Abstract
Rhizopus oryzae NBRC 4697 was selected from among promising candidates as a biocatalyst for biodiesel production. This microorganism was immobilized on to polyurethane foam coated with activated carbon for reuse, and, for biodiesel production. Vacuum drying of the immobilized cells was found to be more efficient than natural or freeze-drying processes. Although the immobilized cells were severely inhibited by a molar ratio of methanol to soybean oil in excess of 2.0, stepwise methanol addition (3 aliquots at 24-h feeding intervals) significantly prevented methanol inhibition. A packed-bed bioreactor (PBB) containing the immobilized whole cell biocatalyst was then operated under circulating batch mode. Stepwise methanol feeding was used to mitigate methanol inhibition of the immobilized cells in the PBB. An increase in the feeding rate (circulating rate) of the reaction mixture barely affected biodiesel production, while an increase in the packing volume of the immobilized cells enhanced biodiesel production noticeably. Finally, repeated circulating batch operation of the PBB was carried out for five consecutive rounds without a noticeable decrease in the performance of the PBB for the three rounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainable alternative energies are urgently required as global warming and energy shortages increase [1, 2]. Among the alternative energies, biodiesel is a renewable, biodegradable, and nontoxic fuel that can be used in diesel cars without requiring modification of existing engines [3]. Biodiesel, a fatty acid methyl ester, is commercially produced through transesterification of vegetable oils or animal fats with alkali catalysts, as these promote a high conversion rate. However, the use of alkalis has several drawbacks, including its energy intensiveness, difficulty of glycerol recovery, and the necessity to remove the alkaline catalyst from the product and to treat the highly alkaline wastewater [4].
Biological biodiesel production using enzymes has recently attracted great attention because of its environmentally friendly nature, easy recovery of products, and mild operating conditions in terms of temperature and pH [5, 6]. In particular, glycerol, which is now used as a valuable intermediate in many industries [7], can be commercialized using a simple recovery process, without requiring washing. However, enzymatic biodiesel production is still considered to be far from commercialization because of the high price of enzymes and the easy deactivation of the enzyme by acyl donors, such as short chain alcohols, such as methanol [8–10]. It has been reported that the enzyme is easily deactivated by methanol when the reaction mixture contains more than 1.5 molar equivalents of methanol to the oil [10, 11]. Stepwise methanol addition is the most widely used method to avoid methanol inhibition [6, 10, 11]. Pre-incubation of the enzyme with methyl oleate [12] and introduction of a solvent-like tert-butanol [13] could be solutions to this problem. This alcohol is considered to be an ideal medium that enhances the miscibility of methanol with vegetable oils, as well as being a regenerating agent for lipase [8, 14, 15].
We have previously suggested the use of a pseudo-two phase partitioning bioreactor (P-TPPB), composed of a hydrophobic first phase (soybean oil) and a hydrophilic second phase, as an attempt to extend the usage of TPPB and have applied it to the production of biodiesel [16]. n-Pentanol was found to be the optimum for the second phase, since P-TPPB containing n-pentanol showed the greatest total biodiesel conversion and highest fatty acid methyl ester content. The enzyme was repeatedly used to produce biodiesel in P-TPPB, while maintaining its activity at over 95 % relative to that of the intact enzyme. Although these attempts have been successful in increasing enzyme stability, the inherent disadvantage of the enzyme process, i.e., high cost, cannot be easily overcome. As an alternative to use of an enzyme in the biological process, whole cell biocatalysts have been applied for economical biodiesel production [4, 17–20]. In this context, the use of whole cell Rhizopus oryzae has been studied extensively, and glutaraldehyde or tert-butanol have been used to improve its stability [21–23]. In particular, after treated with glutaraldehyde, R. oryzae IFO 4697 could maintain high activity even after being reused for 35 batches [23]. Whole cells are believed to be promising for more economical production of biodiesel, when compared with enzyme-based production.
However, there are some other issues that should be discussed for industrial application of whole cells. A low conversion rate can mar the economic advantage of whole cells [4]. Whole cell R. oryzae requires 24 and 72 h to achieve approximately 25 and 80 % conversion, respectively [5]. Using Jatropha oil, R. oryzae IFO 4697 (presently NBRC 4697), immobilized on to a polyurethane foam-based support, required 60 h to attain a biodiesel conversion of 80 % [19]. Yeast whole cells required 165 h to obtain a 71 % conversion [24]. R. oryzae IFO 4697 treated with glutaraldehyde required about 70 h for 60–80 % biodiesel conversion during each batch operation, although it maintained stability for 35 batches [23]. Mass transfer resistance that may be one of the reasons for a low conversion rate, should also be taken into consideration [25] because reactants (oil and methanol) and products (biodiesel and glycerol) may easily cross the cell wall, while cell components remain inside the wall. In addition, methanol inhibition on intracellular lipase was reported to be one of the reasons for a low conversion rate [26, 27].
