Abstract
The optimization of tannase production by Lactobacillus plantarum CIR1 was carried out following the Taguchi methodology. The orthogonal array employed was L18 (21 × 35) considering six important factors (pH and temperature, also phosphate, nitrogen, magnesium, and carbon sources) for tannase biosynthesis. The experimental results obtained from 18 trials were processed using the software Statistical version 7.1 using the character higher the better. Optimal culture conditions were pH, 6; temperature, 40 °C; tannic acid, 15.0 g/L; KH2PO4, 1.5 g/L; NH4Cl, 7.0 g/L; and MgSO4, 1.5 g/L which were obtained and further validated resulting in an enhance tannase yield of 2.52-fold compared with unoptimized conditions. Tannase production was further carried out in a 1-L gas-lift bioreactor where two nitrogen flows (0.5 and 1.0 vvm) were used to provide anaerobic conditions. Taguchi methodology allowed obtaining the optimal culture conditions for the production of tannase by L. plantarum CIR1. At the gas-lift bioreactor the tannase productivity yields increase 5.17 and 8.08-fold for the flow rates of 0.5 and 1.0 vvm, respectively. Lactobacillus plantarum CIR1 has the capability to produce tannase at laboratory-scale. This is the first report for bacterial tannase production using a gas-lift bioreactor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tannin acyl hydrolase (E.C. 3.1.1.20), commonly referred as tannase, catalyzes the hydrolysis of ester bonds present in gallotannins. converting them into free molecules of gallic acid (GA) and one molecule of glucose. Tannase additionally acts on substrates such as chlorogenic acid, (2)-epicatechin gallate, and (2)-epigallocatechin-(3)-gallate [1].
Tannase is an industrially important enzyme used in large volumes for wide applications. Use of tannase is centered in food, leather, and pharmaceutical industries. Nowadays, the main application of tannase is in elaboration of instantaneous tea, acorn wine, and gallic acid production. Also, tannase is used as clarifying agent in juice and coffee-flavored beverages [2, 3] and gallic acid is used in pharmaceutical industry as an intermediary compound for the synthesis of trimethoprim; in chemical industry it is used as substrate for the chemical or enzymatic synthesis of propyl-gallate and other antioxidant compounds with varying applications. Studies have demonstrated that gallic acid posses important therapeutic properties [4–6].
The main sources for production of industrial enzymes are microorganisms due to their biochemical diversity [7]. It is well known that majority tannase producer microorganisms are fungi [12] and the use of bacteria as tannase producers has received little attention. Few bacterial species such as Bacillus sp., Corinebacterium sp., Lactobacillus sp., and Serratia sp. have been reported as tannase producers [8–10].
Fermentation processes are influenced by physicochemical and environmental factors that affect the culture conditions. The main factors influencing the tannase production process are quality and quantity of carbon source [11], presence of inducers, pH, temperature, aeration, etc. [12]. Hence, the definition and/or optimization of cultural conditions on bioprocess are necessary. In that sense, classical experimental designs methods have been mainly used. Classical experimental designs involve altering one independent factor at a time keeping all others constant. These procedures are time-consuming, require more experiments, and cannot provide information about mutual interactions [13]. To solve these problems, statistical tools and related experimental design help to obtain more information about the best cultural conditions.
Taguchi method of orthogonal array design of experiments was proposed in the late 1940s [14]. The method is considered as a fractional factorial design that allows statistically and ideally obtaining similar information to a full factorial experimental design, but without so much experimental trials. The statistical analysis of variance (ANOVA) is performed to see which process factors are statistically significant. Finally, an experimental confirmation to verify the defined process factors obtained from Taguchi methodology is necessary [15]. The results obtained from small-scale experiments are valid over the scale up schedule [16]; hence, the method has shown a great success for improving the quality and processes in industry [17]. The application of Taguchi methodology to microbial enzyme production has been less exploited, particularly in tannase production.
