Abstract
For the construction of an efficient copper waste treatment system, a cell surface display strategy was employed. The copper adsorption ability of recombinant bacterial strains displaying three different copper binding peptides were evaluated in LB Luria–Bertani medium (LB), artificial wastewater, and copper phthalocyanine containing textile dye industry wastewater samples. Structural characteristics of the three peptides were also analyzed by similarity-based structure modeling. The best binding peptide was chosen for the construction of a dimeric peptide display and the adsorption ability of the monomeric and dimeric peptide displayed strains were compared. The dimeric peptide displayed strain showed superior copper adsorption in all three tested conditions (LB, artificial wastewater, and textile dye industry wastewater). When the strains were exposed to copper phthalocyanine dye polluted wastewater, the dimeric peptide display [543.27 µmol/g DCW dry cell weight (DCW)] showed higher adsorption of copper when compared with the monomeric strains (243.53 µmol/g DCW).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been shown that the discharge of heavy metals into the environment from petrochemical, agricultural, and mechanical industries keeps increasing [1, 2]. As such, the effects of heavy metal pollution on the ecosystem have become one of the major concerns of society. In recent years, various water soluble reactive dyes have been developed, and their production has increased to make up about 15–25 percent of the total dye production [3]. It has been reported that phthalocyanine dyes, one of the metallic dyes, are highly toxic due to its high copper content [4]. Copper is widely used as an anti-fouling agent in various industries, and its accumulation in living tissues can cause serious illnesses of high mortality and morbidity [5]. According to the Environmental Protection Agency, the maximum copper concentrations should not reach over 1.3 mg/L [5, 6]. Because traditional physicochemical methods such as chemical oxidation and ion exchange evaporative recovery are ineffective, especially for low concentrations of metals, so the development of novel heavy metal cleanup methods is required [7, 8].
Over the last several decades, recombinant DNA technology has been employed to develop novel bioremediation systems. Various genetically modified microorganisms have been constructed by expressing heavy metal-specific proteins to adsorb them out of inorganic waste [9]. It has also been reported that microorganism-based adsorbent can be constructed by expressing metal binding peptides on the surface of the microorganism [10].
Cell surface display (CSD) allows the expression of proteins on the surface of the bacterial cells with the help of an anchoring motif [11]. Various anchoring motifs including LamB, OmpA, FhuA, OmpC, and TraT have been widely used by many researchers in various Gram-negative bacteria [9, 11]. Escherichia coli OmpC is one of the most well-characterized anchoring motif proteins. The OmpC molecule comprises 16 transmembrane, antiparallel β strands, which are able to produce a β-barrel structure surrounding a large channel, and are interlinked by seven internal loops and eight external loops [12, 13]. Considering the high copy level of OmpC (2 × 105 molecules per cell), it is considered to be one of the most appropriate anchoring motifs for peptide surface expression [14, 15]. Heavy metal binding peptide displayed microorganisms can be considered one of the most promising candidates for the development of an affordable and environmentally friendly heavy metal wastewater treatment system [16].
In this study, three different copper binding peptides (CBP) were displayed on the surface of E. coli using OmpC as the anchoring motif. Copper adsorption of these three recombinant strains was evaluated in culture medium, artificial wastewater, and copper phthalocyanine containing textile dye industry wastewater samples. The dimer of the best copper binding peptide was constructed and displayed on the surface of E. coli. The efficiency of copper adsorption by the dimeric peptide was also evaluated in all the three conditions.
Materials and methods
Bacterial strains and media
The bacterial strains used in this study are listed in Table 1. The strains were cultivated in LB medium (10 g/L bacto-tryptone, 5 g/L bacto-yeast extract and 5 g/L NaCl) supplemented with antibiotics (100 mg/L ampicillin) at 37 °C with vigorous shaking at 250 rpm.
Plasmid construction
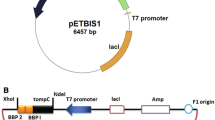
The three copper binding peptide genes were attached to the C-terminus of ompC genes by truncating it at loop8 (993 bp) and amplified using peptide sequence incorporated oligonucleotides (Table 2). The polymerase chain reaction (PCR) was conducted with an MJ Mini Personal Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA) using the Expand High Fidelity PCR system (Roche Molecular Biochemicals, Mannheim, Germany). The three copper binding peptides (NAKHHPR, NRWHHLE, and SPHHGGW) were selected based on biopanning experiment. The PCR products were cloned into the pET21a plasmid using NdeI and BamHI restriction enzymes to construct pETCP1, pETCP2, and pETCP3 (Fig. 1a). Expression of OmpC-peptides was induced under control of the T7 promoter by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG). These plasmids were transformed into a chemically competent E. coli BL21 (DE3) strain for further studies.