Recent research has shown that biodiesel production could be dramatically increased using a genetically modified microorganism, Aspergillus oryzae expressing Fusarium heterosporum lipase [26]. The use of a packed-bed reactor system containing this microorganism, with a residence time of 140 min per pass and stepwise addition of 4.25 molar equivalents of methanol to oil for 6 passes, resulted in a final biodiesel conversion of 96.1 %. However, this recombinant microorganism requires water (optimum 5 wt% based on oil) to produce biodiesel. When 1 % water was used, the biodiesel conversion was less than 15 % even after 96 h reaction [28]. In a repeated batch operation using this microorganism, the effluent from a batch operation formed two layers in the product tank, and the bottom layer containing both glycerol and water was recycled with the oil layer into the packed-bed reactor to supply water to the following batch operation, which would result in an excessive accumulation of glycerol in the packed-bed reactor [26].
We previously isolated a microorganism from grease-contaminated soil and chemically mutated the microorganism to increase its lipase activity [2]. In addition, a novel two-step process, which combines the advantages of using whole cells and using enzyme, was suggested to generate a saving as compared to commercial enzyme usage and to alleviate deactivation of the enzyme by methanol [2]. However, a low biodiesel conversion by the microorganism remained a problem. Moreover, after the whole cells had been used, they were disposed, that is, they were not reused. In addition, the development of a whole cell biocatalyst should have been accompanied by development of a bioreactor system, because bioreactor systems increase the feasibility of commercial biodiesel production using whole cell biocatalysts. For example, the advantages of a packed-bed bioreactor includes continuous removal of glycerol and excess alcohol, effective reuse of the biocatalyst, and protection of the biocatalyst from mechanical shear [15].
In this study, we focused on improving each step of the bioprocess involved in biodiesel production using whole cells, and suggested novel findings that could be applied in each step. Available promising microorganisms were evaluated as biocatalysts to maximize biodiesel conversion. Biocatalyst preparations, including immobilization and drying were also evaluated. We investigated the degree of inhibition of methanol on whole cells, and studied the effects of various molar ratio of methanol to soybean oil on the activity of whole cells. Finally, we constructed a packed-bed bioreactor and discussed the results of its operation.
Materials and methods
Microorganisms and chemicals
After conducting a literature search, we chose three available promising microorganisms to test as candidates for biodiesel production. Some recombinant microorganisms were excluded due to exclusive right by strain developers. The first candidate microorganism was Serratia marcescens JYM110 (JYM110), which had been isolated from grease-contaminated soil and was chemically mutated twice using ethyl methane sulfonate (EMS; Fluka, Japan) to enhance intracellular lipase activity, and consequently, biodiesel conversion [2]. The second organism was R. oryzae NBRC 4697 (NBRC 4697, formerly, IFO 4697), which has been widely used as a biocatalyst in biodiesel production [17, 18]. The third organism was R. oryzae ATCC 10260 (ATCC 10260), which is also known to be an excellent microorganism for biodiesel production [19, 20]. The medium composition for JYM110 was 1.0 g/L yeast extract, 2.0 g/L NaCl, 0.4 g/L MgSO4·7H2O, 0.5 g/L (NH4)2SO4, 0.3 g/L K2HPO4, 0.3 g/L KH2PO4 [2], and that for the other two microorganisms was 70 g/L polypeptone, 1.0 g/L NaNO3, 1.0 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, according to the suggestions of the relevant culture collection institutes. Soybean oil was purchased from a domestic supplier (CJ, Korea); 99 % of the oil was triglycerides, composed of 51.8–56.0 % linoleic acid, 22.0–27.1 % oleic acid, 9.6–11.5 % palmitic acid, 6.2–11.1 % linolenic acid, and smaller percentages of other acids. Methanol (Showa, Japan) was used as the acyl donor. Palmitic acid methyl ester, oleic acid methyl ester, linoleic acid methyl ester, and stearic acid methyl ester were purchased from Sigma-Aldrich (USA) to use as standards in the identification and measurement of the components of biodiesel. The other chemicals used were all of analytical grade.
Analysis
To prepare a calibration curve for the components of biodiesel, each of the previously mentioned methyl esters was dissolved in chloroform (Wako, Japan) to concentrations of 100–1,000 mg/L. After shaking, 1 μL of the dissolved sample was injected into a gas chromatograph (GC; HP 5890, USA) equipped with an FID detector and HP-5 column (30 m × 0.32 mm × 0.25 μm film thickness). The temperature of both the injector and the detector was 250 °C and that of the column was increased from 150 to 250 °C at 5 °C/min after the oven temperature was initially maintained for 2 min. Helium was used as a carrier gas. Methyl heptadecanoate (Fluka, Japan) was used as an internal standard for gas chromatography analysis. Samples were taken every 24 h and centrifuged at 12,000 rpm (Combi-514R Hanil, Korea) for 15 min at 4 °C. The upper layer (oil layer) was separated and 0.01 g of the layer was mixed with 10 mL of chloroform. After shaking vigorously, 1 μL of the solution was injected into the GC.