Tannase production processes have been carried out mainly using shake flasks in both submerged fermentation and solid-state culture. Fewer laboratory studies have been carried out in laboratory-scale fermenters [5, 33, 34]. Most industrial and laboratory-scale bioreactors are still conventional stirred tanks. Recently, gas-lift bioreactors have received increased attention due to their simple construction and less shear stress imposed on shear sensitive cells compared with stirred tanks [35]. Gas-lift bioreactors cover a wide range of gas–liquid or gas–liquid–solid pneumatic contacting devices that possess the characteristic of fluid circulation in a defined cyclic pattern through channels built specifically for this purpose. Those advantages make gas-lift bioreactors suitable for applications in biotechnology. However, until now gas-lift bioreactors have not been reported for production of microbial tannases.
In the present work, Taguchi methodology was used to optimize the cultural conditions of Lactobacillus plantarum CIR1 to improve the tannase production and the optimal conditions were tested in a gas-lift bioreactor. This is the first report for tannase production using a gas-lift bioreactor under anaerobic conditions.
Materials and methods
Microorganism
The bacterium L. plantarum CIR1 was previously isolated from plum fruit juice and molecular characterized [18]. The strain was maintained at 35 °C, for 48 h, using MRS agar (Man-Rogosa-Sharpe) containing (g/L): beef extract, 5; peptone, 10; yeast extract, 5; dextrose, 20; K2HPO4, 2; sodium citrate, 2; sodium acetate, 5; MgSO .4 7H2O, 0.1; agar, 1.2; and Tween 80, 1.0. Inoculum was prepared by propagating the cryo-preserved strains in 20 mL sterile seed medium (composition same as maintenance medium excluding agar) in 25 mL flask for 24 h at 35 °C (Fisher Isotemp® Incubator Senior Model, USA) under anaerobic conditions.
Taguchi methodology
Figure 1 depicts the schematic representation of the designed methodology. The Taguchi method involves establishment of large number of experimental situation described as orthogonal array to reduce experimental errors and to enhance their efficiency and reproducibility of the laboratory experiments. Each phase has its own objective interconnected in sequence wise to achieve the overall optimization process.
Design of experiments (phase 1)
In phase 1 the first step is to define the factors to be optimized in the culture medium. These factors must have significant impact on microbial tannase biosynthesis. The factors that have critical effect on the tannase production are carbon, nitrogen, and phosphate sources, magnesium, pH, and temperature [19]. In this work the cultural conditions proposed by Das Mohapatra [20] were reproduced (Table 1). The next step was the matrix design with the appropriate orthogonal array for selected factors and levels. Three levels for each factor (except pH) were tested (Table 1) and the size of experimental was represented by the array L18 that indicates 18 experimental trials. The three levels of the factors were assigned with a layout of L18 (21 × 35).
Submerged fermentation (phase 2)
Submerged fermentation was carried out in 25 mL anaerobic flask containing 20 mL of media. Production media was prepared by varying the composition (g/L) of carbon source (tannic acid: 5, 10 and 15); nitrogen source (NH4Cl: 3.5, 7 and 10.5); phosphate source (KH2PO4: 1.5, 3 and 4.5); metal ion (MgSO4: 0.5, 1 and 1.5) [20]. The initial pH was tested at two levels 5.0 and 6.0 and the fermentation was performed at three different temperatures (30, 35, and 40 °C) (Table 1). Fermentation was carried out for 36 h and 1 mL of sample from each trial was withdrawn [18]. The tannase activity was presented as the mean value of three individual assays.
Experimental data analysis and prediction performance (phase 3)
After conducting the experiments, the experimental data were analyzed using the software Statistica 7.1 (Statsoft, Tulsa, OK, USA). Results for influence of individual factors on tannase production and their performance at optimal conditions using Taguchi approach were obtained. In Taguchi methodology, the measure of quality is calculated by the deviation of a characteristic from its target value and a loss function [L(y)] is estimated for the deviation as L(y) = k × (y–m)2, where k represents a constant of proportionality, m represents the target value and y is the experimental value obtained for each trial. Usually, there are three categories for quality characteristics in Taguchi methodology, i.e. the lower the better, the higher the better, and the nominal the best. In this work were used the loss function the higher the better that can be written as L(y) = k × (1/y 2) and represented in Eq. (1).
where the factor −10 ensures that this ratio measures the inverse of “bad quality” and n represents the number of samples.