For the construction of the dimeric peptide, the ompC gene was PCR amplified using Cudim1_Frw and Cudim1_Rev, and cloned into pET21a using NdeI and BamHI restriction enzymes. The gene coding SPHHGGW peptide was cloned downstream of the ompC gene using BamHI and XhoI restriction enzymes. The constructed plasmid was digested with BamHI and SalI, and ligated with BamHI and XhoI digested peptide genes to construct the pETC3x2 plasmid (Fig. 1b). The pETC3x2 plasmid was transformed into the BL21 (DE3) strain for further studies.
Expression study by SDS-PAGE
The recombinant E. coli strains were cultured overnight in LB medium at 37 °C, and the cultures were then diluted 100-fold in the same medium. When optical density at 600 nm (OD600) reached 0.6, IPTG was added into the culture broth at different concentrations of 0–1 mM and incubated for 6 h. The harvested cells were sonicated by iced sonicator (30 s ON, 30 s OFF, 12 cycles) and the cells were centrifuged at 8000 rpm to remove cell debris. The outer membrane fractions are isolated from the pellet by adding 10 mM Tris–HCl (pH 7.5) and the suspended cells were kept in 4 °C overnight to lift the membrane fractions to the solution and analyzed by 12 % (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) [17]. Fractioned protein samples were stained with Coomassie brilliant blue R-250 (Bio-Rad Laboratories, Hercules, CA, USA).
Analysis of copper bioadsorption
The recombinant E. coli strains were grown overnight in LB medium supplemented with 100 mg/L ampicillin at 37 °C. The overnight cultures were diluted 100-fold in fresh LB medium, and incubated until OD600 reached 0.5. The cells were induced with IPTG, and the strains were then cultured for 6 h at 30 °C. The strains were washed twice with 0.85 % (w/v) of NaCl, and the cells were incubated in copper sulfate containing LB medium, artificial wastewater, and copper phthalocyanine containing textile dye industry wastewater samples. All the solution’s pH was maintained at 6. Various concentrations of copper sulfate (0.3–1.0 mM) were supplemented into LB medium. The artificial wastewater used contained 0.7 mM of copper, cobalt, lead, and zinc [18]. The industrial wastewater was collected near a factory that manufactures copper phthalocyanine dye in Mumbai, India. The strains were washed twice with 0.85 % (w/v) NaCl, and digested with 70 % (w/v) nitric acid overnight at room temperature. The amount of adsorbed copper was analyzed using an atomic absorption spectrophotometer (AA-7000, Shimadzu, Kyoto, Japan) [19].
Molecular modeling studies
The homology modeling of three peptides was carried out using Modeler. A search for potential structural templates was carried out using the Blastp tool against the protein databank (PDB) with default parameters [20]. With the help of the align 2D tool of Modeler, we aligned our query with a template sequence obtained from the PDB. Based on the sequence alignment and template protein structure, homology models were constructed with the default parameters. Furthermore, the constructed model structure was analyzed for centroid distances to analyze the copper coordination. The Blastp search for other short peptides was carried out for the structural template, with 1OEH/1OEI serving as the best template when compared with the other structural templates [21]. Using the same strategy, the homology model for the other short peptides was also constructed and analyzed for possible copper coordination.
Biopanning
A cyclic random heptapeptide phage display library (Ph.D.-C7C™, Phage Display Peptide Library Kit, E8120S) was obtained from NEB (New England Biolabs, Beverly, MA). A 10 μl sample of the phage library (~2 × 1011 virions with library diversity of 2 × 109 unique clones as claimed by NEB) was mixed with 60 mg of copper powders suspended in 990 μl of Binding Buffer (50 mM Tris–HCl, 150 mM NaCl, pH 7.5 and increasing concentration of Tween-20 from 0.1 to 0.6 % with 0.1 % increment for each round of screening). Following incubation with gentle shaking at room temperature for 1 h, the phage/copper mixture was centrifuged at 16,000g. The collected pellet was washed with 1 ml of Binding Buffer and centrifuged as above, five times. The copper pellet was suspended in 1 ml of Elution Buffer (0.2 M Glycine–HCl, pH 2.2), and the copper pellet was suspended in 0.5 ml of Elution Buffer, subjected to ultrasonication at 28 kHz for 10 min followed by centrifugal collection of the eluted phages (twice). The collected phages were incubated in a microcentrifuge tube filled with Binding Buffer lacking copper for 1 h and centrifuged. The collected phages from the supernatant after the second round of negative screening were isolated on agar plates, and 32 individual phage clones were randomly sampled. The phage DNA was extracted using a QIAprep M13 Kit (27704, Qiagen) and used for DNA sequencing to retrieve peptide sequences displayed on the phage surface.
