Abstract
The optimal temperature for the microbial polysaccharide fermentation is no higher than 30 °C, which is economically undesirable due to additional cooling cost. To solve this problem in the case of welan gum production, we obtained the high-temperature-tolerant-producing strain, Sphingomonas sp. HT-1 by atmospheric and room-temperature plasma-induced mutation. Using HT-1, we obtained a concentration and 1 % aqueous viscosity of 26.8 ± 0.34 g/L and 3.50 ± 0.05 Pa s at a comparatively higher optimal temperature (37 °C). HT-1 was further characterized to understand the mechanism by which these properties are improved. Results indicated that high yield could be attributed to the following: (1) enhanced intracellular synthesis, demonstrated by an increase in the activities of key enzymes, and (2) accelerated cross-membrane substrate uptake and product secretion, indicated by improved membrane fluidity and permeability. Temperature tolerance could be attributed to the overexpression of the investigated heat shock proteins and oxidative stress proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Welan gum is a microbial polysaccharide with many attractive properties [1]. Given its high and stable viscosity in aqueous solutions at a broad range of temperatures (up to 150 °C) and pH values (2–12), welan gum has great commercial potential in food, medicine, concrete additives, oil recovery, and some other fields [2–4]. However, its application is greatly limited by production costs [5, 6]. In particular, the normal temperature for existing industrial manufacturing system of welan gum is 30 °C; as such, a large amount of cooling water has to be consumed in the fermentation, thereby causing high energy consumption problem. In summer in China, ambient temperature often exceeds the limit favourable for the survival of a producing strain; as a result, welan gum production is subjected to seasonal shutdown. However, to our knowledge, few studies have been conducted to improve fermentation temperature for welan gum biosynthesis. Therefore, an efficient strain exhibiting tolerance to high temperatures should be identified and cultured for large-scale biosynthesis of welan gum.
The atmospheric and room-temperature plasma (ARTP) mutagenesis is a technique newly applied to microbial breeding and other biotechnological fields. For instance, ARTP has been successfully applied to improve the properties of several strains, such as Streptomyces avermitilis, Enterobacter aerogenes, and Clostridium beijerinckii [7–9]. In contrast to traditional breeding methods with low efficiency, low stability, and reduced safety, ARTP requires low gas temperature, high concentration of active species, and flexible operation [10, 11]. Hence, this technique could be performed to screen-effective strains.
Welan gum synthesis is considered as a membranous mechanism because substrates should be transported into cytosol and products should be secreted out of cells [12]. As a natural barrier for microbes, cell membrane regulates the transport of extracellular and intracellular substances. Therefore, increased membrane fluidity and permeability could increase both the uptake of extracellular substrates and product secretion [13]; as a result, an efficient welan gum production is developed. Indeed, improved productivity of other strains has been correlated with enhanced membrane fluidity and permeability [12].
Furthermore, under elevated temperature conditions, strains are exposed to deleterious threats affecting their physiological characteristics [14]. To prevent such adverse conditions, cells have developed defence or adaptive mechanisms involving the molecular chaperones—heat shock proteins exemplified by Hsp20 and Hsp40, and detoxifying enzymes such as superoxide dismutase (SOD), catalase (CAT), as well as peroxidase (POD). The specific expression of these chaperones and antioxidant proteins can enhance the tolerance of cells against high temperature [15]. Therefore, cell-rescue and antioxidant proteins in HT-1 should be overexpressed to confer the observed high-temperature tolerance on the mutant.
With these expectations, we aimed to generate the mutant of Sphingomonas sp. by ARTP and improve welan gum biosynthesis at high temperatures. After the desired mutant was obtained, changes in key enzyme activity, membrane fluidity, permeability, heat shock protein expression, and antioxidant protein activity were investigated to obtain insights into welan gum biosynthesis and cellular defence against high temperatures. To the best of our knowledge, this study is the first to demonstrate the physiological mechanism of strain improvement for welan gum biosynthesis. This study also provided information that described the development and improvement of the production processes of other biopolymers.
Materials and methods
Microorganisms
The Sphingomonas sp. CGMCC1737 used as the parent strain was deposited in the China General Microbiological Culture Collection Center.