For significant factors their percentage of contributions were determined from
The interaction of the factors was estimated as shown in Eq. (3) and expressed as percentage of severity index (%SI).
where x L y L represent the interaction factors at the corresponding levels and k is a constant that represents the differences between maximum and minimum combined factor average effects for all pairs of interaction.
Validation (phase 4)
To validate the Taguchi methodology some fermentation experiments were further performed for tannase production using obtained optimal culture conditions (Table 7).
Tannase assay
The tannase activity was evaluated by the spectrophotometric method reported by [21]. The method is based on the formation of chromogen between gallic acid (released by the action of tannase on methyl gallate) and rhodanine (2-thio-4-ketothiazolidine). For determining the tannase activity four solutions were prepared: citrate buffer (50 mM, pH 5), methyl gallate (0.01 M in citrate buffer 50 mM, pH 5), rhodanine (0.667 % w/v in methanol), and KOH (0.5 N). Tannase assay procedure includes the addition of 0.25 mL of crude enzyme to the same volume of methyl gallate. This was followed by the addition of 0.30 mL of rhodanine and 0.20 mL of potassium hydroxide solution with incubation at 30 °C for 5 min after each addition. Reaction was diluted with 4 mL of distilled water and again incubated at 30 °C for 10 min. The color formation was read at 520 nm using a spectrophotometer (VELAB, VE-5600UV). Tubes for blank and control were used simultaneously for each sample. One unit of tannase was defined as the amount of enzyme able to release one µmol of gallic acid formed per minute under assay conditions (temperature and time).
Tannase production in gas-lift fermentor
The production of tannase using the optimized culture conditions by Taguchi methodology was validated in a 1-L gas-lift fermentor (Fig. 2) with a working volume of 0.7 L. The fermentation was initiated with a 1.0 % inoculum. Nitrogen gas (industrial grade) was used for agitation and anaerobically conditions of culture media. Two gas flow conditions were sparged (0.5 and 1.0 vvm). Fermentation was carried out for 24 h and 5 mL of sample was withdrawn at 4-h intervals. Samples were filtered through a 0.45-μm membrane to separate the L. plantarum cells and to measure tannase activity, tannic acid consumption, and gallic acid accumulation. The biomass was determined as mentioned below.
Substrate hydrolysis and gallic acid accumulation
Analysis for tannic acid hydrolysis and gallic acid accumulation by L. plantarum CIR1 was carried out on a VarianProStar HPLC system (Varian ProStar 330, Varian, USA) equipped with a Star800 Photo Diode Array detector. Separation of compounds was carried out using an octadecyl silane column (5 µm × 250 mm × 4.6 mm) and a mobile three-phase gradient system (A: methanol, B: acetonitrile, C: acetic acid (3 %)) at 31 °C and a flow rate of 1.4 mL min−1 with an injection volume of 10 µL. Detection was carried out at 280 nm.
Biomass estimation
The bacterial cell growth was determined by measuring the dry cell weight. The biomass concentration was determined by filtering the sample media using a 0.45-µm membrane previously put at constant weight. The membranes were dried at 50 °C for 24 h and then weighed. Estimation of biomass concentration was determined using Eq. (4):
Kinetic parameter estimation
Kinetic parameters were estimated as follows:
Biomass production was estimated as X (g/L) by the Velhurts-Pearl logistic equation [22] originally developed for population growth and adapted by [23] for the production of fungal tannase:
where µ is the maximal specific growth and X max is the equilibrium value for X where dX/dt = 0 the solution for Eq. (5) is given below:
X 0 refers to the value of X when t = 0. Eq. (6) is generally used for experimental data when Eq. (5) is proposed. Minimal square error values are found as a function of parameters X 0, X max, and µ.
