Abstract
In a complete nitrification sequencing batch reactor (CNSBR), where ammonium containing wastewater (200–1,000 mg N/L) is completely oxidized to nitrate up to 2.4 kg NH4 +–N/m3 d, both ammonia oxidizers and nitrite oxidizers were enriched in the sludge granules. Quantitative fluorescence in situ hybridization analyses of the sludge granules of the CNSBR showed that ammonia oxidizers and nitrite oxidizers occupied 31 and 4.2% of total bacteria, respectively. Most of the nitrite oxidizers were Nitrobacter species (95% of the nitrite oxidizers) and the remainder was Nitrospira species. The population of nitrite oxidizers was significantly higher than that of partial nitrification SBR (PNSBR) where most of the ammonium was oxidized to nitrite. The PNSBR had 37% (ammonia oxidizers) and 0.4% (nitrite oxidizers) of total bacteria. Comparative study with CNSBR and PNSBR revealed that free nitrous acid, rather than free ammonia, played a critical inhibition role to wash out nitrite oxidizers from the reactor. The concentrations of free ammonia and nitrite as well as free nitrous acid in the CNSBR selected Nitrobacter as the dominant nitrite oxidizers rather than Nitrospira.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Novel nitrogen removal technologies, such as single reactor system for high activity ammonium removal over nitrite (SHARON) and anaerobic ammonium oxidation (ANAMMOX), have several advantages by reducing aeration for nitrification and external carbon source for denitrification, and less sludge production [1–3]. Both processes rely on partial nitrification of ammonium to nitrite. Nitrite accumulation by partial nitrification can be achieved by selectively suppressing the numbers or activities of nitrite oxidizers. Partial nitrification occurs when ammonia oxidizers prevail over nitrite oxidizers in a mixed culture nitrification system. It can be obtained by raising reaction temperature [2], lowering dissolved oxygen level [4], and imposing selective inhibition condition for nitrite oxidizers by free ammonia (NH3) and free nitrous acid (HNO2), which are unionized forms of ammonium and nitrite, respectively [5, 6]. The equilibrium shifts nitrite to free nitrous acid as the pH decreases and vice versa for ammonium to free ammonia. Both free ammonia and free nitrous acid are known to have inhibitory effect on nitrite oxidizers [5, 6]. However, their relative inhibition contributions to nitrite oxidizers in a nitrification system are still uncertain. The finding of the responsible compound for the inhibition and suppression of nitrite oxidizers will provide valuable information for the engineered wastewater treatment systems for high nitrogen containing wastewater, like sludge dewatering liquor and landfill leachate, to achieve stable partial nitrification to nitrite.
A sequencing batch reactor (SBR) is preferred to continuous flow stirred tank reactor to achieve nitrite accumulation because it can utilize both free ammonia and free nitrous acid for the inhibition of nitrite oxidizers in a same SBR cycle by keeping high concentrations of substrate (free ammonia) and product (free nitrous acid) at the initial and final aeration phases of an SBR cycle, respectively [7]. The SBR can achieve nitrite accumulation through partial nitrification (PNSBR) and nitrate through complete nitrification (CNSBR) by manipulating SBR operation. If the profiles of nitrogen compounds in a cycle of CNSBR and PNSBR are monitored and their difference is compared to each other, the relative effect of free ammonia and free nitrous acid on the inhibition of nitrite oxidizers can be analyzed.
The objective of this study is to find out the most effective component which selectively inhibits nitrite oxidizers to wash out from the reactor by a comparative study with CNSBR and PNSBR. For the purpose profiles of nitrogen compounds in the CNSBR were monitored and analyzed for nitrification kinetics. In addition, the microbial communities of the nitrifying bacteria in the CNSBR were analyzed by fluorescence in situ hybridization (FISH) and compared to that of the PNSBR to estimate the effect of inhibition compounds (free ammonia, free nitrous acid) and the substrate (nitrite) on the microbial distribution or selection of specific nitrite oxidizer in the SBR.