Medium and cultivation conditions
The seed medium (pH 7.2–7.4) was prepared with the following contents (per litre): glucose, 20 g; yeast extract, 1 g; peptone, 3 g; K2HPO4·3H2O, 2 g; and MgSO4, 0.1 g. The agar medium (pH 7.2–7.4) contained the following contents (per litre): glucose, 10 g; beef extract, 3 g; peptone, 10 g; NaCl, 5 g; and agar, 20 g. The selection agar plates contain the same composition as the agar medium except that the concentration of glucose was 60 g/L. The fermentation medium (pH 7.2–7.4) contained the following contents (per litre): glucose, 50 g; yeast extract, 8 g; K2HPO4·3H2O, 3 g; and MgSO4, 0.4 g.
The mutant of Sphingomonas sp. CGMCC1737 was inoculated into 135 mL of seed medium in a 1-L flask and incubated at 37 °C for 16 h with shaking at 200 rpm. The seed culture was transferred to a 7.5-L bioreactor (BioFlo 110, New Brunswick Scientific, USA) with 4.5 L of fermentation medium. The pH was adjusted to 7.4 with 3 M NaOH. The fermentation culture was incubated at 37 °C with an agitation speed of 600 rpm for 66 h.
Analytical methods
The dry cell weight (DCW) was determined from 10 mL of cell sample suspensions harvested by centrifugation, washed with distilled water, and dried at 105 °C. The glucose content was measured using a biosensor equipped with a glucose oxidase electrode (SBA-40C, Shandong Academy of Sciences, China). The concentration of welan gum was precipitated by two volumes of alcohol, recovered by centrifugation, and then dried at 60 °C to a constant weight. The viscosities of welan gum (1 % aqueous and 1 % KCl, 0.5 % aqueous at pH 2 and pH 10, 25 °C) were measured using a rotational viscometer (NDJ-1, Shanghai Hengping Scientific Instrument Company, China) with rotor no. 4 at 60 rpm. Each experiment was repeated thrice, and the experimental errors were <4 %.
Mutant screening
The implantation sources were produced by ARTP instrument (ARTP-II, Tsinghua University, China). For the mutation of Sphingomonas sp., 20 μL of the culture (OD600 = 1.0) was dipped into a sterilized stainless steel plate (5.0 mm in diameter) and dried in sterile air for a few minutes. The metal plate with the bacterial cells was then treated with a helium plasma jet. The apparatus was operated at a helium gas flow rate Q He = 15.0 standard litres per minute and RF power input of 100 W. Under these operating conditions, the plasma temperature on the surface of the metal plate was 25–30 °C. The time for the treatment of Sphingomonas sp. by ARTP was 2 min [8].
After the treatment, the metal plate was washed with sterilised saline solution, and the suspension was spread on selected agar plates. In the first step of mutation, the plates were incubated at 37 °C. After inoculation, cultures were incubated for 66 h in a shaker at a predetermined temperature with a shaking speed of 200 rpm. Enriched cultures were then streaked on the same selection agar plates at 37 °C. Colonies showing large shapes and fast growth were selected. For the second step of implantation, the plates were incubated at 37 °C. Afterwards, cultures were incubated in a 500-mL flask containing 100 mL of fermentation medium and shaken at 200 rpm for 66 h at 37 °C. Strains were selected based on their welan gum production.
Assay of enzyme activity
Cells were collected at the exponential phase (16 h) of fermentation at different temperatures and washed twice with 0.85 % NaCl solution. Harvested cells were suspended in 100 mM Tris–HCl (pH 8.0) and disrupted by sonication at 4 °C. The cell debris was removed by centrifugation at 10,000×g for 10 min at 4 °C. The cell-free extracts were used for enzyme activity measurement. The activities of phosphoglucomutase (PGM), UDP-glucose pyrophosphorylase (UGP), UDP-glucose dehydrogenase (UGD), and dTDP-glucose pyrophosphorylase (TGP) in the cell extracts were measured according to a previously described method [16–19]. Enzyme activities were determined by the appearance or disappearance of NADH or NADPH at 340 nm. In all of the cases, one unit of activity was defined as the amount of enzyme catalysing one micromole of NADH or NADPH per minute. Total protein concentration was determined by the Bradford method [20]. Each experiment was repeated three times, and the experimental errors were all <4 %.
