Abstract
Human cell lines have attracted great interest because they are capable of producing glycosylated proteins that are more similar to native human proteins, thereby reducing the potential for immune responses. However, these cells have not been extensively characterized and cultured under serum-free suspension conditions. In this work, we describe the adaptation, growth, and cryopreservation of the human cell lines SK-Hep-1, HepG2, and HKB-11 under serum-free suspension conditions. The results showed that both HKB-11 and SK-Hep-1 adapted to serum-free suspension cultures in FreeStyle and SFM II, respectively. Kinetic characterization showed that the HKB-11 and SK-Hep-1 cells reached cell densities as high as 8.6 × 106 and 1.9 × 106 cells/mL, respectively. The maximum specific growth rates (μ max) were similar for both cells (0.0159/h for HKB-11 and 0.0186/h for SK-Hep-1). The growth limitation of adapted cells does not appear to be associated with glucose or glutamine depletion, nor with the formation of lactate in inhibitory concentrations. However, in both cases, ammonia production reached concentrations that are considered inhibitory to mammalian cells (2–5 mM). The adapted cells were also successfully cryopreserved under serum-free formulations. The SK-HEP-1 and HKB-11 cells that were adapted to serum-free suspension conditions might be suitable for use in the manufacturing of recombinant proteins, thereby eliminating the potential for the introduction of adventitious process contamination and greatly simplifying downstream protein purification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biopharmaceuticals are a class of therapeutic products that are produced by biotechnology and are usually used to compensate for the deficiency or absence of an enzyme that is important for the functioning of the body to combat diseases such as cancer and rheumatic and neglected diseases [1–4]. In 2013, more than 425 biotechnology products were approved in the United States and the European Union, of which 140 were recombinant proteins; 40 of these are considered blockbuster drugs, i.e., providing revenues of greater than $1 billion/year, and another 18 have sales between $500 million and $1 billion [4, 5]. Despite the growing commercial importance of these products for the treatment of disease, there are two major concerns related to the production process that may affect safety, efficiency, and product half-life: glycosylation pattern and the presence of adventitious agents [6, 7]. Studies indicate that 43 % of all approved biopharmaceuticals are commercially produced in mammalian cells, more specifically, in murine cells, such as Chinese hamster ovary cells (CHO) [3]. Although murine cell lines produce proteins with similar human post-translational modifications and correct folding, non-human cell lines contain two highly immunogenic epitopes, 5-glycolylneuraminic acid and Gal1-3Galβ1-(3)4GlcNAc termination (the α-galactose epitope). The formation of immunogenic sialic acid occurs because human cells have lost the ability to express the enzyme CMP-N-acetylneuraminic acid hydroxylase (Cmah), which is responsible for the conversion of N-acetylneuraminic acid in N-glycolylneuraminic acid. Therefore, these immunogenic epitopes can affect therapeutic effectiveness because all humans have antibodies against these terminations [7].
Human cell lines have emerged as a new and powerful alternative for biopharmaceutical production because they are capable of producing glycosylated proteins that are more similar to native human proteins, reducing the potential for immune responses against non-human epitopes. The PER.C6 cell line (Crucell Holland BV and DSM Biologics) is one of the most promising alternatives to CHO cell-based recombinant protein production and most likely will be the most used platform for the production of vaccines, enzymes, and monoclonal antibodies [8–14]. PER.C6 is an extensively documented human embryonic retina cell line that was immortalized with the adenovirus E1 gene and is easily cultivated in serum-free suspension cultures [9, 15–17]. Productivities of greater than 30 g/L have been reported, and many companies have already licensed PER.C6 cells for large-scale production and for the domestic production of therapeutic candidates [17, 18]. The HT-1080 cell line is a human fibrosarcoma-derived cell line that has been used by Shire company to produce four commercial therapeutic proteins: Dynepo (epoetin delta), Elaprase (iduronate-2-sulfatase), Replagal (α-galactosidase A), and VPRIV (glucocerebrosidase) [19]. The manufacturer uses gene activation technology, introducing a promoter upstream of the gene of interest [20]. Another promising human cell line, HKB-11, was developed by fusing 293S cells and 2B8 cells (derived from Burkitt’s lymphoma) using polyethylene glycol [21]. This hybrid cell has the characteristics of both HEK-293 cells (easy transfection and high levels of protein expression) and B cells (efficient secretion) and was developed to solve aggregation problems that were experienced when using 293S in serum-free suspension cultures [21, 22]. Some glycoproteins, such as recombinant coagulation factor VIII (FVIII), are difficult to express due to their size and complexity, resulting in a two to threefold lower expression than other recombinant proteins produced in mammalian cells. FVIII expression in HKB-11 cells was eightfold higher than that in HEK-293 cells and 30-fold higher than that in BHK-21 cells [22]. Another study showed higher transfection efficiency and expression (approximately twice) of interleukin and antibodies in HKB-11 cells than in 293S cells [21]. Other proteins, such as IgG, kinase receptor, TGFβ, IL-4, ICAM-1, and FVIII-BDD, have also been expressed successfully [21, 23–25]. Despite its potential for protein expression, no commercial products related to this cell type are yet available.
