Abstract
Reducing power such as NADH is an essential factor for acetone/butanol/ethanol (ABE) fermentation using Clostridium spp. The objective of this study was to increase available NADH in Clostridium beijerinckii IB4 by a microbial electrolysis cell (MEC) with an electron carrier to enhance butanol production. First of all, a MEC was performed without electron carrier to study the function of cathodic potential applying. Then, various electron carriers were tested, and neutral red (NR)-amended cultures showed an increase of butanol concentration. Optimal NR concentration (0.1 mM) was used to add in a MEC. Electricity stimulated the cell growth obviously and dramatically diminished the fermentation time from 40 to 28 h. NR and electrically reduced NR improved the final butanol concentration and inhibited the acetone generation. In the MEC with NR, the butanol concentration, yield, proportion and productivity were increased by 12.2, 17.4, 7.2 and 60.3 %, respectively. To further understand the mechanisms of NR, cathodic potential applying and electrically reduced NR, NADH and NAD+ levels, ATP levels and hydrogen production were determined. NR and electrically reduced NR also improved ATP levels and the ratio of NADH/NAD+, whereas they decreased hydrogen production. Thus, the MEC is an efficient method for enhancing the butanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Butanol is an important chemical intermediate for the precursor of many industrially chemicals in food, chemical and pharmaceutical industries [1, 2]. Due to its favorable physicochemical properties for blending with or directly substituting for fossil gasoline, butanol also is considered as a potential superior liquid transportation fuel [3–6]. Bio-based butanol, produced from acetone–butanol–ethanol (ABE) fermentation by solventogenic Clostridium spp., is preferred as its green renewable feature [1, 7, 8].
Nevertheless, the application of fermentative butanol is still limited due to several draw backs such as its toxicity, low final butanol concentration, yield and productivity, and undesirable selectivity [1, 9]. In order to improve the economic viability of butanol fermentation, efforts have been taken to overcome the above mentioned obstacles. Researchers have successfully carried out many methods to improve butanol fermentation performance, including utilization of mixed substrates [10–12], screening of hyper-butanol strains [7, 13, 14], utilization of high cell concentrations in continuous fermentation [15, 16], multi-stage continuous fermentation [17], the addition of organic acids [18, 19] and in situ butanol recovery [1].
In the metabolic pathways of ABE fermentation, NADH, a cofactor which is used as a reducing power, is generated by glucolysis and consumed along with the production of reduced products. It plays an important role in cofactor-dependent production systems. Manipulation of the redox balance has been demonstrated to change metabolic flux for butanol production [20]. Thus, it is significant to provide available NADH to achieve a redox balance in cellular metabolism to enhanced butanol production.
Electron carriers such as neutral red (NR), benzyl viologen (BV), methylene blue (MB), and methyl viologen (MV), have been widely used to alter metabolite patterns in butanol fermentation. The addition of NR can induce ethanol and butanol production without acetone produced [21]. On the other hand, a 36 % increase of the ferredoxin NAD reductase and an 83 % decrease the hydrogenase activity inhibited the hydrogen production. Rao and Mutharasan reported that significant metabolic shifts were caused after addition of BV at low concentrations in a C. acetobutylicum batch culture. Electron flow was altered to direct carbon flow from organic acids to alcohol production accompanied by decreased hydrogen production [22]. Moreover, in Clostridium acetobutylicum strain ATCC 824 batch culture, the use of MB caused an increase of 88 % in acetone production and 43 % in butanol production [23]. MV, which is known as a commercial herbicide “paraquat”, can intercept electrons from PFOR (pyruvate: ferredoxin oxidoreductase) and donate them to NAD+ via the ferredoxin-NAD+ reductase [24]. MV was reported to enhance the butanol yield by 9.4 % and decrease the acetone yield by 80 %, and at the same time, the hydrogen production was also decreased by a half [24].
