Abstract
Bacillus subtilis TD6 was isolated from Takifugu rubripes, also known as puffer fish. Cellulase from this strain was partially purified by ammonium sulphate precipitation up to 80% saturation, entrapped in calcium alginate beads, and finally characterized using CMC as the substrate. For optimization, various parameters were observed, including pH maximum, temperature maximum, sodium alginate, and calcium chloride concentration. pH maximum of the enzyme showed no changes before and after immobilization and remained stable at 6.0. The temperature maximum showed a slight increase to 60 °C. Two percent sodium alginate and a 0.15 M calcium chloride solution were the optimum conditions for acquisition of enzyme with greater stability. K m and V max values for the immobilized enzyme were slightly increased, compared with those of free enzyme, 2.9 mg/ml and 32.1 μmol/min/mL, respectively. As the purpose of immobilization, reusability and storage stability of the enzyme were also observed. Immobilized enzyme retained its activity for a longer period of time and can be reused up to four times. The storage stability of entrapped cellulase at 4 °C was found to be up to 12 days, while at 30 °C, the enzyme lost its activity within 3 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulosic material is an abundant renewable source that can serve as a substrate for production of chemicals and fuel ethanol by chemical or enzymatic conversion. Utilization of cellulose as a nutrient source requires the enzyme cellulase, which cleaves β-1,4-glycosidic bonds in the polymer for release of glucose units [1, 2].
The main problems associated with use of cellulase on an industrial scale include the difficulty of their separation from the solution and their inactivation by organic solvents and extreme pH or temperature. Thus, immobilization can be considered as the way out of these problems. For many industrial applications, enzymes and cells can be immobilized, via very simple and cost-effective protocols, in order to be re-used over very long periods of time [3]. The use of an immobilized enzyme permits its recovery and reuse, resulting in an economically feasible process [3–5].
Cellulase has been immobilized by several physical and chemical methods, including cross-linking [6], copolymerization [7], fiber ultra-filtration [8, 9], aqueous two-phase systems [10], and by use of an enzyme carrier, such as non-porous ultrafine silica particles [11]. Enzymes can be immobilized using a variety of natural and synthetic supports. The choice of support and/or technique is dependent on the nature of the enzyme, its substrate, and its application.
Entrapment of enzyme in calcium alginate is an important method for immobilization. Alginates are commercially available as water-soluble sodium alginates. Entrapment within calcium alginate is recognized as a rapid, nontoxic, inexpensive, and versatile method for immobilization of enzyme as well as cells [12]. Alginate is a natural polysaccharide derived from marine plants and its basic structure consists of linear unbranched polymers containing β-(1, 4)-linked d-mannuronic acid (M) and α-(1, 4)-linked l-guluronic acid (G) residues. It can form thermally stable and biocompatible hydrogel in the presence of calcium cations [13, 14]. The use of alginate as an immobilizing agent in most applications rests in its ability to form heat-stable strong gels which can develop and set at room temperatures. Alginate forms gels with most di- and multivalent cations. Monovalent cations and Mg2+ ions do not induce gelation while ions like Ba2+ and Sr2+ will produce stronger alginate gels than Ca2+. However, it is the alginate gel formation with calcium ions which has been of interest in most applications [15].
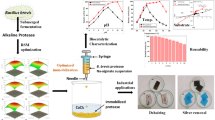
The present research focused on immobilization of partial purified cellulase from a new strain of Bacillus subtilis, TD6, isolated from Takifugu rubripes fish using calcium alginate as a support material. Various parameters, including pH maximum, temperature maximum, and concentration of sodium alginate and calcium chloride solution were observed. As the purpose of immobilization, repeated use of the immobilized cellulase was also studied with storage stability.
Materials and methods
Microorganism
Bacillus subtilis TD6, obtained from Takifugu rubripes gut, was used in this study. It was maintained on CMC medium composed of (g l−1): Carboxymethyl cellulose, 10; K2HPO4, 4; Na2HPO4.2H2O, 4; MgSO4.7H2O, 0.2; CaCl2, 0.001; FeSO4.7H2O, 0.004; Tryptone, 2 at pH 6.0.