Cell immobilization
R. oryzae NBRC 4697 was found to be the best microorganism among candidates for biodiesel production (please refer to Sect. 3.1). Because this microorganism is a filamentous fungus, attachment is a more favorable immobilization method than entrapment or cross linking. Five different polyurethane foams were purchased from domestic manufacturers and the one coated with activated carbon, with 50 pores per square inches and 5-mm thickness (Woongjin, Korea), was found to be the most efficient for cell attachment. After the polyurethane was cut to 6 × 6 mm squares, the pieces were used for cell immobilization.
Whole cell biocatalyst preparation
After polyurethane pieces were placed into a 500-mL flask containing 100 mL of culture medium, they were autoclaved, and then inoculated with R. oryzae NBRC 4697. The cells attached to the polyurethane pieces by covering them with filaments and inserting filaments into the polyurethane foams during cultivation at 30 °C and 250 rpm for 90 h. The immobilized cells were harvested and washed with distilled water and dried via three different methods, viz., natural drying at room temperature, vacuum drying (Isotemp 280A, USA),or freeze dryings (Operon FDCF-12012, Korea).
Packed-bed bioreactor (PBB)
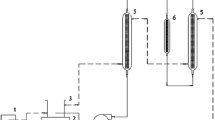
The PBB was made of transparent acryl with a 3.2 cm inner diameter; a small amount of glass wool was placed at the bottom and top of the PBB. The PBB was wrapped with heating tape to maintain 30 °C during operation. The reaction mixture was placed on a hot plate maintaining at 30 °C and vigorously stirred by a magnetic bar. The reaction mixture was transferred from a 250 mL flask to the bottom of the PBB with a pump (Masterflex 7524-55, USA) and the effluent from the PBB circulated back to the flask. Samples were taken from the flask to determine biodiesel conversion.
Results and discussions
Selection of microorganisms
To determine the optimal microorganism for biodiesel production, three promising microorganisms were evaluated in terms of biodiesel conversion. In the case of S. marcescens JYM110, 1 g of freeze-dried cells was added to an equimolar mixture of soybean oil and methanol (0.1:0.1 M). In the case of the other two fungi, the microorganisms were immobilized on to polyurethane foam and then freeze-dried before being used to produce biodiesel. As stated in the following section, the number of polyurethane pieces introduced into the flask was determined based on the amount of cells immobilized per piece. The amount of cells immobilized on to polyurethane foam was determined to be 1.02 g/100 pieces for R. oryzae NBRC 4697 and 0.91 g/100 pieces for R. oryzae ATCC 1026. Accordingly, we placed 98 for R. oryzae NBRC 4697 and 110 pieces of polyurethane foams for R. oryzae ATCC 1026 into the flask containing an equimolar mixture of soybean oil and methanol. As shown in Fig. 1, R. oryzae NBRC 4697 yielded 25.3 % biodiesel conversion after 24 h, while S. marcescens JYM110 and R. oryzae ATCC 10260 yielded 13.9 and 9.2 %, respectively. Because the molar ratio of methanol to soybean oil (MRMTS) was 1.0, the theoretical maximum biodiesel conversion was 33.3 %. Therefore, R. oryzae NBRC 4697 reached a maximum biodiesel conversion of 76.0 % in 24 h. Although S. marcescens JYM110 was previously developed in our laboratory and, being a bacterium, is easier to handle as compared to fungi, such as R. oryzae, we concluded that R. oryzae NBRC 4697 was the best microorganism for biodiesel production given that it demonstrated a markedly higher biodiesel conversion than did the other two microorganisms.
Cell immobilization and drying
A whole cell biocatalyst was successfully applied in the production of biodiesel in our previous study [2]. However, after the cells were used for biodiesel production, they were not recovered or reused, which is a critical drawback from an economical point of view. In this study, R. oryzae NBRC 4697 was immobilized to allow its reuse. When considering that this microorganism is a filamentous fungus, an attachment method using porous inert supports was chosen from among several immobilization methods. Cells were immobilized on to five different types of porous polyurethane foams, dried at room temperature for 24 h, and used for biodiesel production. Polyurethane foam coated with activated carbon was chosen as the best support, since it showed optimal cell attachment when observed with the naked eye and yielded the highest biodiesel conversion (data not shown). The amount of cells immobilized on to a piece of support was 10.2 mg/piece and this is more than twice that used (3.68 mg/piece) for a recombinant microorganism [26]. Cell immobilization was poor when uncoated polyurethane foam was used, and coating the polyurethane foam with activated carbon resulted in a clear enhancement (data not shown), which indicates that immobilization supports should be developed and selected strategically. In the study using the recombinant strain, the amount of wild-type microorganism immobilized on to a piece of support was 5.43 mg/piece [26], which implies that the type of microorganism and also the type of support used substantially influences cell immobilization.