Microbial electrolysis cell (MEC) is another method to supply electrons for a microbial metabolism which works at a biocathode by external power input. Several researchers have used MECs for hydrogen production, reduced chemicals production and bioremediation [25, 26]. The mechanisms of extracellular electron transfer (EET) between biocathodics and cells are direct and indirect electron transfer [27]. Direct electron transfer needs the bacteria in biocathodes have electrochemically activity. Most of the bacteria have been reported to be Gram negative, while most Gram-positive bacteria have not shown a strong capacity for EET [28]. Therefore, many electron carriers have been used in MECs to facilitate this indirect EET between biocathodics and cells [29, 30].
In this paper, butanol production enhancement was studied by the utilization of MEC. Our hypothesis is that a raised NADH/NAD+ level caused by a biocathodic would induce high butanol production, yield and proportion. In order to evaluate the effects of electron carriers in MEC on ABE fermentation, firstly, potential applying was performed without electron carriers. In addition, the effects on hydrogen production, NADH and NAD+ level, the NADH/NAD+ ratio and ATP level were also investigated.
Materials and methods
Strain and culture conditions
The strain Clostridium beijerinckii IB4 was used in this work. It was a mutant derived from C. beijerinckii NCIMB 8052, which was selected by low-energy ion implantation [2]. C. beijerinckii IB4 was grown at 35 °C for 12 h in a yeast extract/peptone/starch (YPS) medium contained (per liter): 3.0 g yeast extract, 5.0 g peptone, 10.0 g soluble starch, 2.0 g CH3COONH4, 2.0 g NaCl, 3.0 g MgSO4·7H2O, 1.0 g KH2PO4, 1.0 g K2HPO4 and 0.1 g FeSO4·7H2O (sterilized at 121 °C for 15 min and cooled to 37 °C); the initial pH was adjusted to 6.0 using 1 M HCl [31]. The inoculation level was 5 % (v/v), and the seed culture was purged with filtered nitrogen gas to remove dissolved oxygen after inoculating in serum bottles.
Clostridium beijerinckii IB4 was cultivated in P2 medium for butanol fermentation, which composed (per liter): 50.0 g glucose, 0.50 g KH2PO4, 0.50 g K2HPO4, 2.2 g CH3COONH4, 0.20 g MgSO4·7H2O, 0.01 g MnSO4·H2O, 0.01 g FeSO4·7H2O, 0.01 g NaCl and 1 g corn steep liquor [32]. It was sterilized at 121 °C for 15 min and cooled to 35 °C before inoculation. In all experiments, a 10 % (v/v) actively growing secondary seed culture was inoculated, and filtered nitrogen gas was purged to remove dissolved oxygen. Except for corn steep liquor, all the chemicals used in this study were of reagent grade. And unless otherwise stated, all the chemicals mentioned above were purchased from either Sinochem (Shanghai, China) or Fluka Chemical (Buchs, Switzerland). While corn steep liquor (84.41 % water, 10.75 % solid material, 2.31 % protein, 2.07 % total sugar, 0.37 % total nitrogen, 0.041 % amino acids, 0.049 % biotin and microelement) was purchased from Anhui BBCA Biochemical Co. (Bengbu, China), and nitrogen was purchased from Nanjing Special Gases Factory (Nanjing, China).
Electron carrier selection
Four electron carriers (NR, BV, MB and MV) were added in serum bottles (100 mL) containing 50 mL P2 medium to select the best one in increasing butanol production. The quantity of each electron carrier was added according to the same amounts of effective site (the concentration of MB is 0.2 mM, and others are 0.1 mM). Then, the addition amount of selected electron carrier was optimized at final concentrations of 0, 0.05, 0.1, 0.2, 0.3 and 0.4 mM. Each condition was performed in triplicate.
Setup of MEC
Batch fermentation was conducted in a microbial electrolysis cell (MEC). The MEC was an H-type reactor with a dual-chamber (300 mL each). It was specially designed for the cultivation of strictly anaerobic bacteria. A three-electrode potentiostatic system was used during the fermentations. The cathode and the anode were both graphite felt electrodes (5 cm × 5.5 cm × 2). The reference electrode was Ag/AgCl, 3 M KCl (CHI111, CH Instruments, Inc., USA), and it was immersed into the cathode compartment. The two compartments were separated by a cation exchange membrane (Naracelltech, Seoul, South Korea). Biogas was collected by one plastic gas (China Dalian Delin Gas Pasking Co., ltd, PR China) connected with rigid plastic pipe fittings inserted in the cathode stopper.