Preparation of crude enzyme
About 100 ml of culture medium was dispensed into 300 ml conical flasks and sterilized at 121 °C for 15 min. The flask was then inoculated with Bacillus subtilis TD6 and incubated at 45 °C 100 rpm for 3 days. The cultures were centrifuged 10,000×g for 20 min. Following collection of the culture filtrate, the enzyme solution was tested based on the DNS method.
Enzyme assays
CMCase activity assay was determined for estimation of endoglucanase activity, as given below [16]. Through the determination of the amount of reducing sugars liberated from CMC solubilized in 50 mM sodium citrate buffer, pH 4.8. This mixture was incubated for 30 min at 50 °C and the reaction was stopped by the addition of DNS solution. The treated samples were boiled for 5 min, cooled in water for color stabilization, and the optical density was measured at 540 nm. The cellulase activity was determined by using a calibration curve for glucose. One unit of enzyme activity was defined as the amount of enzyme that released 1 μmol of glucose per minute.
Determination of protein content
The SMARTTM BCA Protein Assay Kit for standard assay was used for protein determination. The purple-colored reaction product of this assay is formed by chelation of two molecules of BCA with one cuprous ion. This water soluble complex exhibits a strong absorbance at 562 nm.
Ammonium sulfate precipitation
The enzyme solution was partially purified using ammonium sulfate precipitation up to 80% saturation. The precipitate fraction of enzyme was then re-dissolved in a 0.05 M sodium phosphate buffer and dialyzed against the same buffer.
Immobilization of cellulase
The partially purified enzyme solution was mixed with sodium alginate solution in a 1:1 ratio. The cellulose–alginate mixture was added drop-wise into calcium chloride solution with continuous shaking at 4 °C. The beads thus formed were washed 3–4 times with deionized water, and, finally, with sodium phosphate buffer 5 mM pH 6.0.
Optimization of working parameters
Concentration of sodium alginate was 0.5–3% and calcium chloride concentration was 0.1–0.3 M. The pH range 5, 6, 7, and 8 was chosen to observe the optimum pH for immobilized CMCase. Temperatures selected for the immobilization were 30, 40, 50, 60, and 70 °C.
For thermal and pH stability, the immobilized enzyme was incubated at various temperatures (30, 40, 50, 60, and 70 °C) and pH (5, 6, 7, and 8) for 6-h. The residual activity was determined based on DNS method [16].
Results and discussion
CMCase was precipitated at 40 to 80% ammonium sulfate saturation, with the highest specific activity of 1.48 U/mg obtained at 60% saturation (Fig. 1).
Effect of various concentrations of sodium alginate and calcium chloride
Various concentrations of sodium alginate were used for acquisition of beads with greater stability. As drops of cellulose–alginate mixed with CaCl2 solution increased, Na+ ions of sodium alginate were replaced by Ca2+ ions of the CaCl2 solution, which finally resulted in formation of Ca-alginate beads [17].
The enzyme specific activity after immobilization was found to be maximum at 2% (w/v) sodium alginate concentration, which is 61% of free enzyme specific activity (Fig. 2). Maximum leakage of enzyme from beads was observed to occur at 0.5% (w/v) sodium alginate. This occurrence might be the result of the larger pore sizes that fragile the Ca–alginate beads. At 3% (w/v) sodium alginate concentration, the activity of the enzyme was found to be low, which might be due to the decrease of pore size, resulting in hindered penetration of the substrate to the beads.
The concentration of calcium chloride for formation of the capsule of the enzyme also varied in order to acquire stable beads capable of maximum specific activity. In this work, 2% (w/v) of sodium alginate was used. CaCl2 at a concentration of 0.15 M was found to retain the highest activity of immobilized CMCase (Fig. 3). At this concentration, the activity of immobilized CMCase was found to be 60% of free CMCase (control). Other research using different enzymes also reported a decrease in activity of alkaline protease when the concentration of CaCl2 is increased [18]. They observed that the pH of CaCl2 solution changed with its concentration, which might affect the activity of the immobilized enzyme.
Effect of temperature and pH on immobilized CMCase
The immobilized enzyme was assayed at various temperatures and pHs. Optimum temperature of immobilized CMCase was found to be 60 °C, higher than that of free CMCase (50 °C), which indicated that the support material retained the tertiary structure of the enzyme at higher temperature (Fig. 4). Although the maximum temperature was 60 °C, the immobilized enzyme was considered more stable at 50 °C, at which 89% of its activity was retained after a 6-h incubation period. On either side of this point, lowered stability was displayed by the immobilized CMCase, nontheless sufficient activity was present at 70 °C during a 6-h incubation period (Fig. 5).
The effect of various pH on the immobilized enzyme activity was reported in Fig. 6. The optimum pH for immobilized CMCase was known to be 6.0, respectively. The surface of the beads has been reported to have a cationic and anionic nature. The charged surface of beads and enzyme produces a charged microenvironment, which might affect the nature of active enzyme and alter the pH of entrapped enzyme [19]. Nonetheless, in this study, the surface of beads had no effect on the active enzyme; therefore, the optimum pH of free and immobilized enzyme remained the same.
The pH stability of immobilized CMCase after a 6-h incubation period at 25 °C indicated that the enzyme retained approximately 90% of its specific activity after incubation at a pH value of 6.0 (Fig. 7). Although maximum stability of the immobilized enzyme was found at pH 6.0, the enzyme was found to be throughly stable at pH 5.0–7.0, where it retained more than 80% of the maximum activity for a 6-h incubation period.
Kinetic constant of immobilized enzyme
The K m and V max values of immobilized CMCase calculated from a Lineweaver–Burk plot were 2.9 mg/ml and 32.1 μmol/min/mL, respectively. These values were higher compare to free CMCase (2.4 mg/mL and 29.94 μmol/min/mL). The increase in K m of entrapped enzyme might be due to inaccessibility of the substrate to the enzyme due to limitation in diffusion of substrate into enzyme beads, which is affected by bead size, pore size, and enzyme loading per bead. The increase in the V max, compared with that of the native enzyme, may be attributed to increased stability of the enzyme after immobilization.
Another research study using a different enzyme also reported an increase in both K m and V max values of commercial immobilized pectinase [20]. Buga et al. [21] also reported the same finding using immobilized polygalacturonase. These findings are consistent with the findings of this study.
Reusability of immobilized enzyme
The activity immobilized CMCase was evaluated using a repeated batch process in order to observe the reuse of the immobilized enzyme (Fig. 8). After the fourth cycle, the enzyme showed 80 and 45% for the second and third cycles, respectively. After the fourth cycle, the remaining activity was less than 10%. This decrease might due to leakage of enzyme from beads along with the process and washing steps at the end of each cycle.
Storage stability of immobilized CMCase
The immobilized CMCase was stored at 30 and 4 °C for 15 days for determination of the stability of immobilized enzyme. The enzyme was more stable at a temperature of 4 °C, which showed 30% loss of activity after 48 h, 45% after 6 days, and 90% after 12 days. Significant loss in enzyme activity of immobilized enzyme was observed at 30 °C, which showed 85% loss of activity after the second day and no activity on the third day.
Conclusion
The enzyme has been partially purified using ammonium sulphate precipitation and immobilized using sodium alginate. The immobilized CMCase showed optimum activity at pH 6.0 and 60 °C, respectively. It has been found to be stable at pH 5.0–7.0 and at 40–60 °C. This work has demonstrated that immobilized CMCase can be used through at least three catalytic cycles.
References
Barr B, Hsieh YL, Ganem B, Wilson DB (1996) Identification of two functionally different classes of exocellulases. Biochemistry 35:586–592
Nishida Y, Suzuki K, Kumagai Y, Tanaka H, Inoue A, Ojima T (2007) Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochimie 89:1002–1011
Mateo C, Palomo JM, Lorente GF, Guisan JM, Lafuente RF (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Technol 40:1451–1463
Cao L (2005) Immobilized enzymes: science or art? Curr Opin Chem Biotechnol 9(2):217–226
Kumar A, Srivastava A, Galaev IY, Mattiasson B (2007) Smart polymers: physical forms and bioengineering applications. Prog Polym Sci 32:1205–1237
Busto MD, Ortega N, Mateos MP (1997) Stabilization of cellulase by cross-linking with glutaraldehyde and soil humates. Bioresour Technol 60:33–37
Yuan X, Shen N, Sheng J, Wei X (1999) Immobilization of cellulas using acrylamide grafted acrylonitride copolymer membranes. J Membr Sci 155:101–106
Ohison I, Tragardh G, Hahn-Hagerdal B (1984) Enzymatic hydrolysis of sodium hydroxide pretreated sallow in an ultrafiltration membrane reactor. Biotechnol Bioeng 26:647–653
Henley RG, Yang RYK, Greenfield PF (1980) Enzymatic saccharification of cellulose in membrane reactors. Enzym Microbiol Technol 2:206–208
Tjerneld F, Persson I, Albertsson PA, Lee JM (1991) Enzyme cellulose hydrolysis in anattrition bioreactor combined with an aqueous two-phase system. Biotechnol Bioeng 37:876–882
Afsahi B, Kazemi A, Kheirolomoom A, Nejati S (2007) Immobilization of cellulase on non-porous ultrafine silica particles. Sci Iranica 14(4):379–383
Fraser JE, Bickerstaff GF (1997) Entrapment of enzymes and cells in calcium alginate. In: Immobilization of enzymes and cells. Humana Press, Totowa, pp 61–66
Blandino A, Macias M, Cantero D (1999) Formation of calcium alginate gel capsules: influence of sodium alginate and CaCl2 concentration on gelation kinetics. J Biosci Bioeng 88:686–689
Blandino A, Macias M, Cantero D (2000) Glucose oxidase release from calcium alginate gel capsules. Enzym Microb Technol 27:319–324
Andre B, Bioencapsulation encyclopedia. http://encyclopedia.oktala.com.br/content/252
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Abida A, Qader SA, Aliya R, Samina I, Abid A (2009) Calcium alginate: a support material for immobilization of proteases from newly isolated strain of Bacillus subtilis KIBGE-HAS. World Appl Sci J 7(10):1281–1286
Roig MG, Rashid DH, Kennedy JF (1995) High-alkaline protease from Bacillus PD92 entrapped in calcium alginate gel: physicochemical and microscopic study. Appl Biochem Biotechnol 55:95–121
Norouzian D (2003) Enzyme immobilization, and the state of art in biotechnology: a review. Iran J Biotechnol 1:197–206
Sartoglu K, Demir N, Acar J, Mutlu M (2001) The use of commercial pectinase in the fruit industry, part 2: determination of kinetic behaviour of immobilized commercial pectinase. J Food Eng 47(4):271–274
Buga ML, Ibrahim S, Nok AJ (2010) Physico-chemical characteristics of immobilized polygalacturonase from Aspergillus niger (SA6). Afr J Biotechnol 9(52):8934–8943
Acknowledgments
It is the outcome of a Manpower Development Program for Energy & Resources Supported by The Ministry of Knowledge and Economy (MKE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andriani, D., Sunwoo, C., Ryu, HW. et al. Immobilization of cellulase from newly isolated strain Bacillus subtilis TD6 using calcium alginate as a support material. Bioprocess Biosyst Eng 35, 29–33 (2012). https://doi.org/10.1007/s00449-011-0630-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0630-z