Results and discussion
Optimization of peptide display conditions
To construct copper adsorbing recombinant E. coli, three copper binding peptides were integrated to the C-terminus of the truncated E. coli outer membrane protein OmpC. The effect of IPTG concentration on fusion protein expression was investigated using various concentrations of IPTG (0.3, 0.5, 0.7, and 1 mM), and the expression level was monitored using SDS-PAGE analysis. Generally, the expression of fusion proteins increased as the IPTG concentration increased up to 0.5 mM, and similar levels of protein expression were observed with higher IPTG concentrations (SI Fig. 1).
Considering a previous report that mentioned that OmpC expression is highly influenced by temperature, the effect of temperature on fusion protein expression was also investigated by culturing recombinant strains at two different temperatures (25 and 30 °C) [19]. Relatively higher fusion protein expression was observed at 30 °C than at 25 °C (data not shown). Based on these results, it was proposed that the optimum condition for fusion protein expression is 0.5 mM IPTG at 30 °C.
Copper adsorption in various environments
To evaluate the copper adsorption ability of recombinant strains, the strains were cultured in LB media supplemented with various concentrations of copper sulfate (0.3, 0.5, 0.7, and 1 mM). The recombinant E. coli, in which only OmpC was overexpressed, was used as the control strain, and 66.5 µmol/g DCW of copper was adsorbed on its surface. While the copper concentration increased to 0.7 mM, the amount of adsorbed copper increased. Copper adsorption levels then decreased at higher copper concentrations. When 0.7 mM of copper was used, 245.7 µmol Cu/g DCW of maximum cupper adsorption was obtained with E. coli (pETCP3). At a copper concentration of 0.7 mM, 142.4 and 183.5 µmol/g DCW of copper were adsorbed by E. coli (pETCP1) and E. coli (pETCP2), respectively (Fig. 2a).
Evaluation of copper adsorption by three recombinant strains in a. LB (Luria–Bertani medium), b artificial polluted wastewater. White color denotes BL21 (DE3), Light gray denotes pETCP1, gray denotes pETCP2, and dark gray denotes pETCP3, c copper adsorption analysis of all monomeric strains in dye polluted wastewater
To evaluate the selectivity of the three copper binding peptides displayed strains, the three strains were incubated in artificial wastewater containing various metal ions including chromium, cobalt, copper, and lead. All of the three tested strains showed an efficient selective adsorption of copper ions from artificial wastewater, and a significantly higher amount of copper was adsorbed than other metal ions (Fig. 2b). In general, the most adsorbed metal ion was copper, followed by lead, chromium, and cobalt. The maximum copper adsorption of 278.0 µmol/g DCW was obtained by E. coli (pETCP3). E. coli (pETCP1) and E. coli (pETCP2) adsorbed 149.1 and 183.5 µmol/g DCW of copper, respectively (Fig. 2b). Based on the high copper specificity of the peptides, it is suggested that peptide displayed recombinant E. coli could be used for selective copper adsorption in wastewater.
The performance of recombinant strains in the real industrial process was evaluated using copper phthalocyanine dye polluted textile wastewater, which was collected near a copper phthalocyanine dye-manufacturing factory in Mumbai, India (Fig. 2c). The maximum copper adsorption of 234.5 µmol/g DCW was obtained by E. coli (pETCP3), followed by E. coli (pETCP1) (132.3 µmol/g DCW) and E. coli (pETCP2) (125.3 µmol/g DCW). These results demonstrated that peptide displayed recombinant E. coli can be applied to the treatment of real industrial wastewater. Based on the results of the copper adsorption studies carried out in the three conditions, it can be deduced that E. coli (pETCP3) is the best strain in terms of copper adsorption ability.