SK-Hep-1 is an immortal human cell line that was derived from the fluid ascites of a patient with liver adenocarcinoma; however, despite its location of origin, this cell does not express liver-specific proteins such as albumin, α-fibrinogen, or γ-fibrinogen [26]; instead, this cell is endothelial. Herlitschka et al. [27] showed that the SK-Hep-1 cell line is an excellent producer of recombinant factor VIII that does not require gene amplification and that expresses this protein at levels greater than those observed in CHO cells. Picanço-Castro et al. [28] also expressed FVIII in hepatic and nonhepatic cell lines using lentiviral vectors with different promoters and concluded that the highest levels of expression were obtained using the CMV promoter in the SK-Hep-1 cell line.
The HepG2 cell line is an immortalized lineage that was obtained from hepatocellular carcinoma with epithelial morphology. This cell is interesting mainly due to its potential for expressing coagulation factors. The HepG2 cell line, for example, naturally produces small amounts of FVIII [27]. In 2008, researchers found that the expression of factor VIII in HepG2 cells was 10 times higher than that obtained in CHO cells [29]. Despite the potential for recombinant protein production, SK-Hep-1 and HepG2 cells have not been properly characterized and cultivated under culture conditions that are suitable for industrial-scale production, that is, in serum-free suspension cultures.
In this paper, we describe the adaptation, growth, and cryopreservation of the promising human cell lines SK-Hep-1, HepG2, and HKB-11 under serum-free suspension conditions. The goal of this study was to evaluate whether these adapted cells possess good characteristics for use as hosts for recombinant protein production. The evaluation of new technologies for biopharmaceutical production is important, and most companies are considering the clinical, quality, and productivity implications of their expression system selection. Biosimilar competitors might use alternative technologies that could undercut the prices of innovative products, thus driving the search for new and improved expression systems [18].
Materials and methods
Human cell lines, culture media, and supplements
The human cell lines SK-Hep-1 (ATCC HTB-52), HKB-11 (ATCC CRL-12568), and HepG2 (ATCC HB-8065) were purchased from the American Type Culture Collection (ATCC) and were maintained under liquid nitrogen. Cells were initially cultured as monolayer in T-flasks (75 cm2, tissue culture-treated flasks) (Greiner Bio-One, Frickenhauser, Germany) in Dulbecco’s modified Eagle’s Medium (Invitrogen, Grand Island, NY) supplemented with 10 % (v/v) characterized fetal bovine serum (Thermo Scientific, US Origin, South Logan, UT) and 1 % penicillin–streptomycin (10,000 U/mL) (Invitrogen, Grand Island, NY) and were maintained under a humidified atmosphere of 5 % CO2 in air, at 37 °C (Form Steri-Cult CO2 incubator, Thermo Scientific). Cells were passaged by trypsinization when 70–90 % confluency was reached at seeding densities of 3–5 × 105 cells/mL. Four chemically defined animal-free formulations were used for serum-free adaptation: FreeStyle 293 Expression Medium (FS, Invitrogen, Grand Island, NY), CD 293 AGT (CD 293, Invitrogen, Grand Island, NY), 293 SFM II (SFM II, Invitrogen, Grand Island, NY), and CDM4-CHO (CDM4, Thermo Scientific, South Logan, UT). All media were supplemented with 1 % penicillin–streptomycin (10.000 U/mL) (Invitrogen, Grand Island, NY). CD 293 and SFM II cells were also supplemented with 1 % GlutaMAX (100X) (Invitrogen, Grand Island, NY). At the end of the adaptation period, serum-free media were supplemented with 1 % Insulin–Transferrin–Selenium (ITS) (Invitrogen, Grand Island, NY), 1 % Pluronic-F68 (Invitrogen, Grand Island, NY), and 10 % Cell Boost 5 (Thermo Scientific, South Logan, UT).
Serum-free suspension adaptation
The FBS-supplemented cultures were adapted to serum-free conditions through sequential adaptation: the cells were passaged into mixtures of serum-containing media (SCM) and serum-free media (SFM) until complete serum-free conditions were reached (100 % SCM to the stages: 75 % SCM/25 % SFM; 50 % SCM/50 % SFM; 25 % SCM/75 % SFM; and 100 % SFM), as shown in Fig. 1. The adaptation process was initiated with cells in the exponential growth phase having a viability of greater than 90 % and at a cell density of 1.25 × 105 cells/mL (n = 1). Cells were passaged every 2–3 days and maintained for 2–3 passages in each proportion of SCM/SFM. The term “passage” in the adaptation process refers to the moment in which the cells have been replated and allowed to grow for 2–3 days.
The cultures that had adapted to SFM were cultivated for five passages and then evaluated regarding cell growth and viability. P1 was considered at the time the cells were first plated in 100 % SFM. The doubling time (DT) was estimated at each passage during this period according to the following equation:
where t is the cultivation time, N is the final cell number, and N o is the initial cell number.