Because a reverse reaction can occur during biodiesel production in the presence of water [17], others have reported drying the immobilized cells at room temperature [14, 20] or freeze drying [22] before using them for biodiesel production. We evaluated three drying methods: natural drying at room temperature, vacuum drying, and freeze drying. On the basis of previous biological biodiesel production studies that used commercial enzyme [9, 16], the total amount of methanol was determined to be a 4.5 molar equivalent to soybean oil. To prevent methanol inhibition, the methanol was arbitrarily divided into four equal aliquots in this study, and each was added to the flask containing 100 pieces of cell-immobilized polyurethane scraps every 24 h. As shown in Fig. 2, the biodiesel conversion after 150 h was 83.7 % for vacuum-drying, and 52.3 and 37.2 % for natural and freeze-drying, respectively. This result revealed that the drying method significantly influences the performance of the immobilized whole cells (IWCs) in biodiesel production and that the whole cell biocatalyst should be prepared by vacuum-drying of cells immobilized onto polyurethane foam that has been coated with activated carbon.
Effect of drying method on biodiesel conversion. After cells were immobilized on to polyurethane foam coated with activated carbon, they were vacuum dried, naturally dried at room temperature or freeze-dried. One hundred cell-immobilized polyurethane pieces were used to produce biodiesel. Error bars represent standard deviation. Filled circle vacuum drying, filled square natural drying, filled triangle freeze drying
When the IWCs were vacuum-dried, the initial biodiesel conversion rate (IBCR) was approximately 1.09 %/h for the first 48 h, but slowed down when the conversion exceeded 60 %, as shown in Fig. 2. The final biodiesel conversion (FBC) reached about 84 % after 150 h and did not increase further thereafter. Figure 3 shows the SEM images of polyurethane before and after cell immobilization, along with photographic images. The polyurethane was completely and evenly covered with cells after immobilization and no pores were observed.
Methanol inhibition
Commercial enzyme for biodiesel production is easily and irreversibly deactivated by methanol [9]. Elaborate methanol feeding methods, including stepwise addition and novel bioreactors such as a P-TPPB, could be good approaches for avoiding methanol inhibition of the enzyme [9–11, 16]. We investigated the inhibitory effect of methanol on IWCs. Each methanol, ranging from 0.5 to 5.0 MRMTS, was added to an individual flask containing the mixture of IWCs and soybean oil, and samples were taken every 24 h to analyze biodiesel conversion. Overall, biodiesel conversion increased until 48 h of operation, but no noticeable increase was observed thereafter, as shown in Fig. 4. The FBC with MRMTS are presented in Fig. 5. The FBC increased with the increase in MRMTS until it peaked at an MRMTS of 2.0, where biodiesel conversion was highest (50.3 %). Over an MRMTS of 2.0, the FBC continuously decreased with the increase in MRMTS and reached its lowest value (3.6 %) at an MRMTS of 5.0. It was clear that the immobilized cells were severely inhibited at an MRMTS of 5.0. However, it was unclear whether the cells were inhibited at an MRMTS of 1.0, and if so, it is required to estimate to what extent the cells were inhibited. In this study, in order to estimate the degree of inhibition by methanol, we first defined “relative conversion” as the actual conversion divided by the theoretical maximum conversion. Then, the degree of inhibition (DI) was estimated by subtracting the relative conversion from 100 %. For example, when the MRMTS is 1.0, the maximum theoretical conversion is 33.3 %; therefore, the relative conversion would be 87.4 % (29.1 % divided by 33.3 %). Accordingly, the DI at an MRMTS of 1.0 is estimated to be 12.6 %. The DI by methanol is also presented in Fig. 5. The DI constantly increased with the increase in MRMTS, reaching 16.8 % at an MRMTS of 1.5, and markedly increasing when the MRMTS exceeds 2.0. The DI reached almost 50 % at an MRMTS of 3.0 and 90 % at an MRMTS of 3.5. These results suggested that stepwise methanol feeding is essential for producing biodiesel at a high FBC using whole cell biocatalysts as well as enzyme [9–11]. When we applied this concept to a widely used commercial enzyme, Candida antarctica lipase (Novozym 435), the DI was about 10 % at an MRMTV (molar ratio of methanol to vegetable oil, a mixture of soybean oil and rapeseed oil) of 0.5 but increased to about 70 and 90 % at MRMTVs of 0.6 and 1.0, respectively [11]. For another commercial enzyme, Thermomyces lanuginosa IM lipase (Lipozyme TL IM), the DI was approximately 90 % at an MRMTS of 1.5 and almost 100 % at an MRMTS of 2.0 [29]. Therefore, it can be said that the enzyme is deactivated much more easily than the whole cells at the same MRMTS.