Materials and methods
SBR for complete nitrification (CNSBR)
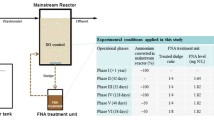
The CNSBR was an air lift reactor, which was made of polymethyl metacrylate, and it had a working volume of 3 L (total height of 100 cm, column diameter of 8 cm). One operation cycle of the SBR took 180 to 300 min depending on the ammonium (NH4 +–N) concentration in the wastewater. A cycle time of the SBR with 200, 400 and 600 mg NH4 +–N/L wastewater was 180 min and it was increased to 300 min with 800 and 1,000 mg NH4 +–N/L wastewater. Each cycle consists of wastewater feeding (2 min), aeration (169 or 289 min), sludge settling (4 min), and effluent discharge (5 min), which were controlled by programmable logic circuit. Sludge settling time was intentionally shortened to promote sludge granulation by excluding poor settling sludge from the CNSBR. Hydraulic retention time (HRT) was maintained at 6–10 h by discharging a half of the wastewater (1.5 L) in the SBR during the discharge. Schematic experimental set-up of the SBR system is shown in Fig. 1. Reactor temperature was maintained at room temperature (23–27 °C). The CNSBR had been operated for 100 days from the start-up. Initially, ammonium (NH4 +–N) concentration in the wastewater was maintained at 200 mg/L for 20 days, and it was increased periodically to 400 mg/L (21–45 days), 600 mg/L (46–70 days), 800 mg/L (71–80 days) and 1,000 mg/L (81–100 days).
Dissolved oxygen concentration of the CNSBR was maintained higher than 2.0 mg/L by adjusting air flow rate and the pH was controlled at higher than 7.0. A half of the theoretical alkalinity demand was provided in the wastewater and additional alkalinity (NaHCO3) was pumped to the reactor by a pH controller (DIK, Korea). In the later phase of the CNSBR when nitratation (nitrite oxidation to nitrate) occurs, the pH was maintained at 7.3–7.6, which is considerably higher than the pH when nitritation (ammonium oxidation to nitrite) occurs.
SBR for partial nitrification (PNSBR)
The PNSBR had exactly the same dimension and operational scheme with the CNSBR except the pH control. For the partial nitrification of ammonium to nitrite, pH was monitored and used to control aeration time based on the differences of carbon dioxide stripping and bicarbonate consumption rates during nitritation and nitratation. During the partial nitrification, pH of the PNSBR was controlled at 7.0 by the addition of sodium bicarbonate. As soon as the ammonium was completely oxidized to nitrite, pH began to increase. As pH reached 7.3, aeration cycle was stopped to prevent further oxidation to nitrate. More detailed experimental condition can be found elsewhere [7].
Fluorescence in situ hybridization
Fluorescence in situ hybridization technique [8, 9] was used to investigate the microbial communities of the ammonia oxidizing bacteria and nitrite oxidizing bacteria in the SBR. Biomass samples were periodically withdrawn from the reactors and fixed in 4% freshly prepared paraformaldehyde solution for 2 to 3 h at 4 °C. The biomass samples were rinsed twice with phosphate-buffered saline (PBS). Each fixed biomass was placed in a small aluminum cup, embedded in Jung OCT compound (Leica Microsystems, Germany) overnight at room temperature to allow the compound to penetrate the biomass samples. Ten samples were frozen at −20 °C. The frozen biomass samples were cut into 20-μm thick vertical slices with a cryostat (Reichart-Jung cryocut 1800, Leica Microsystems) at −20 °C. Each slice was placed on a slide coated with 0.1% gelatin in the presence of 0.01% chromium potassium sulfate and dried overnight. The specimen was dehydrated by successive 50, 80, and 98% ethanol washes (3 min each), air dried, and stored at room temperature. The probe sequences, as well as the specificities of the probes and the hybridization conditions, are shown in Table 1. Oligonucleotides were synthesized and fluorescently labeled with a hydrophilic sulfoindocyanine dye (Cy-3 or Cy-5) or fluorescein isothiocyanate (FITC) at the 5′ end (MWG Biotech, Germany).
In situ hybridizations were performed in a hybridization incubator using hybridization buffer (0.9 M NaCl, 20 mM Tris hydrochloride (pH 7.2), 0.01% sodium dodecyl sulfate and formamide at the concentrations shown in Table 1 at 46 °C for 2 h). The probe concentration was 20 μg/mL. Hybridization was followed twice by a stringent washing step at 48 °C for 10 min with 50 mL of pre-warmed washing buffer [20 mM Tris hydrochloride (pH 7.2), 0.01% sodium dodecyl sulfate, NaCl at the concentration listed in Table 1]. The washing buffer was removed by rinsing the slides with distilled water and the slides were air dried. The slides were mounted to avoid bleaching and examined with an Axioplan epifluorescence microscope (Carl Zeiss, Germany) and MRC-1024 (Biorad, UK) CLSM, equipped with Kr/Ar lasers (excitation wave length 494 and 650 nm) and HeNe lasers (550 nm). For quantitative analysis of FISH images, about 10 images were scanned and averaged by image processing software (IMT i-Solution, version 3.0, Korea).
