Abstract
This paper describes the performance of a novel bio-reactor system, the membrane-integrated fermentation reactor (MFR), for efficient continuous fermentation. The MFR, equipped with an autoclavable polyvinylidene difluoride membrane, has normally been used for biological wastewater treatment. The productivity of the MFR system, applied to the continuous production of pyruvic acid by the yeast Torulopsis glabrata, was remarkably high. The volumetric productivity of pyruvic acid increased up to 4.2 g/l/h, about four times higher than that of batch fermentation. Moreover, the membrane was able to filter fermentation broth for more than 300 h without fouling even though the cell density of the fermentation broth reached 600 as OD660. Transmembrane pressure, used as an indicator of membrane fouling, remained below 5 kPa throughout the continuous fermentation. These results clearly indicate that the MFR system is a simple and highly efficient system that is applicable to the fermentative production of a range of biochemicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Continuous fermentation using cell recycle with a membrane has been a popular method for obtaining high productivity [1, 2]. Although good results have been obtained in investigations using ceramic and organic polymer membranes, these membranes both have inherent problems. Ceramic membranes are autoclavable but require high operating pressures during filtration and are prone to fouling. Organic membranes, as well as being subject to fouling problems, are not autoclavable and thus present a risk of contamination. These drawbacks limit their application, particularly for large-scale fermentation production.
In biological processes for wastewater treatment, membrane bioreactors (MBRs) have been used commercially for the past 20 years [3], based on the separation of activated sludge using membranes [4]. Membrane fouling is a serious problem affecting the performance of MBR processes, so studies into membranes with anti-fouling properties and high permeability have been carried out [5]. Such membrane systems have not been utilized in MBRs for fermentation processes but they nevertheless have potential applications.
This study focuses on the use of a polyvinylidene difluoride (PVDF) membrane in a MBR for fermentation. This membrane material has been shown to have anti-fouling properties and to be highly permeable during MBR operation and these properties strongly suggest that it may be able to solve the problems encountered in membrane-mediated continuous fermentation processes. We found that the PVDF membrane is autoclavable and then used it to develop a novel MFR system. Continuous pyruvic acid fermentation was used to evaluate the MFR performance and a remarkable improvement in the volumetric productivity of pyruvic acid was demonstrated. The MFR system was capable of running for long periods, producing pyruvic acid at high productivity, without membrane fouling or contamination.
Materials and methods
Microorganism and culture conditions
Torulopsis glabrata NBRC 0005 (formerly known as IFO 0005), a pyruvic acid producing yeast [6], was obtained from the NITE Biological Resource Center (Chiba, Japan). A basal fermentation medium described previously [7] was used with slight modifications, containing (per liter of distilled water) 100 g glucose, 2 g (NH4)2SO4, 0.5 g MgSO4·7H2O, 0.3 g soybean hydrolyzate, 20 mg CaCO3, 8 mg nicotinic acid, 1 mg pyridoxine–HCl, 50 μg biotin and 30 μg thiamine-HCl. Batch fermentation experiments were carried out at 30 °C in a 2 l fermentor (Able-Biott, Tokyo, Japan). The initial fermentation medium volume was 1 l, the agitation speed was set at 800 rpm, and air was sparged at 1 l/min. The inoculum medium was the basal fermentation medium supplemented with 20 g/l CaCO3. 50 ml of inoculum was incubated at 30 °C for 24 h on a shaking incubator at 100 rpm before inoculation of the fermentor. 4 N NaOH was added automatically to maintain pH at 5.5.
Analyses
Samples were withdrawn for the analyses of pyruvic acid, ethanol, glucose and cell concentrations at desired intervals during the fermentation. Samples were centrifuged (8,000×g, 5 min), and the supernatants analyzed for pyruvic acid and glucose. The concentration of pyruvic acid was determined using a high performance liquid chromatography (HPLC) system (Prominence series: Shimadzu, Kyoto, Japan) equipped with a conductivity detector. A shim-pack SPR-H column (Shimadzu) was used with 5 mM p-toluenesulfonic acid as the mobile phase at a flow rate of 0.8 ml/min. 5 mM p-toluenesulfonic acid, 20 mM Bis–Tris, 0.1 mM EDTA·2Na was used as the reaction phase at a flow rate of 0.8 ml/min. The column temperature was maintained at 45 °C. Before HPLC analyses, samples were filtered through a 0.22 μm membrane. The concentration of glucose was analyzed using an F-kit (Roche, Basel, Switzerland). The ethanol concentration was measured by gas chromatography (GC2010, Shimadzu) equipped with a flame ionization detector under the following conditions: capillary column TC-1 (0.53 mm i.d. by 15 m) (GL science, Tokyo, Japan); temperature of column, injector and detector, 45, 200 and 250 °C, respectively; flow rate of helium carrier gas, 3 ml/min. Cell concentration was measured by optical density at 660 nm (OD660).
Results
Development of the membrane-integrated fermentation reactor system
A diagram of the MFR system is shown in Fig. 1 . Continuous fermentation was performed in a 2 l fermentor (working volume 1.5 l) equipped with a microfilter element (described in detail below) submerged in the fermentation broth. A system suitable for continuous fermentation was developed as follows. Feed was added to the fermentor at various dilution rates, as indicated. Permeate flow through the filter was controlled by a peristaltic pump, with permeate removed at the same rate as the rate of addition of feed plus base to keep a constant broth volume (1.5 l) in the fermentor. Permeability was evaluated by measuring transmembrane pressure throughout the continuous cultivation.