Biodiesel production with different molar ratios of methanol to soybean oil (MRMTS). To initiate biodiesel production, methanol (ranging from 0.5 to 5.0 M) was added to a flask containing 100 cell immobilized polyurethane pieces and 0.1 M of soybean oil. Error bars represent standard deviation. Filled circle 0.5, filled square 1.0, filled triangle 1.5, filled inverted triangle 2.0, filled diamond 2.5, open circle 3.0, open square 3.5, open triangle 4.0 open inverted triangle 4.5, open diamond 5.0
In order to determine the appropriate mode of stepwise methanol addition, we considered two criteria: the IBCR and DI by methanol. For the first 24 h, an MRMTS of 1.5 showed the highest IBCR of 1.53 %/h, followed by 1.40 %/h at an MRMTS of 2.0, and 1.20 %/h at an MRMTS of 2.5. The DI by methanol was 16.8, 24.5, and 48.8 % at MRMTS of 1.5, 2.0, and 2.5, respectively. On the basis of this analysis, the best level of MRMTS for biodiesel conversion was thought to be 1.5. Since the total MRMTS is 4.5, it could be divided into three equal aliquots. In the following experiments, one aliquot of methanol was initially placed into the mixture of IWC and soybean oil to trigger biodiesel production, and the other two aliquots were fed into the mixture every 24 h. As shown in Fig. 6, the IBCRs were 1.55 %/h for 24 h of operation, and 1.21 %/h for 48 h of operation. Although the IBCR slowed down as the operation progressed, the biodiesel conversion increased constantly. These results implied that stepwise methanol addition is essential to mitigate methanol inhibition of IWC.
Biodiesel production with stepwise methanol addition in a batch flask operation. Total methanol, 0.45 M, was equally divided into three aliquots and an aliquot was fed to the flask containing 100 cell-immobilized polyurethane pieces and 0.1 M of soybean oil to trigger biodiesel production. The other two aliquots were fed to the flask every 24 h thereafter. Arrows and error bars represent the addition of an aliquot and standard deviation, respectively
Packed-bed bioreactor operation
We selected a packed-bed bioreactor (PBB) as the bioreactor for biodiesel production as per previous practices [13, 15, 26]. PBBs have many advantages against flask-type bioreactors including the availability of multiple operation modes, and the ability to effectively reuse the biocatalyst, continuously remove glycerol and excess alcohol, and protect the biocatalyst from mechanical shear [15]. In this study, as shown in Fig. 7, the mixture of soybean oil and methanol in a flask was fed to the PBB and circulated back to the flask. Accordingly, this system can be considered a circulating batch system. The IWCs were vacuum-dried, and then placed in the PBB. The packing volume of immobilized cells was 75 cm3 and the feeding rate was 1.25 mL/min (approximately 25 min of retention time). These conditions were used as the standard against which the functioning of the system under other operating conditions was compared. In order to investigate the inhibitory effect of methanol on the performance of the PBB, all the methanol, i.e., 4.5 MRMTS, was mixed with soybean oil, and this mixture was fed to the PBB under controlled conditions. Since this mixture was composed of 0.1 M of soybean oil (87.2 g) and 0.45 M of methanol (12.8 g), the initial volume of the mixture was approximately 120 mL. The biodiesel conversion was below 5 % up to the end of the PBB operation, implying that methanol inhibited the IWCs severely (data not shown). Therefore, stepwise methanol is also required for PBB operation. The total amount of methanol (4.5 MRMTS) was divided into three equal aliquots, and each was fed into the flask every 24 h, as we had done in the batch flask operation. The IBCR was almost constant as 0.39 %/h until 96 h of operation, but it slowed down after this time as shown in Fig. 8. As the operation proceeded, the contents of glycerol and biodiesel in the reaction mixture increased, while those of soybean oil and methanol decreased, which may decrease mass transfer rate of soybean oil and methanol into IWCs, due to decreasing their concentration gradients between the bulk phase and the surface of IWCs. The viscous biodiesel and glycerol surrounding IWCs may also hinder mass transfer of soybean oil and methanol into the IWCs. Consequently, BCR at some point begins to decrease and finally reaches zero, after which biodiesel conversion no longer increases. We operated the packed-bed bioreactor for 192 h at which the biodiesel conversion was 52.4 %.
