Abstract
A conventional process for ethanol production involving liquefaction followed by simultaneous saccharification and fermentation (SSF) under the yeast fermentation conditions, was investigated at 30 and 35% dry solid (DS) of Indian broken rice and pearl millet feedstocks. The study followed the typical conventional process currently in use by the Indian Ethanol Industry. Liquefaction was carried out using a thermostable alpha amylase, and whereas SSF with a glucoamylase with additional side activities of pullulanase and protease under the yeast fermentation conditions. To measure the enzyme efficacy in the liquefaction process, fermentable sugar and liquefact solubility (brix) were monitored at the end of the liquefaction process. The liquefact was subjected to SSF with yeast. Addition of an acid fungal protease at a concentration of 0.1 kg per metric ton of grain during SSF was observed to accelerate yeast growth and ultimately, ethanol yield with both feedstocks. With both concentrations of feedstocks, the fermentation efficiency and ethanol recovery were determined. This study assesses the potential of these enzymes for ethanol production with higher dry solid concentration (≥30% w/w DS) of both these feedstocks in the conventional process to achieve higher plant throughput without compromising fermentation efficiency and ethanol recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common biofuel is bioethanol, accounting for more than 90% of total biofuel usage. Conventional production involves a widely used process based on enzymatic conversion of starchy biomass into sugars, and/or fermentation of 6-carbon sugars followed by final distillation of ethanol to fuel grade. Considerable attention has been given to the first stage of ethanol production from various sugar substrates such as molasses or sugar cane juice [1]; starchy materials like rice, millet, corn, sorghum, wheat, potato and cassava [2]. The simultaneous yield of by-products (e.g., animal feed) from this ethanol production process reduces the net production cost of ethanol (http://www.iea.org/Textbase/techno/essentials.htm). Pearl millet, broken rice and sorghum are the major starchy materials used by Indian ethanol producers for producing potable alcohol [3] and biofuel (http://www.icrisat.org/text/research/grep/homepage/sgmm/chapter12.pdf). Indian ethanol producers use these raw materials based on availability and cost since these are seasonal grains [3, 4].
Increasing price of crude oil and other fossil fuels have stoked interest in alternative fuel sources around the world [5, 6]. Fuel alcohol production from starch needs constant process improvement in the biomass conversion to fuel alcohol for making it economically viable. The emerging very high gravity (VHG) fermentation technology is one such measure to increase the fermentation rate and concentration of ethanol, and to minimize waste effluent [7]. VHG technology is now commonly used to increase concentration of starchy grains in the feedstock (≥30% w/w DS) and to increase plant throughput [8].
Most biofuel production processes involve conversion of grain starch to fermentable sugar followed by conversion into ethanol. It consists of three steps, starch liquefaction (80–125 °C), saccharification (55–65 °C) and sugar fermentation (32–35 °C) to ethanol [9]. Recent technological improvements have eliminated one enzymatic step of a separate process of saccharification at 55–65 °C, which avoids high osmolarity stress in the initial stage of yeast fermentation and reduces the risk of contamination during VHG fermentation technology [8]. The improved biological process now involves liquefaction and SSF (simultaneous saccharification and fermentation). The latter process involves the saccharifying enzyme hydrolyzing the liquefied starch into fermentable sugars under yeast fermentation conditions with simultaneous fermentation of sugars to ethanol [10].
Saccharomyces cerevisiae is a yeast species that can produce high concentrations of ethanol and tolerate these high concentrations without detriment to its own growth under appropriate environmental and nutritional conditions [11]. Its growth is supported and prolonged when assimilable nitrogen levels are maintained at an adequate level along with very low oxygen levels during VHG fermentation [12]. It reduces the costs of distillation, water, labor and increases alcohol production at any given plant capacity [11]. Concurrently VHG technology lowers energy cost per liter of alcohol, bacterial contamination risk, and capital costs. In addition, an increased harvest of high protein-spent yeast is achieved during VHG fermentation [7]. VHG fermentation technology using wheat mash, sugarcane juice and molasses resulted in production of up to 23% (v/v) alcohol in batch fermentations [13]. While production of fuel alcohol is highest with sugarcane [14], the quest for applying VHG technology to maximize ethanol yield from other substrates continues.
