Abstract
As a discarded lignocellulosic biomass, chestnut shell is of great potential economic value, thus a sustainable strategy is needed and valuable for utilization of this resource. Herein, the feasibility of biological processes of chestnut shell with Dichomitus squalens, Phlebia radiata and their co-cultivation for lignin-modifying enzymes (LMEs) production and biodegradation of this lignocellulosic biomass was investigated under submerged cultivation. The treatment with D. squalens alone at 12 days gained the highest laccase activity (9.42 ± 0.73 U mg−1). Combined with the data of laccase and manganese peroxidase, oxalate and H2O2 were found to participate in chestnut shell degradation, accompanied by a rapid consumption of reducing sugar. Furthermore, specific surface area of chestnut shell was increased by 77.6–114.1 % with the selected fungi, and total pore volume was improved by 90.2 % with D. squalens. Meanwhile, the surface morphology was observably modified by this fungus. Overall, D. squalens was considered as a suitable fungus for degradation of chestnut shell and laccase production. The presence of LMEs, H2O2 and oxalate provided more understanding for decomposition of chestnut shell by the white-rot fungi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is composed of cellulose, hemicellulose and lignin. The prospects of utilization of this abundant renewable resource have stimulated research in biotechnology on the microbial degradation or modification of lignin by the relevant microorganisms and their enzymes. More attention has been recently devoted to the utilization of agronomic residues, also termed “lignocellulosic biomass”, which are the most potential and renewable biological resources. These feed stocks are widely employed in elimination of waste that represents a source of pollution, and production of useful by-products [1]. Chestnut shell, which is a common waste derived from food processing industry, has recently attracted much attention. This material has been used to prepare adsorbent to remove pesticides, copper, lead, cadmium and other heavy metal cations [2–4]. The extracting component of chestnut shell has been reported to have antioxidant activity and hepatoprotective effects [5, 6]. In addition, this lignocellulosic biomass was used to produce mono- and oligosaccharides [1]. Though reported above, it is uneasy to fulfill practical application owing to the rigid barrier of chestnut shell that contains a high proportion of lignin. Hereby, a green and sustainable process should be advocated in the utilization of chestnut shell biomass.
The known physical and chemical treatments of lignocelluloses biomass may exist some potential problems, like high-energy consumption and environmental pollution, thus economic and environmental benefits are subjected to restriction. Nevertheless, biological treatment is widely explored and considered as an alternative solution for utilization of biomass feedstocks [7, 8], especially pretreatment with the white-rot fungi. Since fungal lignin-modifying enzymes (LMEs), mainly including laccase (Lac), manganese peroxidase (MnP) and lignin peroxidase (LiP), play an important role in decomposition of complex lignin structure [9]. Biotreatment of chestnut shell with white-rot fungi should be a feasible and eco-friendly process, but only few works focus on this issue. The earlier study exhibited that co-cultivation of Dichomitus squalens and Phlebia radiata has been selected as a potential strategy in lignocellulolytic biodegradation with high enzyme activity relying on their synergistic effect [10]. Gómez et al. [11] also compared the potential of chestnut shell and barley bran for laccase production by Coriolopsis rigida under solid-state conditions. The above studies verify that microbial treatment is a considerable and low-cost approach for the utilization of this lignin-containing biomass.
Lignin-modifying enzymes (LMEs), such as Lac and MnP, whose molecular weight are >35 kDa, are difficult to pass through the micropore structure of complete timber [12]. Only if cell wall is deeply damaged, LMEs can play a full role in the degradation of lignocelluloses, accordingly, some auxiliary media of small molecules are required. In fungal degradation, oxalate might have multiple functions, including an inhibitor of lignin peroxidase, an electron donor of NADH, a source of superoxide, and a chelator for stabilizing manganic ions [13]. MnP was reported being capable of oxidizing oxalate to form extracellular hydrogen peroxide [14]. Moreover, reactive oxygen species (ROS) produced by Basidiomycetes remain unidentified in most cases, but hydroxyl radical (·OH) is likely a participant, which may be the major oxidant that initiate decay within the secondary wood cell wall due to the great destructiveness to lignocelluloses [15]. It improves the accessibility of ligninolytic enzymes in the internal structure by corroding the lignified cell wall. Extracellular formation of hydrogen peroxide (H2O2) is important not only for catalysis by LMEs, but also for ROS generation, including ·OH, superoxide and hydroperoxyls via Fenton reaction (H2O2 + Fe2+ + H+ → H2O + Fe3+ + ·OH) [9]. Besides, surface areas and pore size distribution of the processed chestnut shell reflect the characteristics of lignin degradation attacked by LMEs.