Wastewater and analytical methods
The synthetic wastewater used in this study had the following compositions: (NH4)2SO4, (200–1,000 mg N/L), MgSO4·7H2O (30 mg/L), KCl (35 mg/L), NaHPO4·12H2O (29 mg/L), CaCl2·2H2O (16 mg/L), KH2PO4 (45 mg/L), FeCl3·6H2O (10 mg/L). For alkalinity supplement, a half of the theoretical NaHCO3 requirement for nitrification (7.1 g as CaCO3/g NH4 +–N) was initially added to the wastewater and additional alkalinity was supplied by pumping during the nitrification to control the pH at a level higher than 7.0 as explained before. During the ammonium oxidation some of the supplied alkalinity was assimilated for biosynthesis. Batch nitrite oxidation activity of the granule was measured as follows. Granule samples from the CNSBR were washed twice with the synthetic wastewater without (NH4)2SO4. The samples were transferred to the Erlenmeyer flasks (500 mL) in a shaking incubator (Vision Scientific, Korea), which had 200 mL of the above synthetic wastewater while (NH4)2SO4 was replaced with 100 mg NO2 −-N/L. The incubator was operated at 30 °C and 250 rpm. All the batch experiments were performed in triplicates.
All the analytical methods were based on the Standard Methods [10]. NH4 +–N was measured by a cation chromatograph (ICS 1500, Dionex, USA) with YK-421 column. Both NO2 −–N and NO3 −–N were measured by an anion chromatograph (DX 500, Dionex) with IonPac AS-11 column. All the ion chromatography samples were measured after passing through a 0.22 μm syringe filter. Dissolved oxygen (YSI 55, USA), temperature and pH (720P, Istek, Korea) of the reactor were continuously monitored.
Results
Complete nitrification in SBR (CNSBR)
After the start-up of the CNSBR with 200 mg NH4 +–N/L, nitrification efficiency increased gradually and continuously. Complete nitrification was achieved in 10 days with 0.8 kg NH4 +–N/m3 day. Figure 2 shows the typical operation cycles of the CNSBR at 70th (Fig. 2 a), and 100th day (Fig. 2 b) after the start-up. After 20 day of the start-up, ammonium was completely nitrified in 100 min to nitrite and nitrate (data not shown). Figure 2a shows the profiles of nitrogen compounds in the CNSBR cycle at 70th day. Ammonium concentration was increased to 600 mg NH4 +–N/L at the load of 2.4 kg NH4 +–N/m3 day. Ammonium was completely nitrified in 2 h to nitrite and nitrate. Free ammonia was decreased from 8 mg N/L to zero with the decrease of ammonium. During the nitrification nitrite was accumulated up to 170 mg N/L, and it decreased to zero by further oxidation to nitrate when ammonium is disappeared. Free nitrous acid level was maintained less than 0.01 mg HNO2–N/L during the cycle. The oxidation rate of nitrite increased sharply as soon as the ammonium concentration decreased significantly. The pH remained at 7.0 during the ammonium oxidation by supplying bicarbonate, but the pH increased gradually to 7.6 during the nitrite oxidation (data not shown). The pH increased as the nitrite oxidation to nitrate does not produce proton ion and carbon dioxide was stripped off during the aeration. Initially, nitrate concentration in the SBR was high because denitrification did not occur in this experiment. In the CNSBR, 600 mg NH4 +–N/L was fully nitrified to nitrate in 3 h.
Figure 2b shows the profiles of nitrogen compounds in the CNSBR cycle at 100th day. The wastewater ammonium concentration had been increased to 1,000 mg NH4 +–N/L and the cycle time also increased to 5 h with the same load (2.4 kg NH4 +–N/m3 day) to achieve complete nitrification. It showed similar trend with Fig. 2a. Most of the ammonium was nitrified in 150 min to nitrite/nitrate and the remaining nitrite was completely oxidized to nitrate in 210 min. Free ammonia was also decreased from 11 mg N/L to zero with the decrease of ammonium. During the nitrification nitrite was accumulated up to 300 mg N/L, and it decreased to zero by further oxidation to nitrate when ammonium was disappeared. Free nitrous acid level was maintained up to 0.02 mg HNO2–N/L during the cycle.