Operation of MEC
The cathode compartment was filled with P2 medium (250 mL), and the anode compartment was filled with 0.1 M PBS (pH 7.0, 250 mL). Cathode potential versus Ag/AgCl reference electrode was determined from a redox peak at −700 mV in a cyclic voltammogram of C. beijerinckii IB4 culture (electron carrier added) with a scan rate of 20 mV/s over the range −300 to −1 mV [33]. MEC was also operated under the constant cathode potential of −700 mV (vs. Ag/AgCl), which was set using an electrochemical workstation (CHI1000C, CH Instruments, Inc., USA) controlled by a personal computer. The computer also monitored the current–time (i–t) curve. To investigate the effects of MEC on ABE fermentation, typical batch fermentation, potential applying without electron carriers, electron carriers, and electrochemical reduced electron carriers processes were gradually performed in the H-type reactor.
Product analysis and hydrogen detection
Optical density (OD) of cell was measured at 600 nm with a spectrophotometer (UV 2100, Unico). The concentration of glucose was measured by SBA-40C biosensor analyzer (Institute of Biology, Shandong Province Academy of Sciences, PR China). The total solvent [acetone, butanol and ethanol (ABE)] was analyzed by gas chromatograph (GC-2010, Shimadzu Scientific Instruments, Japan) equipped with a flame ionization detector and an InterCap WAX column (0.25 mm × 30 m, GL Sciences Inc., Japan). Organic acids (acetic acid and butyric acid) were analyzed by high-performance liquid chromatography (HPLC, Chromeleon server monitor, P680 pump, Dionex, USA) with a mobile phase of H2SO4 (0.005 mol/L) at a flow rate of 1.0 mL/min.
Biogas (hydrogen and carbon dioxide) was collected together and quantified by the increased gas volume. The hydrogen gas composition percentage α0 was analyzed by a hydrogen analyzer (HY-OPTIMA 700, H2scan, USA). Then, hydrogen production was calculated using the ideal gas law Eq. 1 and Avogadro’s Hypothesis Eq. 2:
Equations 1 and 2 can be combined into Eq. 3.
where P is the absolute pressure, atm; V i is the total volume of gas, V H2 is the volume of hydrogen, L; n i and n H2 the number of moles of total gas and hydrogen; R is the gas constant, 0.0821 atm/K/mol; and T is the temperature in K.
The reported values are averages of triplicate experiments for each condition.
Enzyme and ATP assays
The intracellular NADH and NAD+ concentrations were measured by the enzyme cycling method [2, 34]. Generally, 1 mL culture broth under each condition was sampled quickly into two microcentrifuge tubes for NADH and NAD+ extraction, respectively. Each of them was centrifuged at 15,000 rpm for 1 min to collect cells. 300 μL 0.2 M NaOH or 300 μL 0.2 M HCl was added to the tubes to resuspend the cells for NADH or NAD+ extraction, respectively. The samples were heated at 50 °C for 10 min and then cooled to 0 °C. The cell lysate was neutralized by adding 300 μL 0.1 M HCl or 300 μL 0.1 M NaOH drop by drop for NADH or NAD+ extraction, respectively. Both microcentrifuge tubes were centrifuged at 15,000 rpm for 5 min to gather the supernatants. All the supernatants were transferred to new tubes and stored at −20 °C. The mixture contained equal volumes of 1.0 M Bicine buffer (pH 8.0), absolute ethanol, 40 mM EDTA (pH 8.0), 4.2 mM MTT and twice as much of 16.6 mM PES was prepared firstly, and then placed it in a 30 °C water bath for 10 min. The reaction system was 50 μL extract, 0.3 mL pure water, 0.6 mL mixture and 50 μL alcohol dehydrogenase (500 U/mL) in 1 mL total volume. And it was enlarged in a cuvette. The reaction was started when alcohol dehydrogenase was added. The absorbance at 570 nm was determined immediately by UV–Vis spectrophotometer for 3 min. NADH and NAD+ titres in the supernatants were determined and the intracellular content per gram cells was calculated by comparing the slope of absorbance to reaction time with standard curves.