Suspension adaptation was performed with the SFM that showed the best results in the previous step. The cells were initially cultivated at a cell density of 5 × 105 cells/mL in glass spinner flasks containing a magnetic shaped-blade impeller (Wheaton Scientific, Millville, NJ); the cells were stirred at 80 rpm (Cellgro Type 45600 agitator, Thermolyne) in a working volume of 20 L for 30–50 days until consistent and reproducible growth was obtained. Subsequently, the suspension adaptation was evaluated in disposable sterile 125-mL plastic Erlenmeyer flasks (Corning, Corning, NY) with a working volume of 20 mL; the cells were stirred at 80 rpm by placing the flasks on an orbital shaker platform (Agitador Mod. 109, Nova Ética), and the cellular growth profile was compared to that obtained in the spinner flasks.
After suspension adaptation, the maximum specific growth rate (μ max) was estimated from the slope of the best-fitting line of the logarithm of viable cell density versus time during exponential growth phase using Origin® software (OriginLab Corporation) (n = 3).
Cryopreservation of adapted cells under serum-free conditions
Cultures containing FBS were cryopreserved in standard freezing medium containing 10 % DMSO (Sigma-Aldrich, St. Louis, MO) and 90 % FBS at a cell density of 3–5 × 106 cells/mL. For the cryopreservation of the SFM-adapted cells, the following five serum-free freezing formulations were tested and compared with the standard freezing formulation: Synth-a-Freeze (Invitrogen, Grand Island, NY); HyCryo (Thermo Scientific, South Logan, UT); ProFreeze-CDM (Lonza Walkersville, Walkersville, MD); Cell Freezing Medium-DMSO Serum-free (Sigma-Aldrich, St. Louis, MO), and a freezing formulation containing 45 % fresh medium, 45 % conditioned medium, and 10 % DMSO. The adapted cells were trypsinized in exponential growth phase and resuspended in the freezing formulations at a final cell density of 1.5 × 106 cells/mL; the cells were maintained at 4 °C for 10–15 min and then placed in a cryodevice (Mr. Frosty™ Freezing Container, Thermo Scientific, South Logan, UT) which was maintained at −80 °C for 24 h for subsequent storage in liquid nitrogen for 15, 30, and 60 days. After this period, the cells were rapidly thawed in a water bath at 37 °C and then resuspended and cultivated in 10 mL of fresh SFM medium in T75 flasks. Survival rate (the ratio of viability before freezing to viability after thawing) and cell viability during the fifth passage after thawing were analyzed (n = 2) to choose the appropriate formulation.
Analytical methods
Cell growth and viability were determined by counting the total number of cells in the presence of 0.4 % Trypan Blue solution (Invitrogen, Grand Island, NY) in a Neubauer chamber (Boeco, Germany) under phase contrast microscopy. Glucose, lactate, and glutamine concentrations were determined using a 2700 YSI Biochemical Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Ammonia concentration was determined using an ion-selective electrode (Orion 720A+, Thermo Scientific, South Logan, UT) according to the manufacturer’s instructions.
Results and discussion
Adaptation to serum-free media in static flasks
Serum-free adaptation was realized in stationary T-flasks through sequential adaptation. Gradual serum reduction increases the chances of satisfactory cell adaptation to serum-free culture [30]. The doubling time (DT) of the HKB-11, SK-Hep-1, and HepG2 cell lines increased with passage number during serum-free adaptation due to the progressive withdrawal of FBS (data not shown). The DTs of the adapting cells were higher than the corresponding values of each control cell (cultivated in DMEM supplemented with 10 % FBS). HKB-11 cells were not able to adapt to serum-free CD293 and SFM II media, and SK-Hep-1 cells were not able to adapt to FreeStyle and CD293 serum-free media. HepG2 cells grew only in the CDM4 serum-free medium, although the DT increased considerably compared to the control cells. Moreover, even in DMEM 10 % FBS, the HepG2 cell line exhibited a high DT (90.1 h, SD 15.4, n = 5), and large agglomerates were formed, preventing individual cell growth; this made subculturing of the cells more difficult. These characteristics are not suitable for industrial application. Consequently, the adaptation process was continued only for the HKB-11 and SK-Hep-1 cell lines. Although many protocols for cellular adaptation to FBS-free conditions have been described in the literature [31–33], not all the cell lines can be adapted using conventional methods, which are generally based on the progressive withdrawal of serum [34].
Cells that survived until the end of sequential adaptation were then cultured for five passages in the appropriate serum-free media. In an attempt to improve cell growth, the serum-free media were supplemented with 10 % Cell Boost 5 (Hyclone), 1 % Pluronic® F68 (Invitrogen), and 1 % ITS (Invitrogen). Cell Boost 5 is a chemically defined animal-free supplement, which provides lipids, amino acids, vitamins, trace elements, cholesterol, and growth factors. ITS promotes glucose and amino acid uptake, lipogenesis, and iron transport; decreases the content of reactive oxygen radicals; and provides cofactors for glutathione peroxidase and other proteins, thus acting as an antioxidant. Pluronic® F-68 is a nonionic surfactant that protects cells from shear forces in suspension cultures, thus alleviating stress and cell death. These supplements were added to supply nutrients that were previously provided by FBS [35–37]. Most mammalian cell lines need additional supplements to satisfactorily survive and proliferate in basal medium, mainly in serum-free formulations [33, 36–38]. This need is exemplified by the significant increase in doubling time of the HKB-11 and SK-Hep-1 cell lines when cultivated in serum-free medium compared to FBS-supplemented medium.
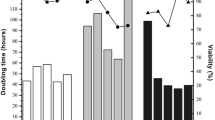
The doubling time for HKB-11 cells increased from 41.0 h (SD 15.9, n = 5) in serum-containing media (SCM) to approximately 57.6 h and 48.1 h at passage 5 in Freestyle and CDM4 media, respectively. SK-Hep-1 cells presented an average DT of 46.7 h (SD 10.3, n = 5) in SCM and of 94.2 and 289.8 h in CDM4-CHO and 293 SFM II media, respectively (Fig. 2). For both cell lines, CDM4-CHO culture was the most promising, presenting average DTs of 56 and 94.2 h for the HKB-11 and SK-Hep-1 cells, respectively. It is worth mentioning that DT values greater than 100 h cannot be considered as truly proliferation. It indicates that probably a portion of cells remained viable and proliferates and a portion of them died, as can be also noticed by the viability values.
Hernández and Fischer [39] performed serum-free adaptation of several mammalian cell lines (myeloma BALB/c, NS0, hybridoma, Vero, COS-1, COS-7, BHK, and HEK-293) through gradual serum withdrawal, the same procedure as that used in this work. In general, it was possible to adapt all cell lines, although COS-7 cells exhibited an increase of 38 % in DT after 11 passages, and VERO cells exhibited a 2.5-fold increase in DT.
To the best of our knowledge, no previous studies have reported the adaptation of SK-Hep-1 and HKB-11 cells to serum-free suspension conditions. Although some studies reported the production of recombinant proteins using adapted HKB-11 cells [21, 22, 24, 25], none described how the adaptation was achieved and no results were presented regarding this issue.
The morphology of HKB-11 and SK-Hep-1 cells is shown in Fig. 3; after adaptation, both cell lines presented a rounded shape in suspension culture and a tendency to form aggregates of varying sizes. As expected, cells that were cultivated in DMEM 10 % FBS adhered to the flask and presented a more elongated shape. SK-Hep-1 cells are predominantly larger and more elongated than HKB-11 cells.
Adaptation to suspension culture
The HKB-11 and SK-Hep-1 cell lines that were adapted to grow in serum-free media were cultivated initially in spinner flasks to evaluate the potential for suspension adaptation. In this condition, only cultures that were grown in CDM4 showed satisfactory and consistent cell growth (data not shown). Based on this result, the cells were also cultured in Erlenmeyer flasks with orbital shaking, and the results were compared. As shown in Fig. 4, HKB-11 cells cultivated in Erlenmeyer flasks exhibited superior cell growth, with maximum cell densities (X max) of 3.42 × 106 and 8.01 × 106 cells/mL, and μ max values of 0.0185 and 0.0278/h when cultured in FreeStyle and CDM4 media, respectively. HKB-11 cells cultured in spinner flasks did not grow in either medium. SK-Hep-1 cells cultivated in CDM4 exhibited an X max of 1.37 × 106 cells/mL and a μ max of 0.0101/h in Erlenmeyer flasks, but did not show satisfactory growth in spinner flasks. In SFM II, SK-Hep-1 cells grew in both spinner and Erlenmeyer flasks, reaching 2.24 × 106 cells/mL (μ max 0.0165/h) and 2.13 × 106 cells/mL (μ max 0.0225/h), respectively.
Cells cultured in suspension under orbital shaking showed good results for the serum-free media tested. In comparison, poorer results were found using spinner flasks equipped with a blade impeller (80 rpm) due to inefficient oxygen transfer and high shear stress. Muller and colleagues [40] also demonstrated that HEK-293 EBNA and CHO-DG44 cell lines that are adapted to serum-free suspension cultures grow better under orbital shaking than in spinner flask cultures.
After suspension adaptation, the cells cultured in Erlenmeyer flasks exhibited doubling times that were similar to or smaller than those observed in serum-containing medium in most serum-free media. DT values were estimated for HKB-11 cells in FreeStyle and CDM4 media as approximately 37.5 and 24.9 h, respectively (34.8 h in SCM). SK-Hep-1 cells cultured in CDM4 and SFM II media presented DTs of approximately 69.3 and 30.8 h, respectively (44.2 h in SCM).
Kinetic and metabolic characterization of adapted cell lines
Kinetic and metabolic characterization was performed only in HKB-11 cells cultured in FreeStyle 293 medium and in SK-Hep-1 cells in SFM II medium because both cells, when cultured in CDM4 medium, did not survive the freezing/thawing procedure. The HKB-11 growth profile presented the absence of a lag phase and an extended growth phase (18 days or 432 h), and cell densities reached as high as 8.6 × 106 cells/mL (SD 0.6 × 106) (432 h), and a µ max value of 0.0159/h (SD 0.0014) was obtained (Fig. 5). After 432 h, cell density decreased, accompanied by a decrease in cell viability. The high cell density reached here is well above the values commonly obtained for other human cell lines, such as HEK 293 and PER.C6 cell lines under serum-free conditions: an X max of 2–3 × 106 cells/mL [10, 41–43]. Loignon et al. [43] cultivated HEK293-6E cells for the production of IFNα2b during suspension in serum-free F17 medium (Invitrogen) supplemented with 0.5 % peptone. During 9 days in fed batch culture, the X max obtained was approximately 3 × 106 cells/mL and the μ max was 0.0148/h.