In this study, D. squalens and P. radiata were employed to comparatively investigate LMEs production of their co-culture and the respective monocultures in the submerged fermentation containing chestnut shell. And H2O2 in the culture broth was examined to assess the radical reaction in lignin decomposition. As well, low-molecular weight (LMW) organic acids were measured for analysis of their functional effects on LMEs. Furthermore, environmental scanning electron microscopy, surface area and pore size distribution of chestnut shell were, respectively, determined to evaluate the physical modification after biological processes.
Materials and methods
Chemicals and materials
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), veratryl alcohol (VA), and standard organic acids like succinic, malic, tartaric, itaconic, oxalic, fumaric and citric acid were purchased from Sigma Aldrich (St. Louis, MO, USA). Other chemicals were of analytical grade unless otherwise indicated.
Commercial chestnut fruits (Castanea mollissima Blume) were obtained from a local market in Hangzhou (China) and peeled manually. Chestnut shell was washed thoroughly with distilled water to remove the dirt, and dried in an air oven at 60 °C for 48 h, then was ground and sieved through a 40-mesh sieve.
Fungi and biotreatments of chestnut shell
Dichomitus squalens DSM 9615 and P. radiata DSM 5111, obtained from the Deutsche Sammulung von Microorganismen und Zellkulturen (Braunschweig, Germany), were maintained on potato dextrose agar (PDA) slants and stored at 4 °C. Before use, the fungi were cultured on PDA plates at 28 °C for 5–7 days. These fungi were inoculated with eight plugs (0.5 cm × 0.5 cm) in 250-mL Erlenmeyer flasks containing 40 mL medium with and without chestnut shell. The medium contained the following compositions (g L−1): 10.0 glucose, 0.5 NH4NO3, 0.8 KH2PO4, 0.2 Na2HPO4, 0.5 MgSO4·7H2O, 0.01 FeSO4·7H2O, 0.01 CuSO4·5H2O, 0.005 MnSO4, 0.001 CaCl2·2H2O, 0.001 ZnSO4·7H2O, 0.0005 thiamine, adjusting initial pH to 6.0.
1 % (w/v) chestnut shell was added into the culture medium and sterilized at 115 °C for 30 min before inoculation. After cooling to room temperature, each flask was then inoculated and incubated at 28 °C for 12 days under 150 rpm shaking speed at 28 °C in a dark chamber. The culturing flasks were plugged with sterile gauze, which allowed air exchange between the atmosphere and flask culture broth.
In this study, experimental designs were divided into three bioprocesses: (1) submerged fermentation grown with D. squalens DSM 9615 for 12 days (DS process), (2) submerged fermentation grown with P. radiata DSM 5111 for 12 days (PR process), (3) submerged fermentation grown with D. squalens and P. radiata for 12 days (DS+PR process). All three experimental groups were supplemented with chestnut shell, and the opposite groups without chestnut shell served as the control. Each group was performed in triplicate. Two milliliters of supernatant from the fermentation broth was removed to determine physiological and biochemical indexes.
Determination of protein, reduced sugar and H2O2
Protein concentration was determined using the Bradford protein quantitative kit (Tiangen, Beijing, China) [16]. H2O2 quantitative assay kit (water compatible; Sangon Biotech, Shanghai, China) was used for analyses of hydrogen peroxide produced [17]. Reducing sugar in fermentation broths was determined using the 3, 5-dinitrosalicylic acid colorimetric method [18].