To measure the intracellular ATP concentrations under each condition, samples were taken immediately and then stored them at −20 °C quickly. When ATP concentrations were measured, 1 mL of cold 30 % (w/v) trichloroacetic acid was added to the samples (4 mL) and mixed thoroughly. The ATP concentrations were determined by the BacTiter-Glo™ Microbial Cell Viability assay kit on the GloMax®Multi+ Detection System (Promega, Madison, WI, USA).
Assays were performed in triplicate.
Results and discussion
Enhanced butanol productivity in a MEC without electron carrier
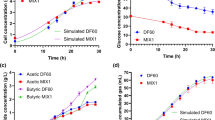
The typical batch fermentation process of C. beijerinckii IB4 in the H-type reactor without potential applying or electron carrier addition was investigated. This strain demonstrated a biphasic fermentation profile, acidogenesis phase and solventogenesis phase. The cell density increased rapidly from 12 h and reached a maximum OD600 of 13.25 at 28 h of cultivation (Fig. 1). After that, the cell density started to decrease. In the fermentation process, the glucose consumption curve (Fig. 2) showed a close relation with the growth curve (Fig. 1). In the acidogenesis phase (0–12 h), the utilization rate of glucose was 0.33 g/L/h, of which was 1.43 g/L/h in the solventogenesis phase (after 12 h) (Table 1). At the end of fermentation, 44 g/L of glucose was consumed in 40 h with 9.36 g/L of butanol and 4.39 g/L of acetone produced (Fig. 3a, b). The highest acetic acid and butyric acid were 3.21 g/L and 0.72 g/L, respectively (Fig. 4a, b). And both of them were reached at 12 h. Moreover, the yield, proportion and productivity of butanol were only 0.213 g/g, 0.667 and 0.234 g/L/h, respectively (Table 2).
Profiles of cell growth in batch fermentations with 50 g/L glucose under four conditions. Typical batch fermentation was used as control; NR(neutral red) of 0.1 mM was used as NR condition; E indicated Electricity of −700 mV cathodic potential; ENR was electrically reduced NR, the NR concentration was 0.1 mM and the cathodic potential was also poised at −700 mV
In order to examine the effects of electron carriers in MEC on ABE fermentation, potential applying without electron carriers was performed. The cathode potential was poised at −700 mV (vs. Ag/AgCl reference electrode) by the cyclic voltammogram method (as previous mentioned), which matched the NR redox potential. It can be observed from Fig. 1 that the cell growth was strongly increased from 12 h, and there was an obvious enhancement compared with the control at 16 h. After 20 h, the cell density started to decrease. From the glucose consumption curve (Fig. 2) and product generate curve (Fig. 3a, b), 42 g/L of glucose was consumed in only 28 h with 9.20 g/L of butanol and 4.15 g/L of acetone produced. So it produced almost the same concentration of products within much shorter time compared with the control. In comparison with the process without potential applying, the glucose consumption rate was highly enhanced (1.50 g/L/h), especially in the acidogenesis phase (0–12 h), of which was 1.08 g/L/h. Actually, OD600 in the acidogenesis phase didn`t showed a big difference between potential applying and the control, while the concentration of products were much higher under the potential. These findings clearly indicated that the accelerated glucose consumption was used to produce solvents faster. However, in the solventogenesis phase, the stimulated cell growth was accompanied by a higher utilization rate of glucose, which demonstrated that more biomass accumulated within the fermentation system facilitated glucose utilization. Moreover, the highest acetic acid and butyric acid were 1.35 and 0.31 g/L, respectively (Fig. 4a, b). Both of them were lower than that in the control at 12 h. Hence, based on these data obtained, the yield, proportion and productivity of butanol were calculated, of which were 0.219 g/g, 0.675 and 0.329 g/L/h, respectively. It's worth noting that the butanol productivity was 40.6 % higher in the MEC without electron carrier than in the control process.