Effect of packing volume on the performance of the packed-bed bioreactor. Total methanol, 0.45 M, was equally divided into three aliquots and an aliquot was fed to the flask containing 0.1 M of soybean oil. The mixture was fed to the packed-bed bioreactor to trigger biodiesel production. The other two aliquots were fed to the bioreactor every 24 h thereafter. Arrows and error bars represent the addition of an aliquot and standard deviation, respectively. Filled circle 75 cm3, filled square 112 cm3, filled triangle 131 cm3
When the packing volume was increased by 50 % (112 cm3), the IBCR and FBC were increased to 0.83 %/h and 84.3 %, respectively; that is, by increasing the packing volume by 50 %, the IBCR and FBC were enhanced by 212.8 and 60.9 %, respectively. The increase of packing volume corresponded to the increase in the amount of IWCs, and the retention time of the reaction mixture in the PBB, which increased the contact frequency or contact time between the reaction mixture and IWCs. However, when the packing volume was increased by 75 % (131 cm3), the increases in the IBCR and the FBC were 228.2 and 63.3 % relative to the control, which suggests that the increase of packing volume from 150 to 175 % relative to the control is not economically practical. When the packing volume was increased further by 100 % (150 cm3), the PBB did not always function appropriately, because the packing volume was too large for the reaction mixture to flow smoothly over the packing. That is, most of the reaction mixture sometimes remained in the PBB and only little flowed back to the flask. These results imply that the packing volume of the IWCs is proportionally related to the IBCR and the FBC, but the practical increase of the packing volume is limited, and depends on the volume of the reaction mixture.
The PBB operation was compared with batch operation in a flask rotating at 150 rpm. The IBCR and the FBC of the PBB operation were 0.83 %/h for the first 24 h and 84.3 % at 196 h of operation, respectively, and 1.55 %/h for the first 24 h and 86.5 % at 144 h of operation, respectively, in the batch flask operation. In addition, it took about 114 h for batch and 154 h for PBB operations to reach 80 % of FBC. However, although the IBCR of PBB was much lower compared with flask batch operation, the FBCs were similar. This result implies that mass transfer of the compounds involved in biodiesel production in the flask is less limited than in the PBB.
In the PBB operation in this study, the feeding flow rate or circulating flow rate could also be an important operating variable. A high feeding flow rate may correspond to a high contact frequency between the reaction mixture and the IWCs. We evaluated two additional feeding flow rates at a constant packing volume (75 cm3) as shown in Fig. 9. When the feeding flow rate was doubled from 1.25 to 2.5 mL/min, both the IBCR and the FBC barely increased. When the feeding flow rate was tripled, both the IBCR and the FBC slightly decreased to 0.35 %/h and 47.5 %, respectively. Overall, the feeding flow rate influenced both the IBCR and the FBC, but its effect was much less than that of the packing volume.
Effect of circulating flow rate on the performance of packed-bed bioreactor. Total methanol, 0.45 M, was equally divided into three aliquots and an aliquot was fed to the flask containing 0.1 M of soybean oil. The mixture was fed to the packed-bed bioreactor to trigger biodiesel production. The other two aliquots were fed to the bioreactor every 24 h thereafter. The packing volume was 112 cm3. Arrows and error bars represent the addition of an aliquot and standard deviation, respectively. Filled circle 1.25 mL/min, filled square 2.5 mL/min, filled triangle 3.75 mL/min
In order to demonstrate the feasibility of PBB for biodiesel production, five repeated circulating batch operations were conducted for 1,000 h, as shown in Fig. 10. The packing volume and feeding flow rate were 112 cm3 and 1.25 mL/min. One of three aliquots of methanol was fed every 24 h for each round of operation. When biodiesel conversion reached about 80 %, the reaction mixture was replaced with a fresh mixture of soybean oil and one aliquot of methanol. The results showed that biodiesel could be stably produced for four consecutive rounds, with an FBC in excess of 75 %, while the IBCR began to decrease noticeably after three rounds, perhaps due to the decrease in intracellular lipase activity. Accordingly, the time required to reach over 75 % of FBC became longer after three rounds, that is, 168 h for 1st round and 216 h for 4th round. In the 5th round, FBC did not reach even 60 % after 280 h of operation. The limitation in mass transfer of viscous and sticky compounds like glycerol, remaining from previous rounds and surrounding IWCs, could be another cause of the decrease in BCR.
Considering that it takes about 80 h to achieve 90 % biodiesel conversion in a flask wherein mass transfer is less limited than that in a PBB, the retention time of the reaction mixture in the PBB should be at least 80 h, which requires an impractically large volume of PBB or an extremely low flow rate, i.e., a PBB volume of 9.6 L at a flow rate of 2 mL/min or a PBB volume of 100 cm3 at a flow rate of 2.1 × 10−2 mL/min. Therefore, we did not operate the PBB in the continuous mode. However, if multiple columns were sequentially connected, continuous operation would be possible [27].