The configuration of the microfilter element is shown in Fig. 2. A flat sheet PVDF membrane with an average pore diameter of 0.08 μm, as described by Henmi et al. [8], was used in the MFR system. The membrane was originally developed for use in a MBR in biological wastewater treatment. Pure water permeability of the membrane, an index of the membrane performance, was virtually unchanged before and after autoclaving (data not shown), confirming that the membrane was autoclavable. The microfilter element was constructed by arranging a porous mesh (3) and the membrane (1), in order, on both sides of a support plate (2), with a recess milled into both sides of the support plate. The membranes were crimped to the support plate with a crimping plate (5) to prevent leakage of fermentation broth. The support plate and crimping plate were stainless steel and the porous mesh was polyethylene terephthalate resin. The effective membrane area of the microfilter element was 120 cm2. The fermentation broth permeate on each side flowed through the porous mesh in the recess and was discharged via a collection pipe (4) to the outside of the fermentor.
Results of continuous pyruvic acid fermentation using the MFR system
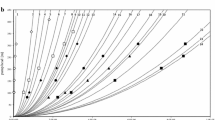
We performed continuous pyruvic acid fermentation using T. glabrata to evaluate the MFR system. T. glabrata was reported to be a superior microorganism for the fermentative production of pyruvic acid [6] and has been utilized for commercial production [9]. In biological wastewater treatment, the concept of critical flux is applied to MBRs as an operational strategy to reduce the flux decline caused by membrane fouling [10]. Below the critical flux value, the transmembrane pressure (TMP) is stable at a value that increases in proportion to the flux. Above the critical flux value, TMP increases drastically and fouling occurs. During MBR operation, even if filtration is performed below the critical flux, the TMP tends to increase during long-term continuous filtration at constant flux. Intermittent filtration is therefore carried out in practice, whereby cleaning of the membrane surface during periods with no filtration brings about long-term filtration with a stable TMP. Therefore, the critical operating flux of a cultivated broth of T. glabrata was determined to facilitate long-term continuous fermentation. Determination of the critical flux was carried out according to the stepwise flux method [11], with several modifications. 1.5 l of a batch cultivation broth of T. glabrata was poured into the fermentor containing the microfilter element and filtered. The permeate was returned to the fermentor and the permeate flux increased stepwise every 15 min while monitoring TMP. Figure 3 shows the TMP measured at each flux value of the cultivated broth. The critical flux was approximately 0.4 m/day (17 l/m2/h) and the operation was therefore subsequently carried out at a flux below 0.4 m/day (a dilution rate of 0.133 h−1) throughout the fermentation.
The MFR containing fermentation medium was autoclaved (121 °C, 20 min) before cultivation. At first, batch cultivation was carried out for 24 h, as described in “Materials and methods”. After the batch cultivation, the operation was shifted to continuous fermentation for 362 h at 30 °C and pH 5.5. Filtered air at 2 l/min was introduced throughout the fermentation to prevent a shortage of oxygen during pyruvic acid production. The filtration condition was set at a dilution rate of approximately 0.1 h−1, and intermittent operation of filtration was carried out, wherein the filtration was stopped for a 1 min interval after 9 min of filtration, according to standard MBR operation (a flux of 0.3 m/day, i.e. below the critical flux value). As a control experiment, conventional batch fermentation was carried out as described in “Materials and methods”.
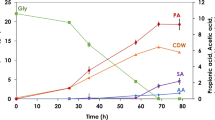
The time profiles of batch pyruvic acid production and continuous production using the MFR system are compared in Fig. 4. In batch production, 41 g/l pyruvic acid was achieved at 49 h and the yield from glucose (p/g ratio) was 0.43 g/g-glucose (Fig. 4a). On the other hand, during the continuous operation, pyruvic acid accumulation resulted in 36 g/l, glucose concentration remained below 2 g/l after 56 h and ethanol, which is the main by-product, was less than 8 g/l. The dissolved oxygen concentrations remained between 0.2 and 0.5 g/l (as approximately 2.5–6% of saturation) (Fig. 4b). The pyruvic acid production reached the range of 3.5–4.2 (3.7 on average) g/l/h, and the p/g ratio was in range 0.35–0.43 (0.39 on average) g/g-glucose (Fig. 4c). The productivity of pyruvic acid by the MFR system improved about fourfold over that of the batch fermentation. Although the cell density of the fermentation broth reached 600 (expressed as OD660), the TMP remained below 5 kPa throughout the operation (Fig. 4d), and no signs of membrane fouling were observed.
Time-courses of batch pyruvic acid production and continuous production using the MFR system. a Batch production; pyruvic acid (closed circles), glucose (open circles), ethanol (open triangles) and cell concentrations (open diamonds). b–d Continuous production, after 24 h batch cultivation. Continuous operation was carried out for 362 h, dissolved oxygen (plus signs), yield of pyruvic acid (closed triangles), productivity of pyruvic acid (closed diamonds) and TMP (open squares). The same symbols as above were used for pyruvic acid, glucose, ethanol and cell concentrations, respectively
Discussion
It is known that continuous cultivation with cell recycle systems using membranes potentially offers high productivity during fermentation. However, industrial application of this system has not been realized because the membranes used in the past showed serious problems, including fouling, low permeability and no heat-tolerance in autoclaving. The present results have demonstrated that a novel MFR system equipped with an autoclavