of orange pigments production provides improvements to red pigments production once the orange pigment has low solubility in the culture medium, can be separated from the fermentation medium and later modified via amino acids reaction.
Conclusions
It was evident the importance of knowing the kinetics aspects on the production of monascorubrin and rubropunctatin by M. ruber. The shaking frequency of 300 rpm provided higher concentration of orange pigments in the culture medium and mo, Yo and C Crit values allowed obtaining results that describe the cell growth vs. cultivation time.
References
Bridle P, Timberlake CF (1997) Anthocyanins as natural food colours-selected aspects. Food Chem 58:103–109
Sabater-Vilar M, Maas RFM, Fink-Gremmels J (1999) Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutat Res 444:7–16
Pennacchi MGC, Rodrígues-Fernández DE, Vendruscolo F, Maranho LT, Marc I, Cardoso LAC (2015) A comparison of cell disruption procedures for the recovery of intracellular carotenoids from Sporobolomyces ruberrimus H110. Int J Appl Biol Pharm 6:136–143
Dufossé L, Galaup P, Yaron A, Arad SM, Blanc P, Murthy KNC, Ravishankar G (2005) Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality? Trends Food Sci Technol 16:389–406
Meinicke RM, Vendruscolo F, Moritz DE, Oliveira D, Ninow JL (2012) Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatal Agric Biotechnol 1:238–242
Lu LP, Zhang BB, Xu GR (2013) Efficient conversion of high concentration of glycerol to Monacolin K by solid-state fermentation of Monascus purpureus using bagasse as carrier. Bioprocess and Biosyst Eng 36:293–299
Bühler RMM, Müller BL, Moritz DE, Vendruscolo F, Oliveira D, Ninow JL (2015) Influence of light intensity on growth and pigment production by Monascus ruber in submerged fermentation. Appl Biochem Biotechnol 10:1–13
Chen JC, Wang J, Wang ZJ, Li YJ, Pang J, Lin HT, Yin SW (2015) Effect of Monascus aged vinegar on isoflavone conversion in soy germ by soaking treatment. Food Chem 186:256–264
Vendruscolo F, Bühler RMM, Carvalho JC, Oliveira D, Moritz DE, Schmidell W, Ninow JL (2016) Monascus: a reality on the production and application. Rev Appl Biochem Biotechnol 178:211–223
Hsu FL, Wang PM, Lu SY, Wu WT (2002) A combined solid-state and submerged cultivation integrated with adsorptive product extraction for production of Monascus red pigments. Bioprocess Biosyst Eng 25:165–168
Meinicke RM, Dutra AC, Vendruscolo F, Moritz DE, Ninow JL (2013) Monascus pigment production in bioreactor using a co-product of biodiesel as substrate. Food Sci Technol 33:9–13
Mostafa ME, Abbady MS (2014) Secondary metabolites and bioactivity of the Monascus pigments review article. Glob J Biotechnol Biochem 9:1–13
Shao Y, Lei M, Mao Z, Zhou Y, Chen F (2014) Insights into Monascus biology at the genetic level. Appl Microbiol Biotechnol 98:3911–3922
Vendruscolo F, Tosin I, Giachini AJ, Schmidell W, Ninow JL (2014) Antimicrobial activity of Monascus pigments produced in submerged fermentation. J Food Proces Preserv 38:1860–1865
Hajjaj H, Blanc P, Groussac E, Uribelarrea JL, Goma G, Loubiere P (2000) Kinetics analysis of red pigment and citrinin production by Monascus ruber as a function of organic acid accumulation. Enz Microb Technol 27:619–625
Jung H, Kim C, Kim K, Shin CS (2003) Color characteristics of Monascus pigments derived by fermentation with various amino acids. J Agric Food Chem 51:1302–1306
Wong H, Koehler PE (1983) Production of red water-soluble Monascus pigments. J Food Sci 48:1200–1203
Lin TF, Yakushijin K, Buchi GH, Demaln AL (1992) Formation of water-soluble Monascus pigments by biological and semi-synthetic processes. J Ind Microbiol 9:173–179
Vendruscolo F, Schmidell W, Moritz DE, Bühler RMM, Oliveira D, Ninow JL (2016) Isoelectric point of amino acid: importance for monascus pigment production. Biocatal Agric Biotechnol 5:179–185
Vendruscolo F, Müller BL, Moritz DE, Oliveira D, Schmidell W, Ninow JL (2013) Thermal stability of natural pigments produced by Monascus ruber in submerged fermentation. Biocatal Agric Biotechnol 2:278–284
Miller GL (1959) Use of dinitrosalicilic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Vendruscolo F, Rossi MJ, Schmidell W, Ninow JL (2012) Determination of oxygen solubility in liquid media. ISRN Chem Eng 1:1–5
Schmidell W (2001) In: Schmidell W, Borzani W, Lima UA, Aquarone E (eds) Transferência de oxigênio em biorreatores, 2nd edn. Edgard Blücher, São Paulo
Luedeking R, Piret EL (1959) A kinetic study of the lactic acid fermentation. Batch process at controlled pH. J Biochem Microbiol Technol Eng 1:393–411
Pirt SJ (1975) Principles of microbe and cell cultivation. A halted press book. Willey, New York
Zabriskie DW, Humphrey AE (1978) Real-time estimation of aerobic batch fermentation biomass concentration by component balancing. AIChE J 24:138–146
Okafor N (2007) Modern industrial microbiology and biotechnology. Science Publishers, Enfield
Acknowledgments
The authors wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—The National Council for Scientific and Technological Development) for financial support (process number 476056/2006-3) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Committee for Professional Development of Higher Education Staff) for a research scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Vendruscolo, F., Schmidell, W., de Oliveira, D. et al. Kinetic of orange pigment production from Monascus ruber on submerged fermentation. Bioprocess Biosyst Eng 40, 115–121 (2017). https://doi.org/10.1007/s00449-016-1679-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1679-5