Analysis of physiological parameters of cell membrane
Transmission electron microscopy
The morphological changes of the wild-type and mutant strains were examined by TEM following a previous method [21]. The samples were prepared by adding 2.5 % (v/v) glutaraldehyde for 30 min. Cell pellets were harvested by centrifugation at 6,000×g for 10 min and mixed with 1.25 % water agar. Then, the agar was cut into 1 mm pieces and fixed in phosphate-buffered 2.5 % glutaraldehyde for 20 min. Then, the pieces were rinsed with phosphate buffer (0.01 M, pH 6.8) four times, post-fixed in phosphate-buffered saline (1 % osmium tetroxide) for 2 h, rinsed with water, and fixed for 2 h in 1 % aqueous uranyl acetate. After dehydration once in ethyl alcohol and thrice in propylene oxide, the agar pieces were embedded in Epon 812 (Spi Supplies, New Chester, PA, USA). The thin sections stained with uranyl acetate and lead citrate were examined under an electron microscope (H7650, Hitachi, Japan).
Fatty acid extraction and analysis
Phospholipid extraction and analysis were conducted according to a previous study [22]. After incubating the cells for 16 h, fatty acids in the phospholipids were prepared and analysed as previously described [23]. Fatty acids were identified with a Shimadzu GC-2012 chromatograph coupled with an Agilent 7890AGC mass spectrometer compared with the standards (analytical grade; Sinopharm Chemical Reagent, China), and their mass spectra were compared with a spectrum database. The relative amounts were calculated from the peak areas. All experiments were performed in triplicate.
Assay of outer and inner membrane permeability
The permeability of the outer membrane was measured by an NPN access assay according to a previously described method [24]. Samples of wild-type and mutant Sphingomonas sp. CGMCC1737 strains were collected at different growth temperatures, rinsed twice by centrifugation at 5,000×g, and then resuspended in 10 mM phosphate buffer (pH 7.2) to OD600 of 0.5. NPN was added to a final concentration of 10 mM into a quartz cuvette containing 2 mL of cell suspension. The sample was mixed by the inversion of the cuvette immediately prior to fluorescence monitoring. Fluorescence was measured using a spectrofluorometer (Cary Eclipse, Varian, USA) with slit widths set to 1 mm as well as excitation and emission wavelengths set to 340 and 460 nm, respectively.
The inner membrane permeability was estimated by measuring the access of ONPG to the cytoplasm as previously described [25]. ONPG was added to a final concentration of 100 μg/mL into a quartz cuvette containing 2 mL of cell suspension. The substrate cleavage by β-galactosidase was monitored by light absorption measurements at 420 nm in a spectrophotometer (UV-2450, Shimadzu, Japan).
Indices of oxidative stress
Cells were collected at the exponential phase (16 h) of fermentation at different temperatures and washed twice with 0.85 % NaCl solution. Harvested cells were suspended in 100 mM Tris–HCl (pH 8.0) and disrupted by sonication at 4 °C. The cell debris was removed by centrifugation at 10 000×g for 10 min at 4 °C. The cell-free extracts were used for oxidative stress protein measurement. The levels of malondialdehyde (MDA) and the activities of SOD, CAT, and POD were measured according to a previously described method [26]. Total protein concentration was determined by the Bradford method [20]. Each experiment was repeated three times, and the experimental errors were all <4 %.
Transcriptional analysis of selected heat shock proteins by RT-PCR
Hsp20 and Hsp40 were investigated as representatives of heat shock proteins by RT-PCR. Cells were harvested by centrifugation. Total RNA was isolated using RNAiso Plus (TaKaRa Biotechnology Company, China) and was employed in the synthesis of cDNA using PrimeScript™ RT Master Mix (TaKaRa Biotechnology Company, China). Real-time PCR (StepOnePlus™ Real-Time PCR System, Applied Biosystems, USA) was carried out with 2 μL cDNA in a 20-μL PCR reaction system (containing 2 × SYBR® Premix Ex Taq Tli RNaseH Plus 10 μL, each primer (25 pmol/μL) 0.4 μL, 50 × ROX Reference Dye 0.4, 2 μL cDNA, and sterile distilled water 6.8 μL). The information collected on these genes was analysed, and their primers were listed as follows: Hsp40-F: CACTGGAAGTGCTGGAACTG and Hsp40-R: GCATCGTTCGGACGTACA; Hsp20-F: GGCAGACGAAATCGACATTA and Hsp20-R: ATTGCCCTGGTTGTTCTCAT; 16S rRNA-F: ATCTCACGACACGAGCTGAC and 16S rRNA-R: TTACCAGCGTTTGACATGGT. 16S rRNA was used as an endogenous control gene. After an initial denaturation period at 95 °C for 30 s, the reaction mixture was cycled 40 times. The PCR conditions used were as follows: 95 °C for 5 s, and 60 °C for 30 s. After 40 cycles, a final extension step was run for 1 min at 60 °C. Melting curves were performed using the dissociation curves software (StepOne™ Software, USA) to ensure that only a single product was amplified. All of the reactions were repeated three times.