Settling characteristics of the granules of CNSBR
Figure 3 shows the time changes of sludge volume index (SVI) of the sludge from the CNSBR. In a SBR, the settling time and the volume exchange ratio have been the two major selection pressures for aerobic granulation [11–13]. As we maintained short sludge settling time (4 min) and large volume exchange ratio (50%), the sludge flocs were transformed gradually into granules. The poor settling sludge had been washed out of the CNSBR. The SVI values became lower and it decreased continuously from an initial value of 130 to about 50 in 50 days due to the formation of granular sludge.
Distribution of ammonia oxidizing bacteria and nitrite oxidizing bacteria in the granules of CNSBR
Relative distribution of ammonia oxidizers and nitrite oxidizers of a microbial community gives much information about their ecological systems. Figure 4 shows the FISH/CLSM images of the granular sludge of the CNSBR at 70th day. Nsm156 (Nitrosomonas species) and Nsv443 (Nitrosospira species) were used for the identification of ammonia oxidizers. Nit3 (Nitrobacter species) and Ntspa662 (genus Nitrospira) were used for the identification of nitrite oxidizers. Figure 4a is the simultaneous hybridization image of Nitrosomonas species specific probe Nsm156 and bacteria-specific probe EUBMIX, which is a mixture of EUB338, EUB338II and EUB338III. The population ratio of specific microbial groups to the total bacteria was calculated from the fluorescence images which specifically bind to the specific microbial group and the total bacteria (EUBMIX) since the probe for the total bacteria also simultaneously hybridize to the specific microbial group. Figure 4b is the simultaneous hybridization image of Nitrosospira species specific probe Nsv443 and EUBMIX. Total number of ammonia oxidizers was about 31% of the total bacteria (EUBMIX). Figure 4c is the simultaneous hybridization image of Nitrobacter species specific probe Nit3 and EUBMIX. Figure 4d is the simultaneous hybridization image of genus Nitrospira-specific probe Ntspa662 and EUBMIX.
Table 2 shows the summarized population distribution of ammonia oxidizers and nitrite oxidizers in the CNSBR at 70th day. From the quantitative FISH image analyses of ammonia oxidizers Nitrosomonas species and Nitrosospira species occupied 26 ± 2 and 5 ± 0.5% of total bacteria, respectively. For the nitrite oxidizers Nitrobacter species and genus Nitrospira occupied 4 ± 0.5 and 0.2 ± 0.05% of total bacteria, respectively. The number of ammonia oxidizers in the CNSBR was about seven times higher than that of nitrite oxidizers. Nitrosomonas and Nitrobacter were the dominant ammonia oxidizers and nitrite oxidizers in the CNSBR.
Discussion
In the CNSBR free ammonia seemed to be a significant inhibition factor to the nitrite oxidizers as the free ammonia concentration reached 2.5–11 mg NH3–N/L with 200–1,000 mg NH4 +–N/L wastewater. It was reported that Nitrobacter was inhibited by free ammonia at much higher concentration (30–50 mg NH3–N/L) than Nitrospira, which is inhibited at 0.04–0.08 mg NH3–N/L [14]. In this case, Nitrospira is expected to suffer much more inhibition by free ammonia than Nitrobacter. However, it is only effective at the early part of the CNSBR cycle. In Fig. 2, it was shown that nitrite oxidation was suppressed when free ammonia level was higher than a certain level by observing nitrite accumulation during the nitrification. Free ammonia inhibition is reversible because nitrite oxidation resumes as soon as the free ammonia level goes down the threshold level. For the validation of the above hypothesis, a batch nitrite oxidation experiment was performed to evaluate nitrite oxidation activity of the sludge granules of the CNSBR in the absence of ammonium. Nitrite was oxidized to nitrate as soon as nitrite was introduced into the sludge of CNSBR (Fig. 5). It clearly showed the instantaneous activity of nitrite oxidizers in the CNSBR in the absence of free ammonia. On the contrary, nitrite did not oxidize to nitrate and it remained constant for 5 h in the PNSBR [7]. It was proved that most of the nitrite oxidizers were washed out of the PNSBR from the FISH analysis. Therefore, it can be assumed that nitrite oxidizing bacteria in the CNSBR was inhibited for some time during the ammonium oxidation. It was also confirmed by the batch nitrite oxidation experiment with the sludge in the CNSBR that nitrite oxidation activity was not inhibited at all in the absence of ammonia.