Enzyme production kinetics
Production of enzyme activity, E (U/L), was estimated according to the Luedeking and Piret model [24]:
where Y E/X (U/g) is the yield production coefficient and k (U/h g) is the secondary coefficient of enzyme production (k > 0) or destruction (k < 0). The solution of Eq. (7), E = E(X), is given below:
with E 0 being the value of E when X = X 0 .
The estimation of parameters for Eqs. (6) and (8) can be obtained using minimization of the sum of squared errors between experimental and calculated values for X(t) and E(t) using the minimization program such as the solver routine from Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA).
Enzyme production and substrate uptake parameters
The specific production rate of enzyme was defined according to [25] as follows:
The specific substrate uptake rate was defined using the next equation:
Y E/X and Y X/S were calculated using the linear correlation between tannase activities and biomass concentration, and biomass and tannic acid concentration, respectively.
The enzyme productivity, Γ obs, was defined for every time (t > 0) as
where E max represents the maximum ratio of enzyme titer and t is the fermentation time. Modified Eq. (11) was used to calculate the gallic acid productivity.
Results and discussion
Optimization of tannase production
Analysis of tannase yield
The production of tannase by LAB has gained importance because of its potential uses and applications in food industry.
Experimental matrix for array L18 (21 × 35) and the experimental means for each trial are showed in Table 2. The results obtained experimentally were analyzed applying the higher the better category to obtain the optimal tannase production. Hence, the mean values were computed using the software Statistica 7.1.
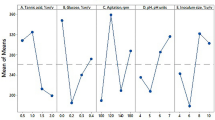
Table 3 shows the average tannase activity and the estimated parameters for every factor at their corresponding levels. Estimated parameters represent the deviation of the mean of the respective factor level from the overall mean and are an index for the influence of the factor level over tannase production. In the present work a maximization of the yield was computed in the software, so for example, for factor tannic acid, the largest yield occurred at level 3.
Figure 3 shows the influence of each individual factor on tannase yield. Increasing the values of factors such as pH, tannic acid, and temperature resulted in an increase in tannase yield. Individually, the higher tannase yield was observed at level 3 of tannic acid factor. NH4Cl induced high tannase production at levels 1 and 3 but not at level 2. According to [20], this may be due to the effect of the others constitutive of culture medium and the different environments generated by the combination of the levels. According to our results, the lower level of phosphate source was necessary to stimulate the bacterial growth and the tannase production. Individually, the magnesium source showed the better effect at level 2. The presence of metal ions, such as magnesium, is necessary for the expression of full catalytic activity of tannase [26].
The interactions between two factors give a better perception into the whole process analysis. Every individual factor may interact with any or all the factors creating the possibility of presence of a large number of interactions. In Taguchi methodology the estimated interactions can be calculated by the severity index (SI). SI allows knowing the influence of two individual factors at various levels of the interaction. In Table 4, the “columns” represent the locations to which the interacting factors are assigned. “Levels” indicate the desirable levels of factors for the optimum interaction. Interaction of 100 % of SI represents a formation of a 90° angle between the lines (factors), while 0 % of SI is for the formation of parallel lines. The highest interaction (SI 68.17 %) was observed between tannic acid and phosphate followed by phosphate and nitrogen (SI 55.80 %), and tannic acid and nitrogen (SI 47.85 %). As we can see, the common factors in those three interactions are tannic acid phosphate and nitrogen sources. Individually, tannic acid was the most influential factor; however, when interacting with phosphate and nitrogen, the tannase yield increases. It was obvious that tannic acid resulted as the major effect factor for tannase production because it is the induction source for tannase production. The phosphate source is an important factor for optimization because it promotes the microbial growth increasing the tannase synthesis level and decreasing the maximum production time [12].