In addition to fermentable sugars yeast-based ethanol production requires other nutrients [free amino nitrogen (FAN), peptides, amino acids, vitamins and minerals such as inositols, zinc and magnesium] to maintain yeast growth to maintain the rate of glucose conversion to ethanol. Nitrogen deficiency retards yeast growth and metabolism, resulting in arrested sluggish fermentation [15–18]. Nutritional supplements, usually inorganic nitrogen such as ammonium salts, are added to the medium prior to fermentation [19, 20], currently being done arbitrarily without monitoring the nitrogen content of the medium and without knowledge of the optimum nitrogen concentrations for yeast growth [21].
The objective of the present study was to evaluate the conventional process of ethanol production from Indian broken rice and pearl millet feedstocks with 30 and 35% dry solid (DS). The comparative impact of acid fungal protease (FERMGEN™) in 30 and 35% DS feedstocks on yeast fermentation was studied.
Materials and methods
Enzymes, reagent and chemicals
SPEZYME® ALPHA [alpha amylase, minimum activity 13,755 AAU (alpha amylase unit)/gm, enzyme activity is determined by the rate of starch hydrolysis measured by decrease in iodine-staining capacity. One AAU of bacterial α-amylase activity is the amount of enzyme required to hydrolyze 10 mg of starch per minute under specified conditions, http://www.genencor.com]; DISTILLASE® ASP [glucoamylase, minimum activity 580 GAU (glucoamylase unit)/gm, one GAU is the amount of enzyme that liberates 1 g of reducing sugars measured as glucose per hour from soluble starch substrate under specified assay conditions, http://www.genencor.com]; FERMGEN™ [acid fungal protease, activity minimum 1,000 SAPU (spectrophotometric acid protease units)/gm, protease activity is expressed in SAPU, one SAPU is the amount of enzyme that liberates 1 μm of tyrosine per minute from a casein substrate under specified assay conditions, http://www.genencor.com]; SPEZYME® FRED [alpha amylase, activity minimum 17,400 LU (liquefon unit)/gm, one LU is digestion time required to produce a color change with iodine solution, indicating a defined stage of dextrinization of starch substrate under the specified conditions, http://www.genencor.com]; and OPTIDEX® L-400 (glucoamylase, activity minimum 350 GAU/gm, one GAU is the amount of enzyme that liberates 1 g of reducing sugars per hour measured as glucose liberated from a soluble starch substrate under the specified assay conditions, http://www.genencor.com). The above enzymes were obtained from Genencor, a Danisco Division. Active Dry Yeast was purchased from AB Mauri India Pvt. Ltd., (MIDC-415 722, India) and urea from Merck (ML7M573074; 60848605001730). Industrial grade Indian broken rice and pearl millet grains were purchased from the local market.
Milling of Indian broken rice and pearl millet
Indian broken rice and pearl millet were milled using laboratory milling (Milcent, Anand, Gujarat-India). Sieve analysis showed that 92–94% of the resulting flour particles passed through US standard 20 mesh-sieves.
Soluble glucose content analysis
Soluble glucose in the flour was extracted into water by dissolving 1.0 g (dry basis) in 99 ml of water and agitating it for 1 h at ambient temperature. The sample was analyzed by HPLC (Agilent Isocratic system 1200, USA) on an Aminex Column HPX-87H (catalog number 1250140, Bio-Rad) at 60 °C with a mobile phase of 0.01 N sulfuric acid at a flow rate of 0.7 ml/min. It was compared with the standard containing glucose (0.5%) to identify and quantify the soluble glucose content analysis.
Starch content analysis
The grain flour were milled so that <10% of particles retained on US 40-mesh sieve. The grain flour was hydrolyzed (liquefied) with alpha amylase, SPEZYME® FRED, followed by saccharification with glucoamylase, OPTIDEX® L-400 [22]. The resulting product was analyzed for glucose using HPLC (Agilent Isocratic system 1200, USA) as described in “Soluble glucose content analysis”.
where, E t is enzyme-treated sample and W e is water-extracted sample.