Enzymes assay
The supernatants of fungal cultures were collected and assayed for LMEs activity at 25 °C after centrifugation at 18,000g for 5 min at 4 °C. Laccase activity was determined by oxidation of 0.5 mM ABTS in 100 mM sodium acetate (pH 4.5) at 420 nm [19]. Lignin peroxidase activity was measured by oxidation of 2.0 mM VA buffered with 100 mM sodium tartrate (pH 3.0) at 310 nm [20], and manganese peroxidase activity was assayed by following the formation of Mn3+–tartrate complex in 100 mM sodium tartrate (pH 4.5) in the presence of 0.5 mM MnSO4, at 238 nm [21]. Peroxidase reactions were started by addition of 0.1 mM H2O2. One unit of enzyme activity was defined as 1 μmol product formed per min, and the specific activity was expressed as units of enzyme activity per milligram of extracellular protein at 25 °C.
Gradient ion chromatography measurements
Organic acids including succinic, malic, tartaric, itaconic, oxalic, fumaric and citric acid were quantified by a Dionex ICS-3000 ion chromatography system (Dionex, Sunnyvale, CA, USA), equipped with an IonPac AS11-HC analytical column (4 × 250 mm), an AG11-HC guard column (4 × 50 mm), and a suppressed conductivity detector. The suppressor current was set to 50 mA, and the detector cell temperature was held at 30 °C. Sodium hydroxide (NaOH) solution was used as the eluent at a constant flow rate of 0.2 mL min−1. A multistep gradient was used for all separations with an initial injection volume of 10 μL. The gradient procedures were as follows: 25 mM from 0 to 27 min, 25–50 mM from 27 to 30 min, 50 mM from 30 to 35 min, 50–25 mM from 35 to 42 min, and 25 mM from 42 to 50 min. Furthermore, inorganic anions, such as Cl−, NO3 −, SO4 2−, H2PO4 − were simultaneously detected to remove interferences on determination of organic anions. After centrifuged for 10 min at 18,000g at 4 °C, the samples were injected through 0.22 μm Millipore filter before detection by gradient ion chromatography.
Environmental scanning electron microscopic analysis
The surface microtopography of all samples with different bioprocesses was investigated using the environmental scanning electron microscopic analysis (ESEM). The centrifuged chestnut shell was first fixed by immersion in 2.5 % (v/v) glutaraldehyde solution overnight and washed thoroughly in 0.1 M phosphate buffer (pH7.0) for three times, followed by fixing for a further 1–2 h in 1 % (v/v) osmic acid solution. Then it was washed thoroughly again and dehydrated using graded ethanol (50, 70, 80, 90, 95, and 100 % in twice) and 100 % isoamyl acetate. All samples were treated by the critical point drying with liquid CO2 and coated with gold in a sputter coater before microscopic examination. The ESEM images of untreated and treated materials were obtained using a Philips XL-30 ESEM (Philips Company, Amsterdam, and The Netherlands) operating at 20 kV.
Surface area and porosity examinations
The bioprocessed chestnut shell was collected by centrifugation, and washed thoroughly with distilled water. Then moisture and atmospheric gas were removed from the samples by application of vacuum and heat below 80 °C. The textural properties of the samples were obtained by performing nitrogen sorption measurements using Quantachrome Autosorb-1-C high performance surface area and pore size analyzer (Quantachrome Instruments, Florida, USA), and analyzed with Autosorb-1 program. Specific surface area was determined by the Brunauer–Emmett–Teller (BET) method, and pore size distribution was calculated using the Barret–Joyner–Halendar (BJH) method [22]. In these experiments, chestnut shell processed in same procedure without the fungi served as the control.
Statistical analysis
All tests were conducted in triplicate; the results are given as mean value ± standard deviation (SD). Trends were considered as significant when the mean values of compared sets were different at P < 0.05.
Results and discussion
Comparison of different microbial treatments on laccase production
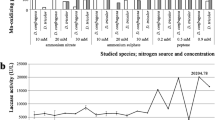
Dichomitus squalens and P. radiata were selected as the fungi for laccase production; the monocultures and co-culture of these fungi were comparatively designed in this study. As presented in Fig. 1, laccase production was evaluated at different culture times under the submerged fermentation with and without chestnut shell. Laccase activities in control groups without chestnut shell decreased significantly from culture time 3–12 days (Fig. 1a), while the production of laccase was dramatically improved in chestnut shell-containing groups cultivated with two white-rot fungi (Fig. 1b). In fact, the structure and composition of different agricultural residues have important influences for the utilization of lignocellulosic materials and LMEs production [10, 23]. Chestnut shell was proven to be a potential substrate and inducer for laccase production by the white-rot fungi owing to its special structure and high proportion of lignin (>30 %). As clearly shown in fig. 1, the improved laccase was greatly attributed by the presence of this substrate, especially for maintaining stable maximal production.