The metabolism of HKB-11-adapted cells was also characterized because the availability of nutrients and the formation of toxic metabolites have been shown to interfere with cell growth regulation and protein production [44, 45]. The initial concentration of glucose (7.6 g/L, 42.18 mM) progressively decreased with time due to cell growth, until exhaustion was reached at 290 h (day 12). Nevertheless, this exhaustion does not appear to be associated with the limitation of cell growth. Due to glucose consumption, lactate was produced at the beginning of the culture period, reaching a plateau value of approximately 1.3 g/L (14.6 mM) at 96 h. This lactate, which is formed by glucose degradation and the conversion of pyruvate by lactate dehydrogenase, can reduce the pH and increase the osmolarity of the medium. In general, lactate concentrations of less than 20 mM (1.78 g/L) do not affect the cells, whereas concentrations in the range 20–40 mM (1.78–3.56 g/L) interfere with productivity; at concentrations above this value, lactate inhibits cell growth [44]. Therefore, lactate did not form at concentrations that are considered inhibitory. After glucose depletion, lactate was consumed until exhaustion at 336 h (day 14). When the glucose was exhausted, lactate was converted to pyruvate to provide a source of energy for the cell. Pyruvate can enter the tricarboxylic acid cycle, releasing energy.
The glutamine provided in the culture media was used in reactions of the tricarboxylic acid cycle; glutamine was converted to its deaminated form glutamate by the loss of ammonia, and glutamate can take part in the tricarboxylic acid cycle, gluconeogenesis, and protein synthesis, among other pathways. Glutamine probably was not consumed in relevant quantities; instead, glutamine concentrations increased initially due to the use of GlutaMAX™ (Invitrogen). GlutaMAX™ contains an l-alanine-l-glutamine dipeptide that promotes greater stability in aqueous medium; this complex does not degrade spontaneously, unlike l-glutamine, which does. GlutaMAX™ is slowly degraded by aminopeptidases released by the cell to form l-alanine and l-glutamine in the culture medium. The result resembles a fed batch operation because glutamine is continuously released into the culture medium but is maintained at low concentrations [46]. Depletion of glutamine was observed only in the HKB-11 culture at the end of the batch and did not interfere in cell growth. Ammonia was produced, due to amino acid consumption (mainly glutamine) throughout the entire culture period, reaching a maximum value of 71.20 mg/L (4.18 mM) at the end of the culture period. Ammonia production increases the pH of the medium and can affect protein glycosylation by interfering with glycan chain branching. The inhibitory concentration of ammonia in mammalian cells is approximately 2–5 mM (34.06–85.15 mg/L) [44, 47]. Inhibitory ammonia concentrations were observed after 200 h in HKB-11 cultures (34.06 mg/L), although cell density decreased only after 432 h, by which time the ammonia concentration had risen to 60.79 mg/L.
As shown in Fig. 6, SK-Hep-1 cells grew exponentially from the beginning of the culture, and this exponential growth phase lasted for 3 days; the cells entered the stationary phase at 96 h (day 4). During 13 days of cultivation, the cells presented an X max of 1.93 × 106 cells/mL (SD 0.1 × 106) at 192 h and a μ max of 0.0186/h (SD 0.0027). After 192 h, the cell density decreased slightly until the end of culture period, without any decrease in cell viability. The cellular growth exhibited by SK-Hep-1 cells can be compared to the murine cell line most commonly used in industrial processes, CHO-K1. Usually, this cell line, which is adapted for serum-free suspension growth, can achieve cell densities of 1–2 × 106 cells/mL in spinner flasks, with a doubling time of approximately 24–30 h [31, 48]. Glucose and glutamine were consumed without reaching complete exhaustion. As expected, lactate was formed as the consequence of glucose metabolism and reached a maximum concentration of 1.4 g/L (15.7 mM) in 96 h. The ammonia concentration in the supernatant increased until 192 h (day 8), at which time it reached a maximum value of 35.59 mg/L (2.09 mM), coinciding with the beginning of the decline in cellular growth. Furthermore, cell growth might have been inhibited when the ammonia concentration reached approximately 30 mg/L (1.8 mM) because the cells entered stationary phase at 96 h. After 192 h, the ammonia concentration started to decrease, reaching 12.21 mg/L (0.72 mM) at 312 h, at which time the cultivation was ended.
The morphology of HKB-11 and SK-Hep-1 cells adapted to suspension culture is shown in Fig. 7. HKB-11 cells reached high cellular concentrations and formed aggregates of various sizes. SK-Hep-1 cell culture reached lower cell densities; however, unlike the HKB-11 cells, the SK-Hep-1 cells grow individually, rarely forming cellular aggregates. Both cell lines presented a rounded shape after suspension adaptation.