Results and discussion
Mutation and selection of strains for high welan gum production at high temperature
Among the various techniques, ARTP mutagenesis was selected and applied in Sphingomonas sp. CGMCC1737. The cells exhibited sensitivity towards ARTP, and survival rate decreased as the treatment time was prolonged. Mutants with altered physiological characteristics are obtained at a survival rate of 10 % [27]. After 2 min of exposure to ARTP, the lethality rate reached 90 %. At this point, the positive and negative mutation rates reached 30.5 and 19.3 %, respectively (data not shown), which could be considered as an effective mutagenesis [7]. HT-1 strain exhibited the most efficient performance; as such, this strain was selected for further characterisation.
After HT-1 was subjected to fermentation for 66 h at 37 °C, the concentration of welan gum increased to 23.5 ± 0.22 g/L. The viscosity and DCW of welan gum reached 2.87 ± 0.06 Pa s and 8.16 ± 0.11 g/L, respectively. Both biomass and productivity of the mutant increased at the elevated temperature. This result indicated that the objective of our direct screening strategy was obtained. To further evaluate the stability of mutant, HT-1 was subjected to flask fermentation. The stability of welan gum production of HT-1 was maintained (data not shown) in the 10 subcultures, suggesting a high genetic stability.
Effect of temperature on welan gum fermentation by the mutant and the wild-type strains
A temperature of 30 °C was used for industrial biosynthesis of welan gum by wild-type strain. However, the mutant obtained in this study has an optimal temperature of 37 °C. Therefore, welan gum fermentation by the wild-type strain and Sphingomonas sp. HT-1 should be compared at these two temperatures. Results of fermentation in a 7.5-L bioreactor fermentation with either of the strains are shown in Fig 1. The maximum concentration of welan gum was higher in the mutant (26.8 ± 0.34 g/L at 37 °C) than in the wild type (21.4 ± 0.35 g/L at 30 °C). Besides, the welan gum viscosities of 1 % aqueous and 1 % KCl in the mutant increased by 41.7 % (3.50 ± 0.05 vs. 2.47 ± 0.05 Pa s) and 11.6 % (3.74 ± 0.04 vs. 3.35 ± 0.05 Pa s), respectively, compared with original strain. However, the welan gum viscosities of 0.5 % aqueous at pH 2 and pH 10 in the mutant (1.73 ± 0.03 and 1.64 ± 0.04 Pa s) were nearly constant with those in original strain (1.72 ± 0.04 and 1.62 ± 0.03 Pa s). This result indicated an improved production and viscosity (1 % aqueous and 1 % KCl) of welan gum. Apparently, biosynthesis of welan gum of the wild-type strain was suppressed at 37 °C (Fig 1a). In addition, the mutant cells at 37 °C grew much better than the wild-type strains at 37 °C (Fig 1b). The wild-type strain exhibited an increase in the lag time of cell growth. The maximum DCW of the mutant at 37 °C reached 8.30 ± 0.12 g/L. Indeed, the DCW of the mutant at 37 °C was higher than that of the wild-type strain at 30 °C. The maximal consumption of glucose was achieved by the mutant at 37 °C (Fig 1c). This result is consistent with the enhanced welan gum biosynthesis and cell growth. Furthermore, welan gum biosynthesis and cell growth were enhanced in the mutant at 37 °C. In addition to the improved temperature tolerance, the mechanism by which welan gum productivity is increased should be elucidated.