Mixed fungal cultivation appears to have advantages over monocultures in considering the synergistic effect [10, 23, 24], but it depended on interactions between species, additional feedstock and the micro-environmental or nutritional conditions. In this work, monocultures showed significantly more laccase formation than co-cultures of D. squalens and P. radiata containing chestnut shell. In fig. 1b, the biological process with D. squalens in chestnut shell-containing culture obtained the highest level of laccase production of 9.42 ± 0.73 U mg−1 at the 12th day, which was 6–15 times higher than other biological processes. It had a potential for improved laccase production after 12 days. As well, the monoculture of P. radiata gained the highest laccase activity of 3.03 ± 0.65 U mg−1 on day 9, which was still less than half of the corresponding activity of DS process with chestnut shell. In contrast, a high laccase activity was detected in co-cultivation of D. squalens and P. radiata at the third day when cultured without chestnut shell, but laccase activities of all inoculating approaches in control groups showed a huge decline from day 3 to day 12 (Fig. 1a).
In chestnut shell-containing cultures, P. radiata seemingly had a negative effect on co-cultivation with D. squalens for laccase production under the condition of this experiment, although its monoculture exhibited higher enzyme activity than mixed fungal cultures. In contrast, D. squalens was proven to be a better fungus than P. radiata for the utilization of chestnut shell. Interestingly, our previous works indicated that there was a synergistic effect on production of laccase through submerged co-cultivation of P. radiata and D. squalens [10]. Considering the significant change of laccase activity in DS+PR group between submerged broths with and without chestnut shell, it indicated that the synergistic effect between D. squalens and P. radiata was possibly inhibited in the culture containing chestnut shell. However, the exact mechanism underlies required future elucidation.
Comparison of different microbial treatments on peroxidase production
MnP and LiP are two important fungal class II peroxidases in lignin biodegradation [25]. Most of the white-rot fungi could form MnP enzyme [26], also including D. squalens and P. radiata [27]. MnP activity was reduced in control groups from day 3 to day 12, but enhanced in the fermentation cultures containing chestnut shell mainly after 3 days cultivation (Fig. 2), which was similar to laccase production. MnP reached the highest level at the 9th day in experimental groups, after which MnP started to decrease for DS and PR process. Obviously, MnP activities in chestnut shell-containing groups were far below all groups without this substrate at the third day (Fig. 2b). Hereby, chestnut shell is not a desirable substrate for MnP production under this condition.
Based on the whole genome sequence of D. squalens [25], it verified that this fungus does not possess any LiP-encoding genes. But three LiP-encoding genes found were presented in white-rot fungus P. radiata [28], and LiP produced by P. radiata was found in batch and semi-continuous cultivations [29]. Surprisingly, no LiP activity was measured in the fermentation broths of all bioprocesses with D. squalens and P. radiata in this investigation, which was in accordance with the previous finding when cultured with agricultural biomass, such as corn stalk, corncob, wheat straw, and rice stalk [10]. The present work inferred that LiP production by P. radiata was possibly inhibited in the submerged fermentation.
Change of residual reduced sugar, hydrogen peroxide and oxalate involved in the degradation of chestnut shell
In the cultures containing chestnut shell, D. squalens and P. radiata produced laccase and MnP throughout the cultivation process. It was clear that the biosynthesis of abundant laccase primarily occurred at the latter stage of culture (from day 9 to day 12). LMEs were affected by nutrition condition of carbon sources [10, 27], meanwhile, reducing sugar consumption was related to fungal growth [30]. To trace the change of available carbon sources consumed by the white-rot fungi, we examined the concentration of residual reduced sugar in fermentation broths. As shown in Fig. 3, the consumption rate of reduced sugar in the control group was faster than the groups cultured with chestnut shell, especially, in the groups inoculated with D. squalens (such as DS, DS+PR). D. squalens gave rise to a steeper consumption of carbon source in cultures without chestnut shell. Possibly, sugar formation derived from the presence of chestnut shell in the culture medium by the degradation of the white-rot fungus was decelerated (Fig. 3b). An accurate correlation between microbial growth, substrate consumption and laccase synthesis was revealed in the submerged non-homogeneous broths [31]. The reduced production of laccase and MnP in the control culture after 3 days was likely due to the decreased available carbon nutrients. Decomposition of chestnut shell compensated for consumable carbon source, which favored the sustainable production of LMEs. In control culture, laccase production was reduced after 3 days instead of being constant, because of lack of substrate induction.