On the other hand, free nitrous acid had been maintained at moderately lower level in the CNSBR than in the PNSBR not to inhibit the growth of nitrite oxidizers significantly by keeping pH relatively higher than that of the PNSBR. The maximum free nitrous acid level was less than 0.02 mg HNO2–N/L in the CNSBR while it reached up to 0.12 and 0.21 mg HNO2–N/L in the PNSBR at 70th and 100th day, respectively [7]. Blackburne et al. [14] reported that the half inhibition concentration of free nitrous acid based on oxygen uptake rate was 0.3 mg HNO2–N/L for Nitrobacter while it was inhibited at 0.01–0.02 mg HNO2–N/L for Nitrospira. Therefore, nitrite oxidizers, like Nitrobacter, could endure the inhibition and survive in the CNSBR. It was confirmed by the FISH analysis of the sludge in the CNSBR.
Comparative study with the PNSBR reveals the effect and significance of free nitrous acid on the inhibition of nitrite oxidizers. The PNSBR had been operated in the same way with the CNSBR at the same condition except the reactor pH. The pH in the PNSBR was controlled at 7.0–7.3, which is somewhat lower than that of CNSBR. As the pH exerts a direct effect on the equilibrium of free nitrous acid, small decrease of pH increases free nitrous acid level significantly [5].
From the previous PNSBR study, most of the nitrite oxidizers were washed out of the reactor after 70 days [7]. They reported that Nitrosomonas species and Nitrosospira species, as the ammonia oxidizers, occupied 33 ± 0.7 and 5 ± 0.1% of the total bacteria in the PNSBR, respectively, from the FISH analysis. For the nitrite oxidizers, only Nitrobacter species with 0.5 ± 0.1% of the total bacteria were present while genus Nitrospira was not detected at all. The total amount of nitrite oxidizers was negligible when compared to that of ammonia oxidizers. It did not show any nitrite oxidation activity and it had almost no Nitrobacter and Nitrospira present in the granules. The different nitrifying microbial communities of the CNSBR and PNSBR did not give any effect on granule formation and they have similar sludge settling characteristics (SVI).
Repetitive inhibition by both free ammonia and free nitrous acid in the PNSBR seemed to be the main cause of the wash-out of nitrite oxidizers. However, as free ammonia concentrations in the PNSBR were maintained less than that of the CNSBR due to lower pH, free ammonia was not the critical factor for the wash out of nitrite oxidizers from the PNSBR even though it could suppress the growth of Nitrospira. Therefore, free nitrous acid is the only responsible inhibitor for the selective wash out of the nitrite oxidizers from the PNSBR.
Nitrosomonas species (Nsm156) were the most dominant ammonia oxidizers in the CNSBR and Nitrosospira species (Nsv443) also could not be ignored. More than 95% of the nitrite oxidizing bacteria was Nitrobacter species (Nit3) and the remainder was Nitrospira species (Ntspa662). The competition of Nitrobacter and Nitrospira in nitrification system has been discussed before [15, 16]. The dominance of Nitrobacter as the nitrite oxidizing bacteria in the CNSBR can be explained by the following reasons: First, Nitrobacter is more resistant to free ammonia than Nitrospira by the fact that Nitrospira is far more sensitive to low concentrations of free ammonia than Nitrobacter. Free ammonia reached up to 11 mg/L at the early cycle of the CNSBR with 1,000 mg/L NH4–N, and it is obvious that Nitrospira had much severe inhibition than Nitrobacter. Secondly, Nitrospira is more sensitive to low concentrations of free nitrous acid than Nitrobacter. Therefore, Nitrobacter has more chance to survive under free nitrous acid inhibition conditions than Nitrospira. Thirdly, Nitrobacter grows faster than Nitrospira when nitrite concentration is high based on K and r-strategist theory [15, 16]. The maximum specific nitrite-oxidizing activities of Nitrospira and Nitrobacter have been reported as 10.5 and 93.8 mg/g NOB h, respectively [16]. In addition, nitrite affinity (K S) value of Nitrospira is significantly lower than that of Nitrobacter, which reflects the observations of the K and r-strategist theory [17, 18]. Nitrite levels in the CNSBR had been maintained relatively high than other continuous flow type wastewater treatment plant where Nitrospira exists as the representative nitrite oxidizers [15]. Nitrobacter could utilize nitrite faster than Nitrospira when nitrite concentration is much higher than the K s. The K s of Nitrobacter is known to 1.2–1.3 mg/L while it was 0.12–0.15 mg/L and 0.9–1.1 mg/L for Nitrospira [14, 19]. The result agrees with the above theory on the point that Nitrobacter has higher specific activity as an r-strategist and Nitrospira has high substrate affinity coefficients (K s) as a K-strategist.