Time profiles of welan gum concentration (a), cell growth (b), glucose concentration (c), in the cultivation of Sphingomonas sp. wild-type strain and mutant at different temperatures in 7.5-L bioreactor. Wild-type strain at 30 °C (filled triangle), wild-type strain at 37 °C (unfilled triangle); mutant at 30 °C (filled circle), mutant at 37 °C (unfilled circle)
Assay of key enzyme activities of the wild-type and mutant strains
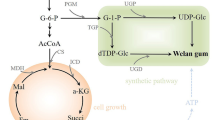
According to the previous study, PGM, UGP, UGD, and TGP were the key enzymes involved in the biosynthesis pathway of sugar nucleotides, which are essential for welan gum production, and the activities of key enzymes might affect the conversion of glucose to welan gum [28]. Considering the ability of mutants to produce welan gum at increased temperature was significantly improved, and the consumption of glucose remarkably increased; we investigated the activities of key enzymes in the wild-type strain and HT-1 strains at 37 °C (Fig. 2). Indeed, the activities of four key enzymes were all significantly increased in the mutant, particularly for PGM (0.18 ± 0.0036 U/mg) and TGP (0.17 ± 0.0051 U/mg). In addition, the activities of four key enzymes in HT-1 at 37 °C were even higher than those in the wild-type strain at 30 °C. This might be the main reason for the increase in the flux from G6P to G1P which leads to the accumulation of intracellular welan gum [28]. Zhang et al. [29] report similar results in which the increased activities of the phosphoglucosamine mutase catalyse a high conversion rate of glucose to curdlan. Thus, key enzymes may exhibit enhanced activities in the mutant at 37 °C for the improved biosynthesis of welan gum.
Assay of membrane fluidity of the wild-type and mutant strains
Cell membrane fluidity is closely related to the membrane fatty acid composition to determine the transport of extracellular substrates and the secretion of products [30, 31]. Considering the enhanced biosynthesis of welan gum in the mutant, we assumed that the cell membrane fluidity could be influenced to regulate the transport of substrates and products. Hence, we investigated the effects of the membrane fatty acid composition of wild-type and mutant strains at 37 °C. Figure 3 shows the changes in the profiles of membrane SFAs and UFAs in the two strains. The content of 14:0 was slightly higher in the mutant than in the wild-type strain. In particular, the contents of 16:0 and 17:0 of the mutant were higher than those of the wild-type strain (Fig. 3a). The increasing proportion of SFAs commonly results in the enhanced membrane fluidity [30–32]. In our study, the enhanced fluidity of the cell membrane in the mutant was observed. Moreover, sharper decrement in 17:1 of the mutant strain was observed (Fig 3b). Furthermore, the contents of 16:1 and 18:1 were similar in the wild-type and mutant strains. The positive effect of cell membrane fluidity is induced by decreased UFAs [30–32]. Therefore, the enhanced membrane fluidity of the mutant may be attributed to the altered SFAs and UFAs of the membrane. Previous studies also proved that Saccharomyces cerevisiae can increase tolerance to environmental stress by regulating the fatty acid composition of the cell membrane [31]. In our study, changes in the fatty acid composition of the membrane were observed in the mutant strain. Therefore, enhanced membrane fluidity contributed to the mutant strain with enhanced tolerance to temperature for the consumption of substrate and the secretion of welan gum at 37 °C.
Assay of membrane permeability of the wild-type and mutant strains
Membrane permeability is another key factor contributing to the maintenance of cell viability and metabolic function. Cell permeability of the mutant and wild-type strains at 37 °C was evaluated on the basis of the permeabilities of outer and inner membranes by using hydrophobic fluorescent NPN and β-galactosidase substrate ONPG probes, respectively. NPN exhibited low fluorescence in aqueous solutions but strong fluorescence quantum yield in the hydrophobic environment of a biological membrane. In this study, NPN entered at specific points where membrane integrity was compromised. The value and increased rate of fluorescence indicate the outer membrane permeability of strains [33]. After ONPG passed through the inner membrane, ONPG combined with β-galactosidase localized in the cytoplasm, resulting in the appearance of a yellow colour. The absorbance at 420 nm and the increased rate of ONPG correspond to the permeability of the inner membrane [25]. In Fig. 4a and b, the value of the fluorescence in the outer membrane of the wild strain was lower than that of the mutant. Similar results were observed in the inner membrane, and the absorbance of the mutant was higher than that of the wild strain (Fig. 4b). The mutant strain exhibited higher permeability of the outer membrane and the inner membrane. This result is consistent with the ethanol tolerance strains exhibiting higher membrane permeability [34]. High membrane permeability induces an enhanced uptake of extracellular substrates and secretion of products [35]. As a consequence, a large amount of extracellular substrates were provided to synthesize welan gum by mutant strains. The efficient secretion of this polymer decreased the accumulation stress of the intracellular substrate and caused an advantageous circle of welan gum biosynthesis.