In terms of antioxidant effect of chestnut shell extract [6] and H2O2-involved in MnP activity [26], hereby, the examination of the content of H2O2 is helpful to reveal the inhibitory effect on MnP production by D. squalens and P. radiata in the submerged culture containing chestnut shell. Moreover, H2O2 is well-known participated in the lignin-containing biomass degradation by the white-rot fungi. Thus, H2O2 was comparatively examined in this work. Results presented in Fig. 4 revealed that H2O2 concentration in chestnut shell-containing submerged cultures with three bioprocesses increased quickly at earlier stage, and reached to the peak value on day 9, then started to decrease after 9th day. Interestingly, the specific activity of MnP reached to the peak value just on that culture time (Fig. 4b). Particularly, the concentration of H2O2 in the culture medium pre-treated by D. squalens alone was always higher than other experimental groups from day 3 to day 12 (Fig. 4a1, b1). As a primary donor, the content of H2O2 partly reflects the situation of free radical generation in broths. In submerged fermentation, LMEs were generated and secreted into the broths; free radical reaction was strengthened quickly. H2O2 production strongly correlated with laccase production. Likely, the free radical attacks triggered by laccase provided a possible source of H2O2. This synergetic effect was used in utilization of hemicellulase for improvement of teak veneer surface color [32]. Simultaneously, H2O2 can initiate enzyme activity of MnP and accelerate the biodelignification of chestnut shell.
Comparison of laccase (Lac) specific activity, MnP specific activity, hydrogen peroxide and oxalate concentration in submerged broths. Lac specific activity, H2O2 and oxalate concentration of DS (1), PR (2), and DS+PR (3) groups using chestnut shell as the substrate were compared in a; MnP specific activity, H2O2, and oxalate concentration of DS (1), PR (2), and DS+PR (3) groups were compared in b. Each data point represents mean ± SD for three independent treatments
Lignin biodegradation is a complex oxidative–reductive process that involves the action of a multienzyme system and the mediation of some small molecules. As been reported, the white-rot fungi also secrete a number of low-molecular mass compounds including oxalic and glyoxylic acids in submerged cultures, oxalic acid during solid-state fermentation of wheat straw and several fatty acids during solid-state fermentation of wood [33]. Therefore, we conducted the determination of low-molecular weight acids among three bioprocesses (Fig. 4a–b) in this work. Among the examined organic and inorganic acids, only oxalate and inorganic anions (Cl−, NO3 −, SO4 2−, and H2PO4 −) were detected by gradient ion chromatography. Other organic acids were observed without detectable quantity, which include succinic, malic, tartaric, itaconic, fumaric and citric acid. As shown in Fig. 4a–b, mass concentration of oxalate rapidly declined at an initial stage of fermentation time from day 3 to day 6, and then fell slowly, even slightly rebounded. Oxalic acid is produced by a wide variety of fungi, including brown-rots, white-rots, mycorrhizae, plant pathogens, and Aspergillus niger. The regulating role played in lignin-containing substrate degradation was specifies-dependent.