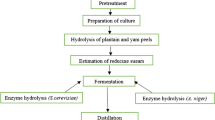
Slurry liquefaction process
Each slurry of 30 and 35% DS was prepared in a 1-l flask separately by adding RO water adjusting its pH to 5.8 using 6 N sulfuric acid. The slurry was subjected to three different liquefaction temperatures as follows: (1) 60 ± 2 °C for 20 min with 30% dosage of 0.6 kg of SPEZYME® ALPHA per MT of starch; (2) jet-cooking process at 108 ± 2 °C for 10 min; and (3) 90 ± 2 °C for 90 min with 70% dosage of 0.6 kg of SPEZYME® ALPHA per MT of starch. The latter is the most typical liquefaction process used in Indian ethanol industries (Fig. 1). At the end of each liquefaction process, the liquefact solubility was analyzed using a refractometer. The fermentable sugar concentration (%) was analyzed by HPLC (Agilent Isocratic system 1200, USA) on an Aminex Column HPX-87H (catalog number 1250140, Bio-Rad) at 60 °C with a mobile phase of 0.01 N sulfuric acid at a flow rate of 0.7 ml/min. A standard containing glucose (0.5%); fructose (0.5%) and maltose (0.5%) was used. The fermentable sugar (%) was calculated as follows:
SSF under yeast fermentation conditions
At end of the liquefaction process, the broken rice and pearl millet liquefacts of 30 and 35% DS were cooled to 32 ± 2 °C from 90 ± 2 °C and subjected to SSF with yeast (Fig. 1), after adding DISTILLASE® ASP of 0.8 kg per MT of starch; urea, 400 ppm; and propagated yeast (6% inoculum of 0.85 × 108 CFU/ml). To assess the importance of FERMGEN™ (proteases) in the yeast fermentation process, SSF was carried out with and without FERMGEN™, 0.1 kg per MT of grain. The flask was covered with a sterile plug and incubated at 32 ± 2 °C in a rotary shaker at 300 rpm. Samples of fermentation slurry were collected at 0, 22, 44, and 55 h of yeast fermentation for assessing ethanol production (% v/v at 20 °C), sugar profile (glucose, fructose, maltose, maltotriose and high saccharides, all % w/v). All analyses were carried out using HPLC (Agilent Isocratic system 1200, USA) on an Aminex Column HPX-87H (catalog number 1250140, Bio-Rad) at 60 °C with a mobile phase of 0.01 N sulfuric acid at a flow rate of 0.7 ml/min. The HPLC was calibrated with standards containing all the above-mentioned items of interest at known concentrations at the beginning of each sample batch. Calibration was verified with a secondary standard after every ten samples and at the end of the batch. The yeast cell counts were also monitored through microscopy.
Ethanol yield, residual starch and sugar analysis
The fermentation slurry was distilled at 80 °C using Soxhlet’s apparatus (Ambassader; B.P. Industries, Delhi-India) in the 55 h of yeast fermentation process. The distilled ethanol (% v/v at 20 °C) was measured using an alcometer. At the same time, residual sugar in the fermented slurry was estimated by the HPLC method as described in “Soluble glucose content analysis”. Residual starch was determined using an enzymatic method with alpha amylase, SPEZYME® FRED and glucoamylase, OPTIDEX® L-400 for the liquefaction and saccharification processes, respectively [22]. Again glucose produced was also estimated by the HPLC method as described in “Soluble glucose content analysis”.
Ethanol recovery and fermentation efficiency
After laboratory-based distillation of the fermented slurry, ethanol recovery (liter per MT of grain), and fermentation efficiency (%) were calculated as follows:
All experiments were done in triplicate and statistical results are presented using Analysis of variance (ANOVA).
Results and discussion
Fermentable sugar production from Indian broken rice and pearl millet
Identifying the initial starch content of substrates (Indian broken rice and pearl millet) is an essential prerequisite to evaluate the yeast fermentation efficiency from starch to ethanol production. The study samples had starch content (%, dry basis) of 68.23 and 60.64 in Indian broken rice and pearl millet, respectively. The fermentable sugar produced through enzymatic liquefaction with SPEZYME® ALPHA was monitored at the end of the three steps of the liquefaction process over a period of 2 h (Fig. 2a). It has been reported that 2 h of liquefaction process is necessary for complete starch hydrolysis of raw starchy grain substrate. It is also documented that a shorter liquefaction process time (0.5 or 1 h) produces inefficient hydrolysis into glucose polymers [23, 24]. An increase in the dosage of alpha amylase reduced the liquefaction process time but may not produce the right substrate for glucoamylase and pullulanase enzymes (due to producing shorter 1–6 linkage branch of glucose polymers). The latter two enzymes are used during saccharification or SSF for producing fermentable sugars [25, 26]. In this study the total dissolved solids (liquefact solubility) were also evaluated at the end of the three steps of the liquefaction process (Fig. 2b). Dissolved solids were higher in 35% DS feedstocks concentration compared to 30% concentration, with Indian broken rice showing higher concentration than pearl millet. This is due to higher fat content in pearl millet (5–8%) which negatively impacts the solubility of pearl millet [27].