Enhanced butanol production in a MEC with an electron carrier
Clostridium beijerinckii are known as the Gram-positive bacteria [2], which have a weak capacity for extracellular electron transfer as mentioned in introduction, so electron carrier should be used. Four electron carriers (NR, BV, MB and MV) were selected for the best in improving butanol production by fermenting in serum bottles. The effects of these electronic carriers on butanol production and cell density by C. beijerinckii IB4 were shown in Fig. 5. It demonstrated that only NR increased both the butanol production and cell growth compared with that in the control condition (no electron carriers). Viologen dyes (BV and MV) inhibited cell growth seriously, especially BV. MB slightly increased C. beijerinckii IB4 growth, however the butanol concentration was lower, the more metabolite flowed to the acetone formation (data not show), Ballongue et al.also found that MB could increase the acetone metabolite flux [23]. Different NR concentrations (0–0.4 mM) were added to find the optimal point. Table 3 showed that the addition of different NR concentrations did not inhibit the cell growth. But butanol production was increased in a narrow window of NR concentration. At 0.1 mM NR, butanol production was the highest. It was interesting that the more NR were added, lower acetone were produced, otherwise, the higher butanol proportion achieved. NR can strongly bind to cell membranes [35]. It has a redox potential of −325 mV which is similar to that of NADH (−320 mV), and its structure is similar to that of flavins. These characteristics suggest that NR could interact with NADH. Studies have showed that it might consume electrons from or offer electrons to steps that involve NADH/NAD+ or perhaps directly transfer electrons with NADH itself [21]. Maybe the higher NAD(H) pool and NADH/NAD+ level gave rise to the butanol production.
In order to study the differences between NR addition and electrically reduced NR, 0.1 mM NR was added into the H-type reactor without electricity. A slightly higher cell growth and faster glucose consumption were obtained. A little more butanol concentration and lower acetone concentration were produced accompanied by 45 g/L glucose used within 36 h. All these differences were observed after 12 h. At 12 h, the maximum acetic acid was higher while the maximum butyric acid was lower than that in the control. So, a slightly higher butanol proportion, yield, productivity and glucose utilization rate were calculated.
A more effective result was shown in MEC with NR (0.1 mM). The cathode potential was also poised at −700 mV (vs. Ag/AgCl reference electrode). The glucose consumption curve was almost coincide between MEC with and without NR, which showed a 36.4 % faster than that of the control. The two cell density curves were similar too. The organic acids were both lower than that of the control in the fermentation process. The differences between MEC with and without NR were the final butanol and acetone concentration, which were 10.50 and 3.86 g/L, respectively. The butanol production was increased by 12.2 % compared with the control. As shown in Table 2, higher butanol proportion and yield were obtained, which were enhanced to 0.715 and 0.250 g/g. Surprisingly, the productivity of butanol reached 0.375 g/L/h, which was enhanced by 60.3 % compared with the control.
Differences of NADH/NAD+ levels, ATP levels and hydrogen production under various conditions
Butanol formation is constrained by NADH limitation, four moles of NADH are consumed (by oxidation to NAD+) per each mole of butanol produced from acetyl-CoA [20]. In order to verify and explore the function of MEC with NR and NR addition compared with that of the control and potential applying without NR, the time courses of the ratio of NADH/NAD+ and the total amounts of NADH and NAD+ under each condition were determined and shown in Figs. 6 and 7, respectively. When cathodic potential was applied only, the ratio of NADH/NAD+ was very similar with that of the control in the whole fermentation process, which rose continuously from 0.32 to 0.45. During the first half of the fermentation process when NR was added, the NADH/NAD+ ratio (0.27 at 12 h) was lower than that of the control, while it became higher and higher (the highest level of 0.54 at 24 h) during the latter half. Electrically reduced NR has successfully reduced intracellular NAD+ to NADH and therefore increased the ratio of NADH/NAD+ through all the fermentation process (from 0.37 at 12 h to 0.49 at 24 h). However, the amounts of NADH and NAD+ were decreased quickly after 12 h under all conditions compared with that of the control. Due to the fermentation results, the decreased amounts of NADH and NAD+ did not affect the distribution or production of the main metabolic products. The trend of the ratio of NADH/NAD+ curve when NR was added demonstrated that NR can consume the reduced power and be reduced in the acidogenesis phase, while in the solventogenesis phase, NR can release the reduced power and be oxidized. MEC can offer continuous electrons to reduce NR, and the electrically reduced NR can increase the available NADH, which can enhance the production of reduced products, such as butanol and ethanol. Hence, higher NADH/NAD+ ratio, more butanol produced, which were also confirmed in Table 2. These results were contradicted with what Meyer and Papoutsakis concluded [36].