Analysis of variance (ANOVA)
The relative influence of selected factors and their interactions in terms of significant factor and interaction influence are depicted in Fig. 4. The contribution of individual factors is the key to any fermentation process. The percentage of contribution for each factor was tested by the ANOVA (Table 5).
It can be seen that pH is the most significant contributory factor; however, when the Eq. (3) is applied for calculate the percentage of contribution the most influential factor results to be tannic acid because of its degrees of freedom. Three factors (phosphate, nitrogen, and magnesium sources) were pooled into the error factor due to their lower significance and influence on the process (Table 6). The most influential factors on tannase production by L. plantarum CIR1 were tannic acid (42.87 %), pH (29.45 %), and temperature (8.19 %).
The factors considered as insignificant were treated as if they were not present (pooling) by combining the influence of the factor with that of the error term. As a rule, a factor is pooled when its contribution is <10 % [27]. However, in the present work was decided to pool the factors with contribution <5 % to keep temperature because was considered as an important factor for tannase production.
The error term or error factor is the row above the total row in Tables 5 and 6. An error of 19.49 % was observed. According to [27], the error term for Taguchi methodology not only represents any experimental error but also includes the influence of two kinds of factors: factors not included in the experiment and uncontrollable factors (noise factors). Hence, a high value of the error factor is not a reflection of the quality of the experiment. A high percent of contribution for error factor does not mean that the experiment is bad or that the factors’ influences are unreliable. Probably, the inoculum and the static condition of fermentation were important factors not considered in this experiment that could have had an important effect on the tannase production. Authors have used similar array (Taguchi L18) for optimization of a bioprocess and obtained lower values for the error factor [20, 28]. However, they did not show the Square Sum (SS) values for the error and did not mention how the percentage of contribution for the error was calculated.
Validation of experiments
Optimum conditions of different factors and their performance in terms of contribution to obtain higher tannase yield were depicted in Table 7. According to the model employed by the statistical software the expected tannase production was 3,272.06 U/L.
In order to validate the experimental methodology, fermentation experiments employing the optimal conditions (pH, 6; temperature, 40 °C; tannic acid, 1.50 g %; KH2PO4, 0.15 g %; NH4Cl, 0.70 g %; MgSO4, 0.10 g %) were performed for the tannase production. The experimental results for validation step showed an increase for tannase yield of 3,143.09 ± 310 U/L. This experimental result was very close to the predicted value (3,272.06 U/L) obtained from the statistical evaluation. It represents an increasing of 2.54-fold compared to its unoptimized conditions (1,239 U/L) [18]. Das Mohapatra [20] reported an increase of 2.18-fold for the production of tannase by Bacillus licheniformis KBR6 using the Taguchi methodology. Kannan and Aravidnan [29] reported that the tannase production by L. plantarum MTCC1407 under submerged fermentation was enhanced to 2.0-fold using the Response Surface Methodology.
Tannase production process in gas-lift bioreactor
In fermentation processes, the bioreactor provides the environment for growth and activity for microorganisms which cause the biological reaction [32]. The production of tannase by L. plantarum CIR1 using the Taguchi optimized conditions (Table 7) was experimented at a gas-lift bioreactor. Two gas flows (0.5 and 1.0 vvm) were sparged to evaluate the capability of L. plantarum to grow and produce tannase under those stress conditions and additionally to predict the fermentation profile using kinetic models.
Kinetic growth
Experimental results revealed that the maximum cell mass concentration of 1.9 g/L was obtained using an agitation rate of 0.5 vvm at 20 h of fermentation (Fig. 5a). Using an agitation rate of 1.0 vvm the maximum growth of L. plantarum was 1.3 g/L at the same time of fermentation (Fig. 5b). In both cases, the L. plantarum growth did not show lag phase. It indicates that the microorganism had well adaptated well to the culture media, especially to tannic acid, and possesses the ability to hydrolyze it rapidly. It can be demonstrated by the specific growth rate values which were 0.38 and 0.61 h−1 for agitation conditions of 0.5 and 1.0 vvm, respectively (Table 8).