Comparisons of the permeabilities of the outer (a) and inner (b) membranes of Sphingomonas sp. wild-type strain and mutant at 37 °C. Wild-type strain (unfilled circle) and mutant (filled circle). a: NPN was a probe of the outer membrane permeability. b: ONPG was a probe of the inner membrane permeability
To further investigate the membrane integrity, we compared the surface morphologies of wild-type and mutant strains at 37 °C by TEM (Fig. 5). The cell membrane of wild-type strain appeared smooth and flat. By contrast, the cell membrane of the mutant strain appeared rough and uneven. The high specific surface area in Propionibacterium acidipropionici induces an efficient transport of substrates and metabolites across the cell membrane [36]. The result indicated that the rough cell membrane of the mutant strain with a larger specific surface area could facilitate the transport of extracellular substrates and the secretion of polysaccharides from the intracellular matrix. Therefore, the efficient transport of substrates and polysaccharides resulted in the enhanced welan gum production of the mutant strain at 37 °C. Hence, the increased membrane fluidity and permeability may contribute to the high welan gum yield of the mutant strain at high temperatures.
Assay of heat shock protein and oxidative stress protein of the wild-type and mutant strains
Synthesis of heat shock proteins and oxidative stress proteins is considered as a significant response of heat-tolerant strains against high temperatures [14]. To investigate the function of such proteins in welan gum production, we selected Hsp20 and Hsp40 proteins as examples of heat shock proteins. SOD, CAT, and POD were selected to represent oxidative stress proteins. The results showed that the expression levels of both Hsp20 and Hsp40 genes were remarkably increased in HT-1 compared with the wild-type strain (Fig 6). Heat shock proteins are molecular chaperones, which regulate membrane ATPase activity to enable the cells to cope with rapid transitions in energy requirements [15]. The overexpression of such proteins in the cell can possibly help conserve intracellular ATP at elevated temperatures; as a result, cells exhibit tolerance to high temperatures [14]. Heat shock proteins were upregulated in the mutant strain that could tolerate high temperatures. The overexpressed proteins help the mutant strain to maintain intracellular ATP for improved cell growth and welan gum biosynthesis. This phenomenon is also associated with ethanol tolerance in different strains [34]. Taken together, the overexpression of heat shock proteins may contribute to temperature tolerance of the mutant strain for better cell growth and welan gum biosynthesis.
The RT-PCR analyses of Hsp40 and Hsp20 genes transcription level in the wild-type strain (unfilled bars) and mutant (filled bars) at 37 °C. The level of transcription was calculated relative to the transcription in culture of wild-type strain. The error bars indicate the standard deviation of three samples taken from the same RNA sample
In addition, heat shock proteins function as redox regulators controlling the levels of intracellular reactive oxygen species (ROSs) [14]. Oxidative stress, which induces the generation of ROSs via a variety of extra-environmental conditions including high temperature, is one of the major stress factors influencing cells during aerobic growth. These ROSs can induce protein or enzyme inactivation and alter membrane fluidity and permeability via lipid peroxidation [15]. Under such circumstances, various antioxidant enzymes, including SOD, CAT, and POD, are often secreted to provide protection. In Table 1, the mutant strain exhibited a significantly lower MDA value of 35 % at 37 °C than the wild-type strain. The mutant strain also exhibited lower lipid peroxidation levels, suggesting a decreased ROS production in the mutant strain compared with the wild-type strain. The remarkable increase in the activities of SOD, CAT, and POD (Table 1) in the mutant strain corresponded to an altered antioxidant protective mechanism. Previous studies indicated that strains with low activities of antioxidant enzymes are more sensitive to the lethal effects of the increased temperatures [37]. Therefore, the antioxidant protection enhanced the growth of the mutant strain that could synthesis welan gum at high temperatures. Cao et al. [38] demonstrated small Hsps that can effectively induce cell protection. Hence, several defence mechanisms and regulatory networks, including the activation of heat shock protein genes, enhancement of antioxidant enzyme activities, induction of Hsps, and resistance to oxidative stress, showed high responses towards elevated temperature. These mechanisms may also provide increased tolerance to high temperatures for the mutant strains. As such, welan gum biosynthesis could be improved.