a Fermentable sugars* (%, w/v); and b brix+ (%) produced at the end of conventional three steps liquefaction process using SPEZYME® ALPHA at 0.6 kg per MT of starch when (grey bars) Indian broken rice and (black bars) pearl millet was used as raw materials at 30 and 35% dry solid (DS) each. The values represent mean values ± SD of three experimental studies. *The p value was found to be 1.46E−06 in case of Indian broken rice while performing ANOVA between 30 and 35% dry solid concentration, whereas in case of pearl millet, p value was found to be 1.62E–06. +The p value was found 7.92E−11 in case of Indian broken rice while performing ANOVA between 30 and 35% dry solid concentration, whereas in case of pearl millet, p value was found to be 1.12E−011
It is documented that 5.9% (w/v) fermentable sugar was produced from 25% DS corn when RSH (raw starch hydrolyzing) enzyme was used at 2.5 kg per MT of corn in the liquefaction process at 48 °C for 2 h [28]. It is also documented that 3.70 ± 0.15% (w/v) fermentable sugar was produced from 30% DS cassava starch treated with SPEZYME® XTRA at 90 °C applied at 0.66 kg per MT of starch [28]. Under these conditions yeast cells are known to experience high osmolarity stress in the initial stage of fermentation. Therefore, it is essential to maintain the total sugars concentration at <20% w/v to avoid sluggish fermentation, which retards the efficiency of ethanol production [29, 30].
Simultaneous saccharification and fermentation
After cooking, the liquefacts of 30 and 35% DS of Indian broken rice and pearl millet feedstocks were cooled to 32 ± 2 °C and subjected to SSF process with DISTILLASE® ASP. These saccharifying enzymes convert the liquefaction end products such as higher molecular weight and long chain saccharides into fermentable sugars. It has been reported that pullulanase activity supports in converting the long chain branches of alpha-1,6 glycoside linkages into the linear dextrins during SSF process that further enhances fermentable sugar generation by glucoamylase reaction, which resulted in shortened hydrolysis time by as much as 37% [31]. In this study, in addition a 6% inoculum of propagated yeast (0.85 × 108 CFU/ml) was added to avoid sluggish fermentation.
A 6% yeast inoculum size was found to play key role in reducing the fermentation time during the high gravity fermentation for the ethanol production [17]. With this high inoculum concentration, higher levels of yeast cell growth were observed in the SSF process within 22 h in both feedstocks concentrations (Fig. 3a), with production of 10.67 ± 0.07 and 11.81 ± 0.08% (v/v at 20 °C) ethanol, respectively, at 30 and 35% DS Indian broken rice feedstock (Fig. 3a). Addition of FERMGEN™ in SSF further enhanced yeast cell numbers (Fig. 3b), with associated ethanol production levels of 12.43 ± 0.04 and 12.22 ± 0.09% (v/v at 20 °C), respectively, at 22 h (Fig. 3b). Yeast growth and ethanol production were similarly enhanced in the pearl millet feedstock by the addition of FERMGEN™ in the 22 h SSF process (Fig. 3c, d).
+Ethanol yield (%, v/v at 20 °C) with (white bars) and without (grey bars) FERMGEN™, +yeast growth profile with (star in the dotted line) and without (star in the straight line) FERMGEN™ addition during SSF* process at 32 ± 2 °C of Indian broken rice having a 30 and b 35% DS; whereas pearl millet having c 30 and d 35% DS. The values represent mean values ± SD of three experimental studies. *DISTILLASE® ASP added at 0.8 kg per MT of starch and urea at 400 ppm. The values represent mean values ± SD of three experimental studies. +The p values were found <0.05 while performing ANOVA between with and without FERMGEN™ at 30 and 35% dry solid concentration, in case of broken rice and pearl millet feedstocks for ethanol and yeast growth profile studies during SSF studies at 22, 45 and 55 h
ANOVA evaluation was performed at different time intervals of yeast growth and ethanol production with and without FERMGEN™ during SSF process, and comparing 30 and 35% DS of the two feedstocks types. All p values were <0.05, indicating a significant difference in yeast growth and ethanol production with and without FERMGEN™. Similar statistical analysis were reported by Gohel et al. [32] in strain selection experiments on Pantoea dispersa for improving chitinase production, and no-cook process studies of ethanol production from Indian broken rice and pearl millet [33].
More rapid yeast cell growth was associated with shorter lag phase in the growth cycle, as observed by Breisha [17]. At low (<5%) yeast inoculum sizes, longer lag phases and lower ethanol production rates during the early stage of yeast fermentation together with increased contamination rate have been observed [34, 35].
Rapid yeast growth was observed up to 22 h of the fermentation cycle followed by a gradual decline with time in both grain feedstocks at both the study concentrations of 30 and 35% DS (Fig. 3). Reflecting the yeast growth, ethanol production at 55 h was found to be 15.14 ± 0.05 and 16.23 ± 0.08% (v/v at 20 °C), and with the addition of FERMGEN™ during the SSF process the production was 16.03 ± 0.02 and 16.41 ± 0.08% (v/v at 20 °C), respectively, in 30 and 35% DS of Indian broken rice feedstock. In pearl millet feedstock, the ethanol production was 12.14 ± 0.05 and 12.67 ± 0.10% (v/v at 20 °C) at 55 h in 30 and 35% DS concentrations, respectively. Addition of FERMGEN™ during SSF for 30 and 35% DS pearl millet resulted in 14.13 ± 0.05 and 14.56 ± 0.02% (v/v at 20 °C) ethanol production, respectively.
It has been reported that addition of acid fungal protease in yeast fermentation of corn feedstock, plays a key role in breaking down complex starch-protein matrix resulting in more free starch for sugar conversion and finally ethanol production. In addition, the protease hydrolyzes protein into amino acids, peptides, and FAN which supports yeast growth during fermentation (http://www.agfdt.de/loads/bi06/pilgrimabb.pdf).
Apart from fermentable sugars, SPEZYME® ALPHA treatment during the conventional liquefaction process of 30 and 35% DS Indian broken rice feedstock produced 30–31 and 36–37% w/v DP4+ (higher saccharides) sugars, respectively, compared to pearl millet, in which 19–20 and 26–27% w/v DP4+ (higher saccharides) sugars were produced (Figs. 4, 5, 6, 7). The yeast was found to have a distinctive sequence of sugar utilization. After consuming glucose, fructose is used, followed by maltose and maltotriose [36]. Higher sugars cannot be metabolized by the yeast. During the SSF process [37], the DP4+ sugars gets converted faster into the glucose (DP1), maltose (DP2) and maltotriose (DP3) by DISTILLASE® ASP enzyme. With the steady and persistent enzymatic action concurrent with yeast fermentation, DP4+ content was found decrease from 30–31 to 0.1–0.2% w/v and 36–37 to 0.2–0.3% w/v in 30 and 35% DS of Indian broken rice feedstock, respectively. The corresponding decreases with pearl millet were 19–20 to 0.2–0.3% w/v DP4+ in 30% DS and 26–27 to 0.3–0.4% w/v DP4+ in 35% DS (Figs. 4, 5, 6, 7). The initial fructose content was about 0.09% (w/v) in Indian broken rice and in pearl millet about 0.4% (w/v). The fructose content decreased to 0.07% (w/v) in the first 22 h in Indian broken rice feedstock of both concentrations and to 0.14% in pearl millet feedstock. Thereafter, the fructose content remained constant in Indian broken rice after 24 h of SSF, but continued to decrease gradually in pearl millet up until 55 h. However, with the addition of FERMGEN™, fructose uptake increased in both broken rice and pearl millet feedstocks.
Sugar profile: a +DP1 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP2 profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; b DP3 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP4+ profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; and c Brix (%) with (white bars) and without (grey bars) FERMGEN™ addition during SSF* process at 32 ± 2 °C of Indian broken rice having 30% DS. The values represent mean values ± SD of three experimental studies. +The p values were found <0.05 while performing ANOVA between with and without FERMGEN™ at 30% dry solid concentration, in case of broken rice feedstock during SSF studies at 22, 45 and 55 h. The p value indicates that there were significant difference in sugars and brix profiles between with and without FERMGEN™ addition
Sugar profile: a +DP1 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP2 profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; b DP3 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP4+ profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; and c Brix (%) with (white bars) and without (grey bars) FERMGEN™ addition during SSF* process at 32 ± 2 °C of Indian broken rice having 35% DS. The values represent mean values ± SD of three experimental studies. +The p values were found <0.05 while performing ANOVA between with and without FERMGEN™ at 35% dry solid concentration, in case of broken rice feedstock during SSF studies at 22, 45 and 55 h. The p value indicates that there were significant difference in sugars and brix profiles between with and without FERMGEN addition
Sugar profile: a +DP1 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP2 profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; b DP3 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP4+ profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; and c Brix (%) with (white bars) and without (grey bars) FERMGEN™ addition during SSF* process at 32 ± 2 °C of pearl millet having 30% DS. The values represent mean values ± SD of three experimental studies. +The p values were found <0.05 while performing ANOVA between with and without FERMGEN™ at 30% dry solid concentration, in case of pearl millet feedstock during SSF studies at 22, 45 and 55 h. The p value indicates that there were significant difference in sugars and brix profiles between with and without FERMGEN™ addition
Sugar profile: a +DP1 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP2 profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; b DP3 profile (%, w/w) with (white bars) and without (grey bars) FERMGEN™, +DP4+ profile with (star in the dotted line) and without (star in the straight line) FERMGEN™; and c Brix (%) with (white bars) and without (grey bars) FERMGEN™ addition during SSF* process at 32 ± 2 °C of pearl millet having 35% DS. The values represent mean values ± SD of three experimental studies. +The p values were found <0.05 while performing ANOVA between with and without FERMGEN™ at 35% dry solid concentration, in case of pearl millet feedstock during SSF studies at 22, 45 and 55 h. The p value indicates that there were significant difference in sugars and brix profiles between with and without FERMGEN™ addition
While the sequence of sugar utilization of yeast remained similar [glucose (DP1) followed by maltose (DP2) and maltotriose (DP3)] (Figs. 4, 5, 6, 7), addition of FERMGEN™ significantly (p < 0.05) enhanced the effective utilization of DP1, DP2 and DP3 during SSF resulting in higher ethanol production (p < 0.05). It has been reported that in barley and cassava feedstocks, residual sugars, sugar uptake rate and fermentation rate of the SSF process were similar to pre-saccharified process at end of fermentation without impacting ethanol production [34].
Ethanol yield after distillation
The final ethanol yield was calculated at the end of the 55 h yeast fermentation through the distillation process. Distilled ethanol yield was estimated with an alcometer (%, v/v) calibrated at 20 °C. Indian broken rice feedstock of 30 and 35% DS (containing 68.23% starch) resulted in ethanol yields of 15.14 ± 0.02 and 16.03 ± 0.04% v/v, respectively, measured at 20 °C. Addition of FERMGEN™ increased the yields to 16.23 ± 0.05 and 16.42 ± 0.05% v/v. The corresponding yields for pearl millet 30 and 35% DS (60.64% starch content) were 12.14 ± 0.07 and 12.60 ± 0.05% v/v without FERMGEN™, and 14.14 ± 0.04 and 14.56 ± 0.02% v/v with FERMGEN™. Based on these distilled ethanol (%, v/v at 20 °C) values, ethanol recovery was also calculated in liters per MT of the grain (Table 1) because this technology can also be used for potable purposes. In comparing the two grain feedstocks, ethanol production was higher with broken rice than with pearl millet, due to higher starch content (68.23 vs. 60.64%).
ANOVA comparisons of ethanol recovery with and without FERMGEN™ comparing 30 and 35% DS of both the feedstocks were done. p values were <0.05, indicating significant difference in ethanol recovery with and without FERMGEN™ (Table 1). Present research study was designed to examine both substrate grains to verify whether the utility of conventional enzymes such as SPEZYME® ALPHA, DISTILLASE® ASP and FERMGEN™ used in low gravity fermentation is also sustained in high gravity fermentation processes for these feedstocks, as this technology and feedstocks are economically more viable for the ethanol industry in India. There is no documented study of high gravity fermentation for Indian broken rice and pearl millet feedstocks in the conventional process.
Fermentation efficiency, residual sugar and starch content
In each feedstock, addition of FERMGEN™, an acid fungal protease resulted in increased fermentation efficiency (Table 2) with minimal residual sugar and starch (Table 3). Theoretically, 100 g of starch should produce 56.7 g of ethanol at the maximum yield, assuming that all starch is completely converted into glucose [33, 38]. With the conventional liquefaction process using SPEZYME® ALPHA followed by SSF using DISTILLASE® ASP in both the feedstocks of the present study, 89–91% fermentation efficiency was achieved (Table 2). Further with the addition of FERMGEN™ along with DISTILLASE® ASP during SSF in both the feedstocks, 93–94% fermentation efficiency was observed (Table 2). Correspondingly residual starch and sugar were lower with FERMGEN™ compared to without FERMGEN™ in SSF (Table 3). p values for fermentation efficiency comparisons with and without FERMGEN™ in both the grain feedstocks and at both DS levels were <0.05, indicating significant increases in fermentation efficiency with FERMGEN™ (Table 2).