Until present, low-molecular weight organic acids were known to involve in lignin degradation [12]. As a major organic acid produced by D. squalens and P. radiata, oxalate was synthesized and secreted to extracellular medium in early stage. It was not accumulated but rather decomposed into carbon dioxide and formate anion radical (CO ·−2 ), and further oxidized to CO2 and superoxide anion radicals (O ·−2 or HOO·) under aerobic conditions [12]. Furthermore, the synthesis and secretion of oxalate at early stage provided glucose metabolism in the submerged fermentation with NADH [34]. Simultaneously, it was an H2O2-dependent reaction to oxidize Mn2+ to Mn3+ by MnP, thus, an organic chelator was needed to stabilize the formation of Mn3+ [35]. The cleavage of oxalate resulted in formation of superoxide, and led to the increase of H2O2, and more oxalate was oxidized to form H2O2 along with the production of MnP, thereby, MnP could be independent of H2O2 in the presence of oxalate. These findings also confirmed the viewpoints on lignin conversion by MnP overviewed by Hofrichter [26]. In general, the strong negative correlation with concentration of H2O2, Lac, and MnP activity demonstrated that oxalate probably plays a key role in LMEs production and biological degradation of chestnut shell.
Surface morphology and pore size distribution of submerged-treated chestnut shell
The activated carbon prepared from chestnut shell and grapeseed was capable as an effective adsorbent for the removal of copper ions from aqueous solutions [4]. The physical structure of chestnut shell should be paid more attention when endured to biological treatments. In this work, we conducted the biomass textural examination and comparison of chestnut shell with and without biotreatments by the selected white-rot fungi. The high magnification images of biomass obtained through ESEM were shown in Fig. 5, which help to visualize the clear surface morphological differences. The compact structures were compared in Fig. 5a–d, a′–d′ at two levels of magnification, and show the exposure of cell lumen with an approximate 10–30 μm diameter and cell wall pits with approximate 1–3 μm diameter (noted by arrows) in the treated substrate belonging to DS group more clearly as compared to other groups (Fig. 5a′–d′). Zeng et al. [7] also discovered that biological pretreatment by Phanerochaete chrysosporium gave rise to the exposure of cellulose microfibrils by scanning electron microscopic (ESM) analysis, and the apparent structure of wheat straw was visibly changed by the fungus. The microscopic analysis in this study revealed that the weak part of solid tissue was easily attacked by extracellular lignin-modifying enzymes (especially MnP or Lac). However, long pretreatment time was generally needed to achieve substantial lignin degradation [8]. Consequently limited biodegradation was observed in the compact structure of chestnut shell after 12 days treatment, that likely due to sclerenchyma tissue of cutin and much more recalcitrant epidermis tissue making this structure resistant to enzymes. In this work, D. squalens was found to demonstrate a better biodegradability on the weak part of chestnut shell.
ESEM images of the surface structure of chestnut shell samples. a–d are the compact structures of control chestnut shell (a), DS group (b), PR group (c), and PR+DS group (d) visualized at ×500 resolution, respectively; a′–d′ are compact structures of the control (a′), DS (b′), PR (c′), and PR+DS group (d′), respectively visualized at ×2,000 resolution. The control group is untreated chestnut shell (a, a′)
Though ESEM examination showed the change of outer structure of chestnut shell, it is still awaiting resolution whether the inner structure or physical property of chestnut shell had changed. As a consequence, specific surface areas of chestnut shell under the treatment of three processes at 12 days were compared by multipoint BET analysis (Table 1). Results revealed that specific surface area in the microbial treatments was far higher than the control group without any treatments. The highest multipoint BET surface area was found to be 1.216 m2 g−1 samples in DS group, which was more than twice the untreated sample. Physical properties of the untreated and the processed samples by D. squalens were shown in Table 2, revealing that specific surface area and total pore volume were efficiently improved by means of biological treatment with D. squalens. Besides, the average pore diameter of DS group was smaller than the control. In addition to the change of surface area, the absorption property should be considered as well. The nitrogen adsorption–desorption isotherms of DS group were provided in Fig. 6. The pore size distribution calculated by BJH method using desorption branch showed two peaks at 17.1 and 1.6 nm of diameter, respectively. Many mesopores below 150 nm in diameter were clearly observed. The pore size distribution was indicative of plenty of small mesopores in diameter below 150 nm formed, which means that biological treatment by fungus D. squalens actually results in the destruction of the complete structure of chestnut shell.