Peptide structure analysis
Sequence analysis against the protein databank using BlastP was carried out. Among the three short peptides, CBP3 (SPHHGGW) shows 83 % identity (query coverage-85 %) with the prion protein octa repeat domain (PHGGGWGQ) that favors copper binding. It was previously reported that the prion proteins with an octa repeat of PHGGGWGQ can bind to copper because the main chain nitrogen atoms of the glycine repeat next to histidine favor copper binding along with it [22, 23] (SI Fig. 2). Similarly, PHHGG residue in CBP3 takes a similar orientation with prion protein, which favors high efficiency binding of copper.
To get deep structural insight of the short peptide sequences that are responsible for the copper binding activity, comparative protein modeling was carried out [24]. Compared with CBP1 and CBP2, the probable copper binding site of CBP3 is better oriented (Fig. 3). CBP1 (Fig. 3a) and CBP 2 (Fig. 3b) show a different orientation that might be due to steric hindrance of the adjacent amino acid. In the case of CBP3, the HHGG residue favors copper binding due to being helped by the side chain nitrogen atom of histidine and the backbone nitrogen of glycine (Fig. 3c). Based on this information, it can be deduced that CBP3 has an appropriate structure for copper binding, consistent with the copper adsorption study.
Copper adsorption by dimeric peptide display
To enhance the copper adsorption ability of recombinant strains, a novel strategy of dimeric peptide display was employed. By attaching two copper binding peptides to the E. coli OmpC anchoring motif, more peptides can theoretically be displayed on the surface of each strain. To realize this idea, a dimer of the CBP3 peptide, which showed the best copper adsorption in the above studies, was attached to the C-terminus of OmpC.
To evaluate the effect of peptide dimer display, the strain was incubated in LB medium at various copper concentrations. In general, when the copper concentration was increased from 0.3 to 0.7 mM, the amount of adsorbed copper also increased (Fig. 4a). When 0.3 and 0.5 mM of copper were supplemented to medium, slightly more copper was adsorbed by the dimer displayed strains than with monomer displayed strains. The maximum copper adsorption of 517.6 µmol/g DCW was achieved with a copper concentration of 0.7 mM. With the monomer displayed E. coli (pETCP3), 245.7 µmol/g DCW of copper was adsorbed (Fig. 4a). At a higher concentration of copper (1 mM), a slight decrease in copper adsorption was observed. Based on these results, it can be deduced that displayed monomeric copper binding peptide was saturated at 0.5 mM copper concentration, while the dimeric peptide had greater room for more copper ion binding. Therefore, the copper adsorption capacity was enhanced by the display of dimeric peptides.
Comparative copper adsorption analysis of pETCP3 and pETCP3x2 in a. LB (Luria–Bertani medium), b artificial polluted wastewater. Dark gray denotes BL21 (DE3), light gray denotes pETCP3, and white color denotes pETCP3x2. c pETCP3 and pETCP3x2 comparative copper adsorption analysis in dye polluted wastewater
The effect of dimeric peptide display on heavy metal selectivity was evaluated by culturing recombinant strains in artificial polluted water. The significant increase of the relative adsorption of copper compared with other metals in artificial wastewater, it can be suggested that the selectivity of the recombinant strain toward copper was enhanced by the display of dimeric peptide (Fig. 4b).
To evaluate the feasibility of the dimeric peptide displayed strain in actual industrial conditions, E. coli (pETCP3x2) was cultured in copper phthalocyanine dye polluted textile wastewater. The dimeric peptide displayed strain adsorbed 543.3 µmol/g DCW of copper, which is more than twofold higher than that obtained with the monomeric peptide displayed strain (Fig. 4c). In the artificial polluted water, the pETCP3 (monomeric strain) removal yield was found to be 39.71 %, whereas the same peptide after the dimeric (pETCP3x2) construction was found to have a removal efficiency of 79.95 %. So the construction of dimeric strain increased the copper removal efficacy by 40.24 %. This result clearly indicates that the newly constructed dimeric peptide displayed recombinant strain from this study can be employed in enhanced selective adsorption of copper from real environmental waste. This system shows higher adsorption of copper, when compared to the other cell surface display systems [8, 12].
Conclusions
Considering the significant levels of environmental heavy metal pollution, the development of economically feasible remediation systems is required. Our newly constructed dimeric copper binding peptide displayed recombinant strain may pave the way for the development of efficient copper biosorption systems. This recombinant strain was also tested in copper phthalocyanine polluted industrial water samples, making a large stride towards moving the development of surface display metal binding peptides from laboratory research to industrial application.