For economic reasons and in order to reduce medium complexity, we also evaluated the cell growth performance in the absence of Cell Boost 5 and ITS supplementation. Figure 8a shows that HKB-11 cell growth in FreeStyle medium supplemented with Cell Boost 5 and ITS was four times higher than that in unsupplemented medium. HKB-11 cells cultivated in FreeStyle without supplementation exhibited an X max of 2.12 × 106 (SD 0.2 × 106) cells/mL and a μ max of 0.0117/h (SD 0.0032); in the supplemented medium, the cells exhibited an X max of 8.6 × 106 cells/mL (SD 0.6 × 106) and a μ max of 0.0159/h (SD 0.0014). Moreover, in non-supplemented FreeStyle medium, cell growth decreased after 142 h (6 days) and exhibited decreased viability after 24 h; however, the supplemented culture continued to grow up to 432 h (18 days), with a viability of approximately 80 % until 384 h. These results demonstrate the importance of these supplements to HKB-11 cell line maintenance.
Figure 8b shows that SK-Hep-1 cells cultured in SFM II without supplementation achieved an X max of 2.45 × 106 cells/mL (SD 0.5 × 106) and a μ max of 0.0144/h (SD 0.0014) in 121 h (5 days); a plateau was maintained until 193,5 h (8 days), after which time cellular growth declined. Throughout the batch culture, viability remained greater than 90 %. When SK-Hep-1 cells were cultured in SFM II supplemented with Cell Boost 5 and ITS, the X max obtained was 1.93 × 106 cells/mL (SD 0.1 × 106) in 192 h (13 days), and a μ max of 0.0186/h (SD 0.0027) was observed; viability was greater than 90 % throughout the batch. After the maximum cell density was reached at 192 h, we observed a decline in cellular growth. Thus, the SK-Hep-1 cell line can be cultivated in SFM II without supplementation, thereby reducing the cost of culture materials and simplifying purification (due the low protein content). In 2004, Wong and colleagues [49] attempted to formulate an animal- and protein-free culture and were able to replace insulin by trace elements that mimic insulin, such as cadmium, nickel, lithium, vanadium, and zinc (the latter was the best solution for hybridomas producing monoclonal antibodies and for the CHO-K1 cell line). von Fircks et al. [50] also succeeded in obtaining insulin-free and serum-free CHO cell systems producing rFVIIa, thereby allowing protein-free cultivation.
Cryopreservation of adapted cells under serum-free conditions
After kinetic and metabolic characterization, the adapted cell lines were cryopreserved using several serum-free formulations. Figure 9 shows the relative survival rates at the time of thawing and the cell viabilities measured during the 5th passage of cultivation after thawing. Synth-a-Freeze and HyCryo presented the best results for HKB-11 cells; the results for survival rate and viability during the fifth passage (5th) (mean value) for Synth-a-Freeze were 83.7 and 82.7 %, respectively, and the corresponding values for HyCryo were 84.4 and 82.9 %, respectively. The freezing formulation containing 45 % MF/MC 45 %/10 % DMSO also showed good results; the survival rate was 81.2 %, and the viability during the fifth passage was 80.2 %.
As also shown in Fig. 9, the survival rates of the 1st batch of SK-Hep-1 cells after thawing were lower than 50 %. In the 2nd batch, HyCryo medium presented SK-Hep-1 survival rates that were greater than 80 % at 15, 30, and 60 days. In general, HyCryo and Cell Freezing Medium-DMSO Serum-Free were the most promising formulations for the cryopreservation of SK-Hep-1 cells cultivated in SFM II medium; the survival rates after thawing and the viability during the 5th passage (mean value) for HyCryo were 63.2 and 59.6 %, and the corresponding values for Cell Freezing Medium-DMSO Serum-Free were 76.7 and 82.8 %, respectively.
Conclusions
It was possible to adapt two of the three human cell lines tested, SK-Hep-1 and HKB-11, to serum-free suspension conditions in Erlenmeyer flasks. The best results for the HKB-11 and SK-Hep-1 cells were obtained in FreeStyle 293 Expression medium and in 293 SFM II medium supplemented with Cell Boost 5 and ITS, respectively. It was possible to cultivate SK-Hep-1 cells in non-supplemented culture, which would simplify purification (due the low protein content) and decrease costs. The adapted cell lines were characterized, and a master cell bank was produced under serum-free conditions for both cell lines. In general, the bioprocess conditions achieved were suitable for manufacturing recombinant proteins because the suspension cultures could be grown at high cell densities and the potential of adventitious contaminations was significantly reduced due to the absence of FBS, greatly simplifying downstream protein purification.