The ESEM data indicated that biological treatments by the selected white-rot fungi actually led to the destruction of chestnut shell. The improvement of BET surface area greatly attributed to lignin decomposition of chestnut shell by the white-rot fungi. Considering that higher laccase activity produced by D. squalens alone at 12 days, it was more aggressive for the removal and/or modification of lignin from this biomass making the accessible surface for hemicellulose and cellulose. The physical characteristics of chestnut shell, especially processed with D. squalens, were vastly modified, which relied on the radical attacks stemmed from Lac and MnP. In recent times, agricultural waste products like coconut shell and chestnut shell were often used as adsorbent to remove pesticides and metal ions in polluted water [2, 36]. Herein, the bioprocess with D. squalens provides an auxiliary method for physicochemical treatment of chestnut shell, that is also a sustainable strategy in biological process of this biomass for laccase production, which is an eco-friendly enzyme with a great potential in industrial application [9, 23]. Further researches are needed in achieving higher yield of laccase, and adsorption material development with the aim of treating pesticides and heavy metal cations.
Conclusions
In summary, biological treatment with D. squalens gave the highest laccase activity with a maximum value of 9.42 ± 0.73 U mg−1 in submerged fermentation. This bioprocess was proven to be more efficient in degradation of chestnut shell, and the surface microstructure of this biomass was obviously modified by the fungus when compared with the control. Laccase, MnP, H2O2 and oxalate were all confirmed to participate in this process. These findings provided us with more understanding for limited knowledge on biodegradation of chestnut shell, and proposed a potential approach for commercial exploitation of this feedstock.
References
Morana A, Maurelli L, Ionata E, Rossi M, La Cara F (2010) Chestnut shell: not only a source of antioxidant compounds. J Biotechnol 150:S325
Vázquez G, Mosquera O, Freire MS, Antorrena G, González-Alvarez J (2012) Alkaline pre-treatment of waste chestnut shell from a food industry to enhance cadmium, copper, lead and zinc ions removal. Chem Eng J 184:147–155
Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater 174:137–143
Özçimen D, Ersoy-Meriçboyu A (2009) Removal of copper from aqueous solutions by adsorption onto chestnut shell and grapeseed activated carbons. J Hazard Mater 168:1118–1125
Noh JR, Kim YH, Gang GT, Hwang JH, Lee HS, Ly SY, Oh WK, Song KS, Lee CH (2011) Hepatoprotective effects of chestnut (Castanea crenata) inner shell extract against chronic ethanol-induced oxidative stress in C57BL/6 mice. Food Chem Toxicol 49:1537–1543
Vázquez G, Fontenla E, Santos J, Freire MS, González-Álvarez J, Antorrena G (2008) Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crop Prod 28:279–285
Zeng JJ, Singh D, Chen SL (2011) Biological pretreatment of wheat straw by Phanerochaete chrysosporium supplemented with inorganic salts. Bioresour Technol 102:3206–3214
Wan CX, Li YB (2011) Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresour Technol 102:7507–7512
Lundell TK, Mäkelä MR, Hildén K (2010) Lignin-modifying enzymes in filamentous basidiomycetes—ecological, functional and phylogenetic review. J Basic Microbiol 50:5–20
Dong YC, Wang W, Hu ZC, Fu ML, Chen QH (2012) The synergistic effect on production of lignin-modifying enzymes through submerged co-cultivation of Phlebia radiata, Dichomitus squalens and Ceriporiopsis subvermispora using agricultural residues. Bioprocess Biosyst Eng 35:751–760
Gómez J, Pazos M, Couto SR, Sanromán MA (2005) Chestnut shell and barley bran as potential substrates for laccase production by Coriolopsis rigida under solid-state conditions. J Food Eng 68:315–319
ten Have R, Teunissen PJM (2001) Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem Rev 101:3397–3413
Shimada M, Akamtsu Y, Tokimatsu T, Mii K, Hattori T (1997) Possible biochemical roles of oxalic acid as a low molecular weight compound involved in brown-rot and white-rot wood decays. J Biotechnol 53:103–113