Conclusion
Atmospheric and room-temperature plasma was applied to generate stable Sphingomonas sp. mutants. An effective mutant HT-1 was identified and characterized for welan gum biosynthesis at a comparatively high temperature. This mutant strain exhibited high activities of key enzymes. Furthermore, the mutant strain was characterized by the improved membrane fluidity and permeability favourable for welan gum production and cell growth. Therefore, high productivity may be induced by enhancing synthesis capacity and facilitating substrate accumulation along with product secretion. Levels of heat shock proteins and antioxidant enzymes proved to be enhanced as well. This result could be accounted for the obtained tolerance against relatively high temperatures. In summary, HT-1 strain generated by ARTP exhibited a higher optimal temperature than the normal temperature used for welan gum fermentation. This enhanced result is important in industrial applications because production costs could be reduced. The study also described the mechanisms by which productivity and temperature tolerance are improved. The results could provide reference for future studies on property modification not only of the welan gum-producing strains but also of other economically important microbes.
References
O’Neill MA, Selvendran RR, Morris VJ, Eagles J (1986) Structure of the extracellular polysaccharide produced by the bacterium Alcaligenes (ATCC 31555) species. Carbohydr Res 145:295–313
Kranenburg R, Boels IC, Kleerebezem M, Vos WM (1999) Genetics and engineering of microbial exopolysaccharides for food: approaches for the production of existing and novel polysaccharides. Curr Opin Biotech 10:498–504
Paris L (2009) Use of thickening agents for producing soft capsules and film production method. US Patent 7612116B2
Li H, Xu H, Xu H, Li S, Ying HJ, Ouyang PK (2011) Enhanced welan gum production using a two-stage agitation speed control process in Alcaligenes sp. CGMCC2428. Bioprocess Biosyst Eng 34:95–102
Li H, Xu H, Li S, Feng XH, Xu H, Ouyang PK (2011) Effects of dissolved oxygen and shear stress on the synthesis and molecular weight of welan gum produced from Alcaligenes sp. CGMCC2428. Process Biochem 46:1172–1178
James GO, Hocart CH, Hillier W, Dean Price G, Djordjevic MA (2013) Temperature modulation of fatty acid profiles for biofuel production in nitrogen deprived Chlamydomonas reinhardtii. Bioresour Technol 127:441–447
Wang LY, Huang ZL, Li G, Zhao HX, Xing XH, Sun WT, Li HP, Gou ZX, Bao CY (2009) Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. J Appl Microbiol 108:851–858
Lu Y, Wang LY, Ma K, Li G, Zhang C, Zhao HX, Lai QH, Li HP, Xing XH (2011) Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem Eng J 55:17–22
Guo T, Tang Y, Xi YL, He AY, Sun BJ, Wu H, Liang DF, Jiang M, Ouyang PK (2011) Clostridium beijerinckii mutant obtained by atmospheric pressure glow discharge producing high proportions of butanol and solvent yields. Biotechnol Lett 33:2379–2383
Laroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2:391–400
Lin SJ, Wen CY, Wang PM, Huang JC, Wei CL, Chang JW, Chu WS (2010) High-level production of erythritol by mutants of osmophilic Moniliella sp. Process Biochem 45:973–979
Thorne L, Mikolajczak MJ, Armentrout RW, Pollock TJ (2000) Increasing the yield and viscosity of exopolysaccharides secreted by Sphingomonas by augmentation of chromosomal genes with multiple copies of cloned biosynthetic genes. J Ind Microbiol Biotechnol 25:49–57
Denich TJ, Beaudette LA, Lee H, Trevors JT (2003) Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Methods 52:149–182
Zhang M, Xiao Y, Zhu RR, Zhang Q, Wang SL (2012) Enhanced thermotolerance and ethanol tolerance in Saccharomyces cerevisiae mutated by high-energy pulse electron beam and protoplast fusion. Bioprocess Biosyst Eng 35:1455–1465
Wei ZH, Wu H, Bai LQ, Deng ZX, Zhong JJ (2012) Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioprocess Biosyst Eng 35:1309–1316
Arrecubieta C, Garcia E, Lopez R (1996) Demonstration of UDP-glucose dehydrogenase activity in cell extracts of Escherichia coli expressing the pneumococcal cap3A gene required for the synthesis of type 3 capsular polysaccharide. J Bacteriol 178:2971–2974
Videira PA, Cortes LL, Fialho AM, Sá-Correia I (2000) Identification of the pgmG gene, encoding a bifunctional protein with phosphoglucomutase and phosphomannomutase activities, in the gellan gum-producing strain Sphingomonas paucimobilis ATCC 31461. Appl Environ Microbiol 66:2252–2258