Figure 5a and b show that in the batch culture as the nitrogen flow rate increased, the cell mass concentration decreased. The explanation may be found in the functional behavior of the gas-lift reactor, where with the increasing of nitrogen flow rate the recirculation velocity increases resulting in a high force shear. Due to this the L. plantarum cells need more energy to survive the high liquid velocity and turbulence medium and also higher maintenance energy is required to sustain against higher liquid velocity causing low yield of biomass concentration. Lactobacillus plantarum CIR1 probably is highly shear-sensitive and the shear stress caused by aeration can adversely affect cell growth.
Tannase production kinetic
The tannase production was found to be started at 4 h of fermentation in both 0.5 and 1.0 vvm nitrogen flow rate agitation (Fig. 6a, b). The maximum tannase activity was found to be 6,675.14 U/L obtained at 24 h of fermentation under a nitrogen flow of 1.0 vvm. The maximal tannase activity (4,272.30 U/L) for the flow rate of 0.5 vvm of nitrogen was obtained at the 24 h of fermentation. These patterns are the result of the effect of nitrogen flow rate because of the high shear situation observed at 1.0 vvm. It probably produces a higher secretion of enzyme or a more active enzyme as consequence of the required supply energy maintenance.
In the present work the maximal production of tannase, for both flows, reached the peak after the stationary phase. Similar pattern was observed in Serratia ficaria [30] intracellular tannase, which reaches the maximum tannase production in the late stationary phase.
Kinetic parameters
The kinetic models, such as logistic model for cell growth and logistic incorporated Luedeking-Piret model for tannase production, were used to predict the kinetic profile. The logistic model predicted the growth kinetic profile of L. plantarum at both 0.5 and 1.0 vvm conditions with R 2 (coefficient of determination) values of 0.982 and 0.978, respectively (Figs. 5 and 6). The product formation kinetic model was able to predict the synthesis of tannase by L. plantarum. The R 2 values for tannase production were 0.911 and 0.885 for the nitrogen flow rates of 0.5 and 1.0 vvm, respectively.
Estimated kinetic model parameters for batch tannase production by L. plantarum CIR1 are depicted in Table 8. The logistic model allows predicting a maximal biomass production of 1.83 g/L in 0.5 vvm and 1.21 g/L in 1.0 vvm. The corresponding values of µ indicate a rapid adaptation and growth of microorganism into the media for both flow rates. Kannan [31] showed a low (0.13/h) specific growth rate for the strain L. plantarum MTCC1407 using orbital agitation and submerged culture.
The ratio of E/t is a measure of the productivity of enzyme in the media. Maximal tannase productivity, Γ E , is estimated per unit of liquid broth (U/L/h). Enzyme productivity for 1.0 vvm was 1.56-fold higher than 0.5 vvm productivity. There was a significant increase in productivity from 34.41 U/L/h (initial unoptimized tannase activity) to 178.01 and 278.14 U/L/h which represents an increase of 5.17- and 8.08-fold for the flow rates of 0.5 and 1.0 vvm, respectively. Those results indicate that tannase production from L. plantarum CIR1 is liable to larger volumes using a gas-lift bioreactor.
In Table 8 is shown the final tannic acid concentration (S 24) for both culture conditions. In the flow rate of 1.0 vvm a final tannic acid concentration of 2.38 g/L was obtained, whereas at 0.5 vvm the final concentration was 4.43 g/L. Low value of biomass yield (0.08 gX/gS) was obtained for agitation rate of 1.0 vvm; hence a slow rate of substrate uptake was also obtained (6.99) despite high consumption of tannic acid. Those results explain the previous discussion about the relationship of tannase production and microbial growth, justifying the hypothesis that L. plantarum employed its energy to survive and not to grow under shear force stress.