Li NJ, Zeng GM, Huang DL, Hu S, Feng CL, Zhao MH, Lai C, Huang C, Wei Z, Xie GX (2011) Oxalate production at different initial Pb2+ concentrations and the influence of oxalate during solid-state fermentation of straw with Phanerochaete chrysosporium. Bioresour Technol 102:8137–8142
Aguirre J, Ríos-Momberg M, Hewitt D, Hansberg W (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol 13:111–118
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Qiu JG, Ma Y, Wen YZ, Chen LS, Wu LF, Liu WP (2012) Functional identification of two novel genes from Pseudomonas sp. strain HZN6 involved in the catabolism of nicotine. Appl Environ Microbiol 78:2154–2160
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Liu W, Chao Y, Liu S, Bao H, Qian S (2003) Molecular cloning and characterization of a laccase gene from the basidiomycete Fome lignosus and expression in Pichia pastoris. Appl Microbiol Biotechnol 63:174–181
Johansson T, Nyman PO (1987) A manganese (II)-dependent extracellular peroxidase from the white-rot fungus Trametes versicolor. Acta Chem Scand B 41:762–765
Ruiz-Dueñas FJ, Martínez MJ, Martínez AT (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol 31:223–235
Chen ZM, Chen BL, Zhou DD, Chen WY (2012) Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ Sci Technol 46:12476–12483
Zhang H, Hong YZ, Xiao YZ, Yuan J, Tu XM, Zhang XQ (2006) Efficient production of laccases by Trametes sp. AH28-2 in cocultivation with a Trichoderma strain. Appl Microbiol Biot 73:89–94
Chi YJ, Hatakka A, Maijala P (2007) Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int Biodeterior Biodegrad 59:32–39
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, John FS, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzym Microb Technol 30:454–466
Chen QH, Krügener S, Hirth T, Rupp S, Zibek S (2011) Co-cultured production of lignin-modifying enzymes with white-rot fungi. Appl Biochem Biotechnol 165:700–718
Hildén KS, Mäkelä MR, Hakala TK, Hatakka A, Lundell T (2006) Expression on wood, molecular cloning and characterization of three lignin peroxidase (LiP) encoding genes of the white rot fungus Phlebia radiata. Curr Genet 49:97–105
Kantelinen A, Hatakka A, Viikari L (1989) Production of lignin peroxidase and laccase by Phlebia radiata. Appl Microbiol Biotechnol 31:234–239
Aggelis G, Iconomou D, Christou M, Bokas D, Kotzailias S, Christou G, Tsagou V, Papanikolaou S (2003) Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res 37:3897–3904
Hou HM, Zhou JT, Wang J, Du CH, Yan B (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39:1415–1419
Iamtasna B, Piyasombatkul T, Prichanont S, Muangnapoh C (2010) Use of hemicellulase in sequence with hydrogen peroxide and laccase for improvement of teak veneer surface color. J Wood Sci 56:184–188
Tanaka H, Koike K, Itakura S, Enoki A (2009) Degradation of wood and enzyme production by Ceriporiopsis subvermispora. Enzym Microb Technol 45:384–390
Pinzari F, Zotti M, De Mico A, Calvini P (2010) Biodegradation of inorganic components in paper documents: formation of calcium oxalate crystals as a consequence of Aspergillus terreus Thom growth. Int Biodeterior Biodegrad 64:499–505
Hakala TK, Lundell T, Galkin S, Maijala P, Kalkkinen N, Hatakka A (2005) Manganese peroxidases, laccases and oxalic acid from the selective white-rot fungus Physisporinus rivulosus grown on spruce wood chips. Enzym Microb Technol 36:461–468
Okafor PC, Okon PU, Daniel EF, Ebenso EE (2012) Adsorption capacity of coconut (Cocos nucifera L.) shell for lead, copper, cadmium and arsenic from aqueous solutions. Int J Electrochem Sci 7:12354–12369
Acknowledgments
We thank Wei Wang (Institute of Quality and Standard for Agriculture Products, Hangzhou, China) for technical assistance in ion chromatography measurements. This work was financially supported by National Natural Science Foundation of China (31171734) and supported by the Program for Zhejiang Leading Team of S&T Innovation (2010R50032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, YC., Dai, YN., Xu, TY. et al. Biodegradation of chestnut shell and lignin-modifying enzymes production by the white-rot fungi Dichomitus squalens, Phlebia radiata . Bioprocess Biosyst Eng 37, 755–764 (2014). https://doi.org/10.1007/s00449-013-1045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1045-9