References
Pazirandeh M, Wells BM, Ryan RL (1998) Development of bacterium-based heavy metal biosorbents: enhanced uptake of cadmium and mercury by Escherichia coli expressing a metal binding motif. Appl Environ Microbiol 64:4068–4072
Guerra FP, Reyes L, Vergara-Jaque A, Campos-Hernández C, Gutiérrez A, Pérez-Díaz J, Pérez-Díaz R, Blaudez D, Ruíz-Lara S (2015) Populus deltoides Kunitz trypsin inhibitor 3 confers metal tolerance and binds copper, revealing a new defensive role against heavy metal stress. Environ Exp Bot 115:28–37
Fu L-Y, Wen X-H, Xu L-J, Qian Y (2002) Removal of a copper-phthalocyanine dye from wastewater by acclimated sludge under anaerobic or aerobic conditions. Process Biochem 37:1151–1156
Silva MC, Corrêa AD, Amorim MTSP, Parpot P, Torres JA, Chagas PMB (2012) Decolorization of the phthalocyanine dye reactive blue 21 by turnip peroxidase and assessment of its oxidation products. J Mol Catal B Enzym 77:9–14
Tunali S, Çabuk A, Akar T (2006) Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J 115:203–211
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141
Igberase E, Osifo P, Ofomaja A (2014) The adsorption of copper (ii) ions by polyaniline graft chitosan beads from aqueous solution: equilibrium, kinetic and desorption studies. J Environ Chem Eng 2:362–369
Ni H, Xiong Z, Ye T, Zhang Z, Ma X, Li L (2012) Biosorption of copper(II) from aqueous solutions using volcanic rock matrix-immobilized Pseudomonas putida cells with surface-displayed cyanobacterial metallothioneins. Chem Eng J 206:264–271
Saleem M, Brim H, Hussain S, Arshad M, Leigh MB, Zia UH (2008) Perspectives on microbial cell surface display in bioremediation. Biotechnol Adv 26:151–161
Bae W, Wu CH, Kostal J, Mulchandani A, Chen W (2003) Enhanced mercury biosorption by bacterial cells with surface-displayed MerR. Appl Environ Microbiol 69:3176–3180
Lee SY, Choi JH, Xu Z (2003) Microbial cell-surface display. Trends Biotechnol 21:45–52
Ravikumar S, I-k Yoo, Lee S, Hong S (2011) Construction of copper removing bacteria through the integration of two-component system and cell surface display. Appl Biochem Biotechnol 165:1674–1681
Xu Z, Lee SY (1999) Display of polyhistidine peptides on the Escherichia coli cell surface by using outer membrane protein C as an anchoring motif. Appl Environ Microbiol 65:5142–5147
Cruz N, Le Borgne S, Hernández-Chávez G, Gosset G, Valle F, Bolivar F (2000) Engineering the Escherichia coli outer membrane protein OmpC for metal bioadsorption. Biotechnol Lett 22:623–629
Link AJ, Tirrell DA (2003) Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J Am Chem Soc 125:11164–11165
Rodríguez Couto S (2009) Dye removal by immobilised fungi. Biotechnol Adv 27:227–235
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Shishir KB, Murthy DVS (2008) Effect of hydraulic retention time and initial nitrate concentration on the performance of an upflow anoxic bioreactor: a factorial design study. Indian Chem Eng 50:27–33
Choi JH, Choi Jong-IL, Lee SY (2005) Display of proteins on the surface of Esherichia coli by C-terminal deletion fusion to the Salmonella typhimurium OmpC. J Microbiol Biotechnol 15(1):141–146
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Zahn R (2003) The octapeptide repeats in mammalian prion protein constitute a pH-dependent folding and aggregation site. J Mol Biol 334:477–488
Hodak M, Chisnell R, Lu W, Bernholc J (2009) Functional implications of multistage copper binding to the prion protein. Proc Natl Acad Sci USA 106:11576–11581
Millhauser GL (2004) Copper binding in the prion protein. Acc Chem Res 37:79–85
Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A (2001) Comparative protein structure modeling using MODELLER. In: Curr. Protoc. Protein Sci. Wiley
Acknowledgments
This work was supported by the 2014 Research Fund of the University of Ulsan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maruthamuthu, M.k., Nadarajan, S.P., Ganesh, I. et al. Construction of a high efficiency copper adsorption bacterial system via peptide display and its application on copper dye polluted wastewater. Bioprocess Biosyst Eng 38, 2077–2084 (2015). https://doi.org/10.1007/s00449-015-1447-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1447-y