Conclusions
R. oryzae NBRC 4697 was selected from among three promising candidates as the biocatalyst for biodiesel production and was immobilized onto polyurethane coated with activated carbon. The amount of cells immobilized was more than twice that in a previous study, which used a recombinant microorganism [26]. For biodiesel production, vacuum-drying was found to be markedly more efficient than natural or freeze-drying, and this finding may be of interest to the other researchers who had prepared immobilized microorganisms through freeze-drying [23, 26]. This study also outlines a novel method to quantify the inhibitory effect of methanol on biocatalysts. The whole cell biocatalyst was severely inhibited by an MRMTS of over 2.0. Since an MRMTS of 1.5 yielded highest IBCR and relatively low DI, the total amount of methanol, 4.5 molar equivalent to soybean oil, was divided into three equal aliquots, each of which was fed to IWCs every 24 h to achieve a high IBCR and FBC. IWCs were packed in a PBB, which was operated in a circulating batch mode. Methanol severely inhibited IWCs in the PBB and stepwise addition of methanol was also required. By increasing the packing volume from 75 to 112 cm3, the IBCR and FBC were enhanced by 212.8 and 60.9 %, respectively. An increase in feeding rate (circulating flow rate) of the reaction mixture slightly promoted biodiesel production, but its effect was minor compared to the effect of an increase in packing volume. The repeated circulating batch operation was performed five consecutive rounds without a noticeable decrease in the performance of the PBB for the first three rounds.
In this study, we outline the novel findings in each step comprising a bioprocess for biodiesel production using whole cells, and these findings will contribute to bioprocess development. Although this study showed the feasibility of a PBB containing IWCs for biodiesel production, relatively low BCR and FBC compared with those of optimized enzymatic biodiesel production processes are still challenging problems. However, we believe that a more elaborate methanol feeding strategy could be a solution to these challenging problems as we have previously shown that enzymatic biodiesel productivity could be enhanced markedly by optimizing methanol feeding [9]. In addition, washing IWCs with some organic solvents, such as tert-butanol [21, 22], could also regenerate IWCs, and thus, elongate the lifetime of the PBB. These factors are subjects for further study. Another issue that should be pointed out is regarding the microorganisms, which are used as biocatalysts and fundamentally determine the reaction rate. Any increase in reaction rate through bioprocess improvement in the absence of biocatalyst development may be limited. A previous study using a recombinant microorganism reported that a biodiesel conversion of 96.1 % was achieved in 14 h in a PBB system [26], and that such efficiency may not be surpassed by other methods while using wild-type microorganisms. Finally, scale-up, which is necessary for commercial biodiesel production, should also be mentioned. The feasible scale-up of PBB can be realized in two ways. One is using a large single PBB, in which the rigidity of supports may be a critical property to prevent compaction of the supports or a pressure drop. The other method involves the use of a large bed packed with many modules, with each module being made up of sequentially connected PBBs [27]. In such a system, the inflow of large volume is divided into small inflows entering modules, reactions occur in each module, and effluents from each module join together to become an effluent of large volume. Innovations that would allow scaling-up of this process should be explored in future studies.
References
Antoni D, Zverlow VV, Schwarz WH (2007) Biofuels from microbes. Appl Microbiol Biotechnol 77:23–35
Jeon DJ, Yeom SH (2010) Two-step bioprocess employing whole cell and enzyme for economical biodiesel production. Korean J Chem Eng 27:1555–1559
Atadashi IM, Aroua MK, Abdul Aziz AR, Sulaiman NMN (2013) The effects of catalysts in biodiesel production: a review. J Ind Eng Chem 19:14–26
Fukuda H, Hama S, Tamalampudi S, Noda H (2008) Whole-cell biocatalysts for biodiesel fuel production. Trends Biotechnol 26:668–673
Ban K, Hama S, Nishizuka K, Kaieda M, Matsumoto T, Kondo A, Noda H, Fukuda H (2002) Repeated use of whole-cell biocatalysts immobilized within biomass support particles for biodiesel fuel production. J Mol Catal B Enzym 17:157–165
Ranganathan SV, Marasimhan L, Muthukumar K (2008) An overview of enzymatic production of biodiesel. Bioresour Technol 99:3975–3981
Shen L, Yin H, Wang A, Lu X, Zhang C, Chen F, Wang Y, Chen H (2014) Liquid phase catalytic dehydration of glycerol to acrolein over Bronsted acidic ionic liquid catalysts. J Ind Eng Chem 20:759–766