Finally, L. plantarum CIR1 tannase could carry out high gallic acid production (Table 8). Productivity of process at 0.5 vvm was 220.46 mg/h. However, a productivity of 359.68 mg/h of gallic acid was obtained using a nitrogen flow of 1.0 vvm; it represents an increase of 1.63-fold using the high gas flow rate.
Conclusion
Latobacillus plantarum CIR1 is an efficient tannase producing bacterium. The Taguchi methodology allowed to obtain the optimal culture conditions. There is an evident influence of tannic acid as the most important factor for the tannase production. The experimental validation of the model is very close to the predicted value. The optimal culture conditions were tested in a 1-L gas-lift reactor. Experimental results allowed to increase the enzyme productivity 8.08-fold compared with unoptimized conditions. This is the first report for the bacterial tannase production under anaerobic submerged fermentation using a gas-lift bioreactor.
References
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:215–260
Aguilar CN, Gutiérrez-Sánchez G (2001) Review sources, properties, applications and potential uses of tannin acyl hydrolase. Food Sci Technol Int 7:373–382
Belmares R, Contreras-Esquivel JC, Rodriguez-Herrera R, Ramirez-Coronel A, Aguilar CN (2004) Microbial production of tannase: an enzyme with potential use in food industry. Lebensm Wiss Technol 37:857–864
Abdelwahed A, Bouhlel I, Skandrani I, Valenti K, Kadri M, Guiraud P, Steiman R, Mariotte AM, Ghedira K, Laporte F, Dijoux-Franca MG, Chekir-Ghedira L (2007) Study of antimutagenic and antioxidant activities of Gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus confirmation by microarray expression profiling. Chem Biol Interact 165(1):1–13
Banerjee D, Mahapatra S, Pati BR (2007) Gallic acid production by submerged fermentation of Aspergillus aculeatus DBF9. Res J Microbiol 2(5):462–468
Yu XW, Li YQ (2008) Expression of Aspergillus oryzae tannase in Pichia pastoris and its application in the synthesis of propyl gallate in organic solvent. Food Technol Biotechnol 46(1):80–85
Treviño L, Contreras-Esquivel J, Rodriguez-Herrera R, Aguilar C (2007) Effects of polyurethane matrices on fungal tannase and gallic acid production under solid state culture. J Zhejiang Univ Sci B 8(10):771–776
Mondal KC, Banerjee D, Banerjee R, Pati BR (2001) Production and characterization of tannase from Bacillus cereus KBR9. J Gen Appl Microbiol 47:263–267
Rodríguez H, de las Rivas B, Gómez-Cordovés C, Muñoz R (2008) Characteriztaion of tannase activity in cell-free extracts of Lactobacillus plantarum CECT 748. Int J Food Microbiol 121:92–98
Milva P, Lucia RL, Roberto A, Alessandro E, Guido P, Monia R, Arianna L, Antonio F, Simone G, Silvano EF (2010) Tannic acid degradation by bacterial strains Serratia spp. and Pantoea sp. isolated from olive mill waste mixtures. Int Biodeter Biodegr 64:73–80
Chávez-González M, Rodríguez-Duran LV, Balagurusami N, Prado-Barragán A, Rodríguez R, Contreras JC, Aguilar CN (2011) Biotechnological advances and challenges of tannase: an overview. Food Bioprocess Tech 5(2):445–459
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragán LA, Ramírez-Coronel A, Contreras-Esquivel JC (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76:47–59
Rao RS, Kumar GC, Prakasham SR, Hobbs PJ (2008) The Taguchi methodology as a statistical tool for biotechnological applications: a critical appraisal. Biotechnol J 3:510–523
Arvidsson M, Gremyr I (2007) Principles of robust design methodology. Qual Reliab Eng Int 24:23–35
Zhou J, Wu D, Guo D (2010) Optimization of the production of thiocarbohydrazide using the Taguchi method. J Chem Technol Biotechnol 85:1402–1406