Summarizing the results, ammonium containing wastewater (200–1,000 mg N/L) could be completely nitrified up to 2.4 kg NH4 +–N/m3 day in the CNSBR. The sludge became granules and the SVI decreased to 50 in 50 days after the start-up. Comparative study with the CNSBR and PNSBR showed that free nitrous acid played a critical role in the inhibition and wash out of nitrite oxidizers from the PNSBR. Considering the concentrations of free ammonia, free nitrous acid, and the substrate (nitrite), Nitrobacter is the dominant nitrite oxidizers in the CNSBR and it was confirmed by the FISH analyses. The above results indicate that the microbial community of nitrifying bacteria can be controlled by the operational strategy of the reactor. Specifically, partial nitrification can be achieved by manipulating the concentration of free ammonia and free nitrous acid and it selectively washes out the total nitrite oxidizers or specific nitrite oxidizers (Nitrospira) from the reactor.
References
Mulder A, van de Graaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol Ecol 16:177–183
Hellinga C, Schellen AAJC, Mulder JW, van Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich wastewater. Wat Sci Technol 137:135–142
van Dongen U, Jetten MSM, van Loosdrecht MCM (2001) The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Sci Technol 44:153–160
Jianlong W, Ning Y (2004) Partial nitrification under limited dissolved oxygen conditions. Proc Biochem 39:1223–1229
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitric acid. J Water Pollut Control Fed 48:835–852
Turk O, Mavinic DS (1989) Stability of nitrite build-up in an activated sludge system. J Water Pollut Control Fed 61:1440–1448
Kim DJ, Seo DW (2006) Selective enrichment and granulation of ammonia oxidizers in a sequencing batch airlift reactor. Proc Biochem 41:1055–1062
Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH (1992) Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol 15:593–600
Amann R (1995) In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes. Mol Microb Ecol Manual 3.3.6:1–15
APHA (1992) Standard methods for the examination of water and wastewater, 18th edn. APHA, Washington DC
Beun JJ, Hendriks A, Loosdrecht MCM, Morgenroth E, Wilderer PA, Heijnen JJ (1999) Aerobic granulation in a sequencing batch reactor. Water Res 33:2283–2290
Beun JJ, Heijnen JJ, Loosdrecht MCM (2001) N-removal in a granular sequencing batch airlift reactor. Biotechnol Bioeng 75:82–92
Tay JH, Liu QS, Liu Y (2001) Microscopic observation of aerobic granulation in sequential aerobic sludge blanket reactor. J Appl Microbiol 91:168–175
Blackburne R, Vadivelu VM, Yuan Z, Keller J (2007) Kinetic characterisation of an enriched Nitrospira culture with comparison to Nitrobacter. Water Res 41:3033–3042
Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M (2001) In situ characterization of Nitrospira-like nitrite oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol 67:5273–5284
Kim DJ, Kim SH (2006) Effect of nitrite concentration on the distribution and competition of nitrite-oxidizing bacteria in nitratation reactor systems and their kinetic characteristics. Water Res 40:887–894
Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R (1999) Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol 65:3690–3696
Manser R (2005) Population dynamics and kinetics of nitrifying bacteria in membrane and conventional activated sludge plants. PhD thesis, Swiss Federal Institute of Technology
Alleman JE (1984) Elevated nitrite occurrence in biological wastewater treatment systems. Water Sci Technol 17:409–419
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, DJ., Seo, DW., Lee, SH. et al. Free nitrous acid selectively inhibits and eliminates nitrite oxidizers from nitrifying sequencing batch reactor. Bioprocess Biosyst Eng 35, 441–448 (2012). https://doi.org/10.1007/s00449-011-0583-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0583-2