Sá-Correia I, Fialho AM, Videira P, Moreira LM, Marques AR, Albano H (2002) Gellan gum biosynthesis in Sphingomonas paucimobilis ATCC 31461: genes, enzymes and exopolysaccharide production engineering. J Ind Microbiol Biotechnol 29:170–176
Silva E, Marques AR, Fialho AM, Granja AT, Sá- Correia I (2005) Proteins encoded by Sphingomonas elodea ATCC 31461 rmlA and ugpG genes, involved in gellan gum biosynthesis, exhibit both dTDP- and UDP-glucose pyrophosphorylase activities. Appl Environ Microbiol 71:4703–4712
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Zhang J, Du GC, Zhang YP, Liao XY, Wang M, Li Y, Chen J (2010) Glutathione protects Lactobacillus sanfranciscensis against freeze-thawing, freeze-drying, and cold treatment. Appl Environ Microbiol 9:2989–2996
Mikhaleva NI, Santini CL, Giordano G, Nesmeyanova MA, Wu LF (1999) Requirement for phospholipids of the translocation of the trimethylamine N-oxide reductase through the Tat pathway in Escherichia coli. FEBS Lett 463:331–335
Sonesson A, Jantzen E, Bryn K, Tangen T, Eng J, Zahringer U (1994) Composition of 2, 3-dihydroxy fatty acid-containing lipopolysaccharides from Legionella israelensis, Legionella maceachernii and Legionella micdadei. Microbiology 140:1261–1271
Loh B, Grant C, Hancock REW (1984) Use of the fluorescent probe 1-n-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:546–551
Lehrer R, Barton A, Ganz T (1988) Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods 108:153–158
Kayali HA, Tarhan L (2004) The effect of glucose and maltose concentrations on pyruvate and ascorbate production, antioxidant enzyme activities and LPO levels in Fusarium equiseti. Process Biochem 39:1519–1524
Hua XF, Wang J, Wu ZJ, Zhang HX, Li HP, Xing XH, Liu Z (2010) A salt tolerant Enterobacter cloacae mutant for bioaugmentation of petroleum and salt-contaminated soil. Biochem Eng J 49:201–206
Li H, Xu H, Xu H, Li S, Ouyang PK (2010) Biosynthetic pathway of sugar nucleotides essential for welan gum production in Alcaligenes sp. CGMCC2428. Appl Microbiol Biotechnol 86:295–303
Zhang HT, Zhan XB, Zheng ZY, Wu JR, Nike E, Yu XB, Lin CC (2012) Improved curdlan fermentation process based on optimization of dissolved oxygen combined with pH control and metabolic characterization of Agrobacterium sp. ATCC 31749. Appl Microbiol Biotechnol 93:367–379
Mykytczuk NCS, Trevors JT, Leduc LG, Ferroni GD (2007) Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog Biophys Mol Biol 95:60–82
Rodriguez VS, Sanchez GA, Martinez RJM, Antonio PJ, Randez GF (2007) Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl Environ Microbiol 73:110–116
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta 1666:142–157
Eriksson M, Nielsen PE, Good L (2002) Cell permeabilization and uptake of antisense peptide–peptide nucleic acid (PNA) into Escherichia coli. J Biol Chem 277:7144–7147
Aguilera A, Peinado RA, Millan C, Ortega JM, Mauicio JC (2006) Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int J Food Microbiol 110:34–42
Lei JJ, Zhao XQ, Ge XM, Bai FW (2007) Ethanol tolerance and the variation of plasma membrane composition of yeast floc populations with different size distribution. J Biotechnol 131:270–275
Suwannakham S, Yang ST (2005) Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol Bioeng 91:325–327
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp Biochem Phys C 143:30–35
Cao B, Loh KC (2009) Physiological comparison of Pseudomonas putida between two growth phases during cometabolism of 4-chlorophenol in presence of phenol and glutamate: a proteomics approach. J Chem Technol Biotechnol 84:1178–1185
Acknowledgments
This work was supported by the National Basic Research Program of China (973) (2013CB733603), the National High Technology Research and Development Program of China (863) (No. 2013AA020301), the National Key Technology R&D Program (2011BAD23B04), the National Nature Science Foundation of China (No. 21106062) (No. 31371732), the Specialized Research Fund for the Doctoral Program of Higher Education (20113221130001), Graduate Student Innovation Project of Jiangsu Province (No. CXZZ13_0463).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, P., Chen, X., Li, S. et al. Screening and characterization of Sphingomonas sp. mutant for welan gum biosynthesis at an elevated temperature. Bioprocess Biosyst Eng 37, 1849–1858 (2014). https://doi.org/10.1007/s00449-014-1159-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1159-8