Phadke MS, Dehnad K (1988) Optimization of product and process design for quality and cost. Qual Reliab Eng Int 4:159–169
Matin KT, Bastani D, Kazemian H (2009) Applying the Taguchi method to develop an optimized synthesis procedure for nanocrystals of T-type zeolite. Chem Eng Technol 32:1042–1048
Aguilar-Zárate P (2013) Definición de las condiciones de producción de una tanasa bacteriana. M.Sc. thesis. Universidad Autónoma de Coahuila, Saltillo, México
Mondal KC, Pati BR (2000) Studies on the extracellular tannase from newly isolated Bacillus licheniformis KBR6. J Basic Microbiol 40:223–232
Das Mohapatra PK, Maity C, Rao RS, Pati BR, Mondal KC (2009) Tannase production by Bacillus licheniformis KBR6: optimization of submerged culture conditions by Taguchi DOE methodology. Food Res Int 42:430–435
Sharma S, Bhat TK, Dawra TK (2000) A spectrophotometric method for assay of tannase using rhodanine. Anal Biochem 279:85–89
Gatto M, Muratory S, Rinaldi S (1988) A functional interpretation of the logistic equation. Ecol Model 42(2):155–159
Aguilar CN, Augur C, Favela-Torres E, Viniegra-González G (2001) Production of tannase by Aspergillus niger Aa-20 in submerged and solid state fermentation: influence of glucose and tannic acid. J Ind Microbiol Biotechnol 26:296–302
Luedeking R, Piret EL (1959) A kinetic study of the lactic acid fermentation. J Biochem Microbiol Technol Eng 1:393–412
Viniegra-González G, Favela-Torres E, Aguilar CN, Romero-Gómez SJ, Díaz-Godínez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Kar B, Banerjee R, Bhattacharyya BC (2003) Effect of additives on the behavioral properties of tannin acyl hydrolase. Process Biochem 38:1285–1293
Roy RK (2001) Design of experiments using the Taguchi approach: 16 steps to product and process improvement. John Wiley and Sons, New York
Prasad KK, Mohan SV, Rao RS, Pati BR, Sarma PN (2005) Laccase production by Pleurotus ostreatus 1804: optimization of submerged culture conditions by taguchi DOE methodology. Biochem Eng J 24(1):17–26
Kannan N, Aravindan R (2012) Evaluation and optimization of food-grade tannin acyl hydrolase production by a probiotic Lactobacillus plantarum strain in submerged and solid state fermentation. Food Bioprod Process 90:780–792
Belur PD, Mugeraya G, Nirmala KR, Basavaraj N (2010) Production of novel cell-associated tannase from newly isolated Serratia ficaria DTC. J Microbiol Biotechnol 20(4):732–736
Kannan N, Aravindan R, Viruthagiri T (2011) Effect of culture conditions and kinetic studies on extracellular tannase production by Lactobacillus plantarum MTCC 1407. IJBT 10:321–328
Ruiz HA, Rodríguez-Jasso RM, Rodríguez R, Contreras-Esquivel JC, Aguilar CN (2012) Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem Eng J 65:90–95
Porruat H, Regerat F, Porruat A, Jean D (1982) Production of tannase (tannin acyl hidrolase E.C. 3.1.1.20) by a strain of Aspergillus niger. Biotecnol Lett 44:583–588
Deschamps AM, Otuk G, Lebeault JM (1983) Production of tannase and degradation of chestnut tannin by bacteria. J Ferment Technol 61:55–59
Znad H, Báles V, Markos J, Kawase Y (2004) Modelling and simulation of gas-lift bioreactors. Biochem Eng J 21:73–81
Acknowledgments
Author Pedro Aguilar-Zárate appreciates the scholarship provided by the Mexican Council for Science and Technology (CONACYT).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Aguilar-Zarate, P., Cruz-Hernandez, M.A., Montañez, J.C. et al. Enhancement of tannase production by Lactobacillus plantarum CIR1: validation in gas-lift bioreactor. Bioprocess Biosyst Eng 37, 2305–2316 (2014). https://doi.org/10.1007/s00449-014-1208-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1208-3