For the general ABE fermentation, the only source of ATP is through the production of acetic and butyric acid besides the glycolytic pathway [36]. Moreover, ATP was reported to affect the cell growth [35]. Therefore, the ATP level was determined, as shown in Table 4, to analyze the differences of the cell growth under various conditions. Under the cathodic potential, the ATP level (5.92 nmol/g) was a little lower than that of the control (6.98 nmol/g). When NR was added, the ATP level (10.28 nmol/g) increased obviously. It's worth noting that MEC with NR increased the ATP level (12.53 nmol/g). This enhancement is caused by the transfer of electrons into the cell. Protons are also transferred into the cytoplasm to maintain charge neutrality, which is broken up by the transfer of electrons. And this process cause ATP synthesis which is driven by a proton gradient [37]. Hence, the higher ATP level is, the more effectively the electrons transfer. But the differences of ATP amounts didn't explain the enhancement of cell growth. Girbal and Soucaille reported that the in vitro phosphotransacetylase and phosphotransbutyrylase (PTB) and butyrate kinase (BK) activities decreased linearly with the intracellular ATP concentration [38]. Under the conditions of NR addition and MEC with NR, the production of acetone was inhibited (Tables 2, 3). Furthermore, the higher ratio of NADH/NAD+ also guided the metabolic flow to butanol and ethanol rather than acetone and organic acids. These two reasons may cause to weaken the metabolic strength of organic acids loop.
Hydrogen, as an end product in clostridia metabolism, is catalyzed by hydrogenases. It is well known that hydrogenase competes with NAD(P) reductase to oxidize the reduced ferredoxin [39]; in another word, hydrogen production competes with butanol production in the typical fermentation. Hydrogen production was detected during all the batch fermentation processes. When NR was added, the hydrogen production(70.2 mM) was decreased obviously compared with that of the control (89.8 mM) (Table 4). Researches have been performing to illustrate that NR can restrain the hydrogenase activity, which can decrease hydrogen production [21]. This can be also understood as a method to avoid reducing power escaping. Electrically reduced NR has the same function, so the hydrogen production in MEC with NR (94 mM) was much lower than that in the MEC (131.7 mM). Surprisingly, when cathodic potential applying, the hydrogen production was stimulated. Producing hydrogen through the electrolysis of water at the cathode requires a potential of at least E 0 = −410 mV (NHE) [40]. It is closed to that of NR [E 0 = −325 mV (NHE)]. This may be the reason of high hydrogen production. In succinate production by Actinobacillus succinogenes, extra hydrogen as a potential electron donor produced more reduced metabolites and gained additional energy for cell growth [35]. Here, electricity also activated cell growth, and it may be due to the generation of hydrogen, the details how electricity and hydrogen stimulated the cell growth is still under studying in our laboratory. Moreover, an active cell growth enhanced by electricity does not need to synthesize ATP through the production of acetic and butyric acid, so the metabolic strength of organic acids loop was weaken obviously under the potential applying.
Conclusions
In this study, the ABE fermentation by Clostridium beijerinckii IB4 was performed in a MEC with NR, which was selected from several electron carriers. The butanol concentration, yield, proportion and productivity were increased by 12.2, 17.4, 7.2 and 60.3 %, respectively. When NR was added in the H-type reactor without potential applying, the concentration of butanol was increased, too. Electricity showed a strong enhancement to the cell growth. The total amount of NADH and NAD+ didn`t show an effect to the metabolic distribution. Higher ratio of NADH/NAD+ led to more butanol production. Both of high ATP level and high NADH/NAD+ ratio weakened the organic acids loop and inhibited the acetone formation. The results of hydrogen production demonstrated the function of NR, which inhibited the hydrogenase activity and kept the reducing power not to escape. In a conclusion, a MEC can enhance butanol production with high concentration, yield, proportion and productivity.