Li L, Du W, Liu D, Wang L, Li Z (2006) Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J Mol Catal B Enzym 43:58–62
Jeon DJ, Yeom SH (2011) Comparison of methods for preventing methanol inhibition in enzymatic production of biodiesel. Korean J Chem Eng 28:1420–1426
Fjerbaek L, Christensen KV, Norddahl B (2009) A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol Bioeng 102:1298–1315
Shimada Y, Watanabe Y, Suglihara A, Tominaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J Mol Catal B Enzym 17:133–142
Samukawa T, Kaieda M, Matsumoto T, Ban K, Kondo A, Shimada Y, Noda H, Fukuda H (2000) Pretreatment of Immobilized Candida antarctica lipase for Biodiesel fuel production from plant oil. J Bioresour Bioeng 90:180–183
Hama S, Yamaji H, Fukumizu T, Numata T, Tamalampudi S, Kondo A, Noda H, Fukuda H (2007) Biodiesel-fuel production in a packed-bed reactor using lipase-producing Rhizopus oryzae cells immobilized within biomass support particles. Biochem Eng J 34:273–278
Talukder MMR, Wu JC, van Nguyen TB, Fen NM, Melissa YLS (2009) Novozym 435 for production of biodiesel from unrefined palm oil: comparison of methanolysis methods. J Mol Catal B Enzym 60:106–112
Chen HC, Ju HY, Wu TT, Liu YC, Lee CC, Chang C, Chung YL, Shieh CJ (2011) Continuous production of lipase-catalyzed biodiesel in a packed-bed reactor: optimization and enzyme reuse study. J Biomed Biotechnol. doi:10.1155/2011/950725
Go YW, Yeom SH (2014) Application of pseudo-two phase partitioning bioreactor (P-TPPB) to the production of biodiesel. Biopro Biosys Eng 37:269–275
Jin G, Bierma TJ, Hamaker CG, Mucha R, Schola V, Stewart J, Wade C (2009) Use of a whole-cell biocatalyst to produce biodiesel in a water-containing system. J Environ Sci Health Part A 44:21–28
Jin G, Bierma TJ, Hamaker CG, Rhykerd R, Loftus LA (2008) Producing biodiesel using whole-cell biocatalysts in separate hydrolysis and methanolysis reactions. J Environ Sci Health Part A 43:589–595
Arai S, Nakashima K, Tanino T, Ogino C, Kondo A, Fukuda H (2010) Production of biodiesel fuel from soybean oil catalyzed by fungus whole-cell biocatalysts in ionic liquids. Enzym Microb Technol 46:51–55
Hama S, Tamalampudi S, Fukumizu T, Miura K, Yamaji H, Kondo A, Fukuda H (2006) Lipase localization in Rhizopus oryzae cells immobilized within biomass support particles for use as whole-cell biocatalysts in biodiesel-fuel production. J Biosci Bioeng 101:328–333
Tamalampudi S, Talukder MR, Hama S, Numata T, Kondo A, Fukuda H (2008) Enzymatic production of biodiesel from Jatropha oil: a comparative study of immobilized-whole cell and commercial lipases as a biocatalyst. Biochem Eng J 39:185–189
Li W, Du W, Liu D (2007) Rhizopus oryzae IFO 4697 whole cell catalyzed methanolysis of crude and acidified rapeseed oils for biodiesel production in tert-butanol system. Process Biochem 42:1481–1485
Sun T, Du W, Liu D (2011) Comparative study on stability of whole cells during biodiesel production in solvent-free system. Process Biochem 46:661–664
Matsumoto T, Takahashi S, Kaieda M, Ueda M, Tanaka A, Fukuda H, Kondo A (2001) Yeast whole-cell biocatalyst constructed by intracellular overproduction of Rhizopus oryzae lipase is applicable to biodiesel fuel production. Appl Microb Biotechnol 57:515–529
Du W, Li W, Sun T, Chen X, Liu D (2008) Perspectives for biotechnological production of biodiesel and impacts. Appl Microb Biotechnol 79:331–337
Yoshida A, Hama S, Tamadani N, Fukuda H, Kondo A (2012) Improved performance of a packed-bed reactor for biodiesel production through whole-cell biocatalysis employing a high-lipase-expression system. Biochem Eng J 63:76–80
Yoshida A, Hama S, Tamadani N, Noda H, Fukuda H, Kondo A (2012) Continuous production of biodiesel using whole-cell biocatalyst: sequential conversion of an aqueous oil emulsion into anhydrous product. Biochem Eng J 68:7–11
Hama S, Tamalampudi S, Suzuki Y, Yoshida A, Fukuda H, Kondo A (2008) Preparation and comparative characterization of immobilized Aspergillus oryzae expressing Fusarium heterosporum lipase for enzymatic biodiesel production. Appl Microb Biotechnol 81:637–645
Xu Y, Du W, Zeng J, Liu D (2004) Conversion of soybean oil to biodiesel fuel using lipozyme TL IM in a solvent-free medium. Biocatal Biotrans 22:45–48
Acknowledgments
This work was supported by Gangwon-do, Korea and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0025572). We greatly appreciate the support. The authors also would like to thank Pansuwan Supaporn and Young Wook Go for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kyeong, J.S., Yeom, S.H. Preparation of immobilized whole cell biocatalyst and biodiesel production using a packed-bed bioreactor. Bioprocess Biosyst Eng 37, 2189–2198 (2014). https://doi.org/10.1007/s00449-014-1196-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1196-3