References
Walsh G (2002) Biopharmaceuticals and biotechnology medicines: an issue of nomenclature. Eur J Pharm Sci 15:135–138
Durocher Y, Butler M (2009) Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol 20:700–707
Berlec A, Strukelj B (2013) Current state and recent advances in biopharmaceutical production in Escherichia coli, yeasts and mammalian cells. J Ind Microbiol Biotechnol 40:257–274
Rader RA (2013) FDA biopharmaceutical product approvals and trends in 2012. BioProcess Intl 11:18–27
Rader RA (2013) An analysis of the US biosimilars development pipeline and likely market evolution. Bioprocess Intl 11:16–23
Grillberger L, Kreil TR, Nasr S, Reiter M (2009) Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J 4:186–201
Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A (2012) Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev 28:147–176
Pau MG, Ophorst C, Koldijk MH, Schouten G, Mehtali M, Uytdehaag F (2001) The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine 19:2716–2721
Jones D, Kroos N, Anema R, van Montfort B, Vooys A, van der Kraats S, van der Helm E, Smits S, Schouten J, Brouwer K, Lagerwerf F, van Berkel P, Opstelten D, Logtenberg T, Boutet A (2003) High-level expression of recombinant IgG in the human cell line PER.C6. Biotechnol Prog 19:163–168
Berdichevsky M, Gentile M, Hughes B, Meis P, Peltier J, Blumentals I, Auninus J, Altaras NE (2008) Establishment of higher passage PER.C6 cells for adenovirus manufacture. Biotechnol Prog 24:158–165
Tchoudakova A, Hensel F, Murillo A, Eng B, Foley M, Smith L, Schoenen F, Hildebrand A, Kelter A-R, Ilag LL, Vollmers HP, Brandlein S, McIninch J, Chon J, Lee G, Cacciuttolo M (2009) High level expression of functional human IgMs in human PER.C6® cells. MAbs 1:163–171
Kruif J, Kramer A, Nijhuis R, Zande V, Blanken R, Clements C, Visser T, Keehnen R, Hartog M, Throsby M, Logtenberg T (2010) Generation of stable cell clones expressing mixtures of human antibodies. Biotechnol Bioeng 106:741–750
Ross D, Brown T, Harper R, Pamarthi M, Nixon J, Bromirski J, Li CM, Ghali R, Xie H, Medvedeff G, Li H, Scuderi P, Arora V, Hunt J, Barnett T (2012) Production and characterization of a novel human recombinant alpha-1-antitrypsin in PER.C6 cells. J Biotechnol 162:262–273
Sanders BP, Edo-Matas D, Custers JHHV, Koldijk MH, Klaren V, Turk M, Luitjens A, Bakker WAM, Uytdehaag F, Goudsmit J, Lewis JA, Schuitemaker H (2013) PER.C6® cells as a serum-free suspension cell platform for the production of high titer poliovirus: a potential low cost of goods option for world supply of inactivated poliovirus vaccine. Vaccine 31:850–856
Fallaux FJ, Bout A, Van der Velde I, Van den Wollenberg DJM, Hehir KM, Keegan J, Auger C, Cramer SJ, Van Ormondt H, Van Der Eb AJ, Valerio D, Hoeben RC (1998) New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 9:1909–1917
Yallop C, Crowley J, Cote J, Hegmans-Brouwer K, Lagerwerf F, Gagne R, Martin JC, Oosterhuis N, Opstelten DJ, Bout A (2008) PER.C6® cells for the manufacture of biopharmaceutical proteins. In: Knablein J (ed) Modern biopharmaceuticals: design, development and optimization, 1st edn. Wiley-VCH, Weinheim
Coco-Martin JM, Harmsen MM (2008) A review of therapeutic protein expression by mammalian cells. Bioprocess Intl 6:28–33
Langer E (2009) On the horizon: new expression systems to become common industry platforms. Biopharm Intl 22:2–4
Swiech K, Picanço-Castro V, Covas DT (2012) Human cells: new platform for recombinant therapeutic protein production. Protein Expr Purif 84:147–153
Moran N (2010) Shire’s replacement enzymes validate gene activation. Nat Biotechnol 28:1139–1140
Cho MS, Yee H, Chan S (2002) Establishment of a human somatic hybrid cell line for recombinant protein production. J Biomed Sci 9:631–638
Mei B, Chen Y, Chen J, Pan CQ, Murphy JE (2006) Expression of human coagulation factor VIII in a human hybrid cell line, HKB-11. Mol Biotechnol 34:165–178
Cho MS, Yee H, Brown C, Jeang K, Chan S (2001) An oriP expression vector containing the HIV-1 Tat/TAR transactivation axis produces high levels of protein expression in mammalian cells. Cytotechnology 37:23–30
Fischer S, Charara N, Gerber Am Wolfel J, Schiedner G, Voedisch B, Geisse S (2012) Transient recombinant protein expression in a human amniocyte cell line: the CAP-T® cell system. Biotechnol Bioeng 109:2250–2261
Tang L, Leong L, Sim D, Ho E, Gu J-M, Schneider D, Feldman RI, Monteclaro F, Jiang H, Murphy JE (2013) von Willebrand factor contributes to longer half-life of PEGylated factor VIII in vivo. Haemophilia 19:539–545
Turner BM, Turner VS (1980) Secretion of alpha-antitrypsin by an established human hepatoma cell line and by human/mouse hybrids. Somatic Cell Genet 6:1–14