References
Wu H, Chen XP, Liu GP, Jiang M, Guo T, Jin WQ, Wei P, Zhu DW (2012) Acetone–butanol–ethanol (ABE) fermentation using Clostridium acetobutylicum XY16 and in situ recovery by PDMS/ceramic composite membrane. Bioprocess Biosyst Eng 35:1057–1065
Jiang M, Chen JN, He AY, Wu H, Kong XP, Liu JL, Yin CY, Chen WF, Chen P (2014) Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochem 49:1238–1244
Dürre P (2007) Biobutanol: an attractive biofuel. Biotech J 2:1525–1534
Jiang Y, Xu CM, Dong F, Yang YL, Jiang WH, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11:284–291
Tracy BP (2012) Improving butanol fermentation to enter the advanced biofuel market. MBio 3:e00518–12
Li TG, Yan Y, He JZ (2014) Reducing cofactors contribute to the increase of butanol production by a wild-type Clostridium sp. strain BOH3. Bioresour Technol 155:220–228
Ezeji TC, Qureshi N, Blaschek HP (2007) Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotechnol 18:220–227
Li L, Ai HX, Zhang SX, Li S, Liang ZX, Wu ZQ, Yang ST, Wang JF (2013) Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour Technol 143:397–404
Zheng J, Tashiro Y, Wang QH, Sonomoto K (2014) Recent advances to improve fermentative butanol production: genetic engineering and fermentation technology. J Biosci Bioeng 119:1–9
Tran HTM, Cheirsilp B, Hodgson B, Umsakul K (2010) Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone-butanol-ethanol production from cassava starch. Biochem Eng J 48:260–267
Abd-Alla MH, Elsadek El-Enany AW (2012) Production of acetone-butanol-ethanol from spoilage date palm (Phoenix dactylifera L.) fruits by mixed culture of Clostridium acetobutylicum and Bacillus subtilis. Biomass Bioenergy 42:172–178
Nakayama SI, Kiyoshi K, Kadokura T, Nakazato A (2011) Butanol production from crystalline cellulose by cocultured Clostridium thermocellum and Clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microbiol 77:6470–6475
Lee JY, Jang YS, Lee J, Papoutsakis ET, Lee SY (2009) Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotech J 4:1432–1440
Jang YS, Lee JY, Lee J, Park JH, Im JA, Eom MH, Lee J, Lee SH, Song H, Cho JH, Seung DY, Lee SY (2012) Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. MBio 3:e00314–12
Kong XP, He AY, Zhao J, Wu H, Jiang M (2015) Efficient acetone–butanol–ethanol production (ABE) by Clostridium acetobutylicum XY16 immobilized on chemically modified sugarcane bagasse. Bioprocess Biosyst Eng. doi:10.1007/s00449-015-1377-8
Jang YS, Malaviya A, Lee SY (2013) Acetone-butanol-ethanol production with high productivity using Clostridium acetobutylicum BKM19. Biotechnol Bioeng 110:1646–1653
Bankar SB, Survase SA, Singhal RS, Granstrom T (2012) Continuous two stage acetone-butanol-ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B5. Bioresour Technol 106:110–116
Martin JR, Petitdemange H, Ballongue J, Gay R (1983) Effects of acetic and butyric acids on solvents production by Clostridium acetobutylicum. Biotechnol Lett 5:89–94
Al-Shorgani NKN, Ali E, Kalil MS, Yusoff WMW (2011) Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. Bioeng Res 5:287–293
Lutke-Eversloh T, Bahl H (2011) Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr Opin Biotechnol 22:634–647
Girbal L, Vasconcelos I, Saint-Amans S, Soucaille P (1995) How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol Rev 16:151–162
Rao G, Mutharasan R (1987) Altered Electron Flow in Continuous Cultures of Clostridium acetobutylicum Induced by Viologen Dyes. Appl Environ Microbiol 53:1232–1235
Ballongue J, Amine J, Petitdemange H, Gay R (1986) Enhancement of solvents production by clostridium acetobutylicum cultivated on a reducing compounds depletive medium. Biomass 10:121–129