Wu et al. [39] used a three-step conventional process of ethanol production from US pearl millet with 65.30% starch and 25% dry solid concentration. Their process involved liquefaction at 95 °C for 45 min followed by 80 °C for 30 min; saccharification at 60 °C for 30 min, and finally yeast fermentation, with an ethanol yield of ~11% v/v at 20 °C, a fermentation efficiency of 90% and residual starch of 3.45%. Zhan et al. [40] used the conventional process for US sorghum containing 68.8% starch and 25% DS concentration and obtained 10.72% v/v ethanol with 85.93% fermentation efficiency. It has been reported that VHG fuel alcohol fermentation can be enhanced with hydrolyzed wheat proteins used as a source of FAN [30]. In barley mash fermentation, FAN is shown to enhance yeast growth and multiplication [21]. It is reported that expression of aspartyl protease on the cell surface of industrial-ethanol producing yeast results in higher yeast cell counts with increased rate of growth, and recombinant strain of S. cerevisiae exhibits higher yield of ethanol and lower residual sugar compared with the parental strain [21].
Conclusions
The present investigation explains the potential of SPEZYME® ALPHA, and DISTILLASE® ASP along with acid protease enzyme (FERMGEN™) for ethanol production with higher dry solid concentration of Indian broken rice and pearl millet feedstocks in the conventional process. This study advances the Indian industries’ search for methods to increase the concentration of feedstocks of Indian broken rice and pearl millet grains (≥30% w/w DS) to achieve higher plant throughput without compromising fermentation efficiency and ethanol recovery.
References
Gough S, Brady D, Nigam P, Marchant R, McHale AP (2007) Production of ethanol from molasses at 45 °C using alginate-immobilized Kluyveromyces marxianus imb3. Bioprocess Biosyst Eng 16:389–392
Sánchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99:5270–5295
Kleih U, Ravi SB, Rao BD, Yoganand B (2007) Industrial utilization of sorghum in India. SAT eJ 3:1–37
Tiwale S (2010) Food grain vs liquor: Maharashtra under crisis. Econ Polit Wkly 14:19–21
Mussatto SI, Dragone G, Guimarães PMR, Silva JPA, Carneiro LM, Roberto IC, Vicente A, Domingues L, Teixeira JA (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28:817–830
Szulczyk KR, McCarl BA, Cornforth G (2010) Market penetration of ethanol. Renew Sust Energ Rev 14:394–403
Bvochora JM, Read JS, Zvauya R (2000) Application of very high gravity technology to the cofermentation of sweet stem sorghum juice and sorghum grain. Ind Crop Prod 11:11–17
Devantier R, Pedersen S, Olsson L (2005) Characterization of very high gravity ethanol fermentation of corn mash. Effect of glucoamylase dosage, pre-saccharification and yeast strain. Appl Microbiol Biotechnol 68:622–629
Suresh K, Sree NK, Rao LV (1999) Utilization of damaged sorghum and rice grains for ethanol production by simultaneous saccharification and fermentation. Bioresour Technol 68:301–304
Nikolić S, Mojović L, Rakin M, Pejin D, Pejin J (2010) Ultrasound-assisted production of bioethanol by simultaneous saccharification and fermentation of corn meal. Food Chem 122:216–222
Thomas KC, Hynes SH, Ingledew WM (1995) Practical and theoretical considerations in the production of high concentration of alcohol by fermentation. Process Biochem 31:321–331
Casey GP, Ingledew WM (1986) Ethanol tolerance in yeasts. CRC Crit Rev Biotechnol 13:219–280
Thomas KC, Hynes SH, Ingledew WM (1994) Effects of particulate material and osmoprotectants on very high gravity ethanolic fermentation by Saccharomyces cerevisiae. Appl Environ Microbiol 60:1519–1524
Jones AM, Thomas KC, Ingledew WM (1994) Ethanolic fermentation of blackstrap molasses and sugarcane juice using very high gravity technology. J Agric Food Chem 42:1242–1246
Bely M, Rinaldi A, Dubourdieu D (2003) Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J Biosci Bioeng 96:507–512
Bisson LV, Butzke CE (2000) Diagnosis and rectification of stuck and sluggish fermentation. Am J Enol Vitic 51:168–177
Breisha GZ (2010) Production of 16% ethanol from 35% sucrose. Biomass Bioenerg 34:1243–1249