Herlitschka SE, Schlokat U, Falkner FG, Dorne F (1998) High expression of a B-domain deleted factor VIII gene in a human hepatic cell line. J Biotechnol 61:165–173
Picanço-Castro V, Heinz S, Bott D, Behrmann M, Covas DT, Seifried E, Tonn T (2007) Recombinant expression of coagulation factor VIII in hepatic and non-hepatic cell lines stably transduced with third generation lentiviral vectors comprising the minimal factor VIII promoter. Cytotherapy 9:785–794
Campos-da-Paz M, Costa CS, Quilici LS, Simões IC, Kyaw CM, Maranhão AQ, Brigido MM (2008) Production of recombinant human factor VIII in different cell lines and the effect of human XBP1 co-expression. Mol Biotechnol 39:155–158
Kim EJ, Kim NS, Lee GM (1999) Development of a serum-free medium for dihydrofolate reductase-deficient Chinese hamster ovary cells (DG44) using a statistical design: beneficial effect of weaning of cells. In Vitro Cell Dev Biol Anim 35:178–182
Sinacore MS, Drapeau D, Adamson SR (2000) Adaptation of mammalian cells to growth in serum-free media. Mol Biotechnol 15:249–257
Schröder M, Matischak K, Friedl P (2004) Serum- and protein-free media formulations for the Chinese hamster ovary cell line DUKXB11. J Biotechnol 108:279–292
van der Valk J, Brunner D, De Smet K, Svenningsen ÅF, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G (2010) Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro 24:1053–1063
Jaluria P, Konstantopoulos K, Betenbaugh M, Shiloach J (2008) Egr1 and Gas6 facilitate the adaptation of HEK-293 cells to serum-free media by conferring enhanced viability and higher growth rates. Biotechnol Bioeng 99:1443–1452
Price PJ, Gregory EA (1982) Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro 18:576–584
Gstraunthaler G (2003) Alternatives to the use of fetal bovine serum: serum-free cell culture. Altex 20:275–281
Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G (2010) Serum-free cell culture: the serum-free media interactive online database. Altex 27:53–62
Hutchings SE, Sato GH (1978) Growth and maintenance of HeLa cells in serum-free medium supplemented with hormones. Proc Natl Acad Sci USA 75:901–904
Hernández YG, Fischer RW (2007) Serum-free culturing of mammalian cells—adaptation to and cryopreservation in fully defined media. Altex 24:110–116
Muller N, Girard P, Hacker DL, Jordan M, Wurm FM (2005) Orbital shaker technology for the cultivation of mammalian cells in suspension. Biotechnol Bioeng 89:400–406
Maranga L, Aunins JG, Zhou W (2005) Characterization of changes in PER.C6TM cellular metabolism during growth and propagation of a replication-deficient adenovirus vector. Biotechnol Bioeng 90:645–655
Sun X, Goh PE, Wong KTK, Mori T, Yap MGS (2006) Enhancement of transient gene expression by fed-batch culture of HEK 293 EBNA1 cells in suspension. Biotechnol Lett 28:843–848
Loignon M, Perret S, Kelly J, Boulais D, Cass B, Bisson L, Afkhamizarreh F, Durocher Y (2008) Stable high volumetric production of glycosylated human recombinant IFNalpha2b in HEK293 cells. BMC Biotechnol 8:1–16
Altamirano C, Gòdia F, Cairó J (2008) Metabolismo de células de mamíferos cultivadas in vitro. In: Moraes AM, Augusto EFP, Castilho LR (eds) Tecnologia de cultivo de células animais: de biofármacos a terapia gênica, 1st edn. Roca, São Paulo
Moretti P, Behr L, Walter JG, Kasper C, Stahl F, Scheper T (2010) Characterization and improvement of cell line performance via flow cytometry and cell sorting. Eng Life Sci 10:130–138
Lifetechnologies (2014) GlutaMAX™ vs. glutamine. http://www.lifetechnologies.com/br/en/home/life-science/cell-culture/mammalian-cell-culture/media-supplements/glutamax-media/glutamax-vs-glutamine.html. Accessed 2 Feb 2014
Altamirano C, Berrios J, Vergara M, Becerra S (2013) Advances in improving mammalian cells metabolism for recombinant protein production. E J Biotechnol 16:1–14
Haldankar R, Kopchick JJ, Ridgway D (1999) Stable production of a human growth hormone antagonist from CHO cells adapted to serum-free suspension culture. Biotechnol Prog 15:336–346
Wong VVT, Ho KW, Yap MGS (2004) Evaluation of insulin-mimetic trace metals as insulin replacements in mammalian cell cultures. Cytotechnology 45:107–115
von Fircks S, Elmer R, Reiter M (2010) Method of producing serum-free insulin-free factor VII. US Patent 2010/0120093 A1, 13 May 2010
Acknowledgments
The authors acknowledge financial support from FAPESP (Process numbers 2012/04629-8 and 2012/02109-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biaggio, R.T., Abreu-Neto, M., Covas, D.T. et al. Serum-free suspension culturing of human cells: adaptation, growth, and cryopreservation. Bioprocess Biosyst Eng 38, 1495–1507 (2015). https://doi.org/10.1007/s00449-015-1392-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1392-9