Rao G, Mutharasan R (1986) Alcohol production by Clostridium acetobutylicum induced by methyl viologen. Biotechnol Lett 8:893–896
Lovley DR (2011) Powering microbes with electricity: direct electron transfer from electrodes to microbes. Environ Microbiol Rep 3:27–35
Steinbusch KJJ, Hamelers HVM, Schaap JD, Kampman C, Buisman CJN (2010) Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ Sci Technol 44:513–517
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis-revisiting the electrical route for microbial production. Nat Rev Microbiol 8:706–716
Cournet A, Délia ML, Bergel A, Roques C, Bergé M (2010) Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem Commun 12:505–508
Fultz ML, Durst RA (1982) Mediator compounds for the electrochemical study of biological redox systems: a compilation. Anal Chim Acta 140:1–18
Gründig B, Wittstock G, Rü del U, Strehlitz B (1995) Mediator-modified electrodes for electrocatalytic oxidation of NADH. J Electroanal Chem 395:143–157
Jin XQ, Zhou H, Wu X, Zhang G, He BF (2008) A rapid screening method of producing strain in acetone-butanol fermentation. Chin J Process Eng 6:1185–1189
Qureshi N, Blaschek HP (2001) Recent advances in ABE fermentation: hyper-butanol producing Clostridium beijerinckii BA101. J Ind Microbiol Biotechnol 27:287–291
Xie XH, Li EL, Tang ZK (2010) Sudden emergence of redox active escherichia coli phenotype: cyclic voltammetric evidence of the overlapping pathways. Int J Electrochem Sci 5:1070–1081
Li J, Jiang M, Chen KQ, Ye Q, Shang LA, Wei P, Yin HJ, Chang HN (2010) Effect of redox potential regulation on succinic acid production by Actinobacillus succinogenes. Bioprocess Biosyst Eng 33:911–920
Park DH, Laivenieks M, Guettler MV, Jain MK, Zeikus JG (1999) Growth and metabolite production neutral red as the sole electron donor for microbial utilization of electrically reduced. Appl Environ Microbiol 65:2912–2917
Charles LM, Eleftherios TP (1989) Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 30:450–459
Pandit AV, Mahadevan R (2011) In silico characterization of microbial electrosynthesis for metabolic engineering of biochemicals. Microb Cell Fact 10:76
Girbal L, Soucaille P (1994) Regulation of Clostridium acetobutylicum metabolism as revealed by mixed-substrate steady-state continuous cultures: role of NADH/NAD ratio and ATP pool. J Bacteriol 176:6433–6438
Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781
Liu H, Grot S, Logan BE (2005) Electrochemically assisted microbial production of hydrogen from acetate. Environ Sci Technol 39:4317–4320
Acknowledgments
This work was supported by the “973” Program of China (Grant No. 2011CB707405), a Project Supported by Program for New Century Excellent Talents in University, Program for Changjiang Scholars and Innovative Research Team in University (Grant NO. 06-A-047), Jiangsu Province Natural Science Foundation for Youth (BK20140940), Jiangsu Province Natural Science Foundation (BK20151532), Jiangsu Key Lab of Biomass-based Green Fuels and Chemicals Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A.-Y. He and C.-Y. Yin contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
449_2015_1508_MOESM1_ESM.tif
Fig.S1 The cyclic voltammetry (CV) curve of ENR (0.1 mM NR) system at a scan rate of 20 mV/s over the range −300 to −1 mV during the batch adaptation of C. beijerinckii IB4 114 x 95 mm (96 x 96 DPI) (TIFF 127 kb)
Rights and permissions
About this article
Cite this article
He, AY., Yin, CY., Xu, H. et al. Enhanced butanol production in a microbial electrolysis cell by Clostridium beijerinckii IB4. Bioprocess Biosyst Eng 39, 245–254 (2016). https://doi.org/10.1007/s00449-015-1508-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1508-2