Dombek KM, Ingram LO (1986) Magnesium limitation and its role in apparent toxicity of ethanol during yeast fermentation. Appl Environ Microbiol 52:975–981
Beltran G, Esteve-Zarzoso B, Rozes N, Mas A, Guillamoan JM (2005) Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J Agric Food Chem 53:996–1002
Bely M, Sablayrolles JM, Barre P (1990) Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J Ferment Bioeng 70:246–252
Guo ZP, Zhang L, Ding ZY, Wang ZX, Shi GY (2010) Improving the performance of industrial ethanol-producing yeast by expressing the aspartylprotease on the cell surface. Yeast 27:1017–1027
Kim TH, Taylor F, Hicks KB (2008) Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour Technol 99:5695–5702
Montesinos T, Navarro JM (2000) Production of alcohol from raw wheat flour by amyloglucosidase and Saccharomyces cerevisiae. Enzym Microb Technol 27:362–370
Neves MA, Kimura T, Shimizu N, Shiiba K (2006) Production of alcohol by simultaneous saccharification and fermentation of low-grade wheat flour. Braz Arch Biol Technol 49:481–490
Ipsita R, Gupta MN (2004) Hydrolysis of starch by a mixture of glucoamylase and pullulanase entrapped individually in calcium alginate beads. Enzym Microb Technol 34:26–32
Kłosowski G, Mikulski D, Czupryński B, Kotarska K (2010) Characterisation of fermentation of high-gravity maize mashes with the application of pullulanase, proteolytic enzymes and enzymes degrading non-starch polysaccharides. J Biosci Bioeng 109:466–471
Jain RK, Bal S (1997) Physical properties of pearl millet. J Agric Eng Res 66:85–91
Shanavas S, Padmaja G, Moorthy SN, Sajeev MS, Sheriff JT (2011) Process optimization for bioethanol production from cassava starch using novel eco-friendly enzymes. Biomass Bioenerg 35:901–909
Beaven MJ, Carpentier C, Rose AH (1982) Production and tolerance of ethanol in relation to phospholipid fatty-acyl composition in Saccharomyces cerevisiae NCYC 431. J Gen Microbiol 128:1447–1455
Thomas KC, Ingledew WM (1990) Fuel alcohol production: effects of free amino nitrogen on fermentation of very-high-gravity wheat mashes. Appl Microbiol Biotechnol 56:2046–2050
Gantelet H, Duchiron F (1999) A new pullulanase from a hyperthermophilic archaeon for starch hydrolysis. Biotechnol Lett 21:71–75
Gohel V, Megha C, Vyas P, Chhatpar HS (2004) Strain improvement of chitinase producing isolate Pantoea dispersa for enhancing its biocontrol potential against fungal plant pathogens. Ann Microbiol 54:503–515
Gohel V, Duan G (2012) No-cook process for ethanol production using Indian broken rice and pearl millet. Int J Microbiol 2012:1–9
Choi GW, Kang HW, Kim YR, Chung BW (2008) Ethanol production by Zymomonas mobilis CHZ2501 from industrial starch feedstocks. Biotechnol Bioprocess Eng 13:765–771
Swinnen IAM, Bernaerts K, Dens EJJ, Geeraerd AH, Van Impe JF (2004) Predictive modelling of the microbial lag phase: a review. Int J Food Microbiol 94:137–159
D’Amore T, Russell I, Stewart GG (1989) Sugar utilization by yeast during fermentation. J Ind Microbiol Biotechnol 4:315–324
Wang P, Singh V, Xue H, Johnston DB, Rausch KD, Tumbleson ME (2007) Comparison of raw starch hydrolyzing enzyme with conventional liquefaction and saccharification enzymes in dry-grind corn processing. Cereal Chem 84:10–14
Gibreel A, Sandercock JR, Lan J, Goonewardene LA, Zijlstra RT, Curtis JM, Bressler DC (2009) Fermentation of barley by using Saccharomyces cerevisiae: examination of barley as a feedstock for bioethanol production and value-added products. Appl Environ Microbiol 75:1363–1372
Wu X, Wang D, Bean SR, Wilson JP (2006) Ethanol production from pearl millet using Saccharomyces cerevisiae. Cereal Chem 83:127–131
Zhan X, Wang D, Bean SR, Mo X, Sun XS, Boyl D (2006) Ethanol production from supercritical-fluid-extrusion cooked sorghum. Ind Crops Prod 23:304–310
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gohel, V., Duan, G. Conventional process for ethanol production from Indian broken rice and pearl millet. Bioprocess Biosyst Eng 35, 1297–1308 (2012). https://doi.org/10.1007/s00449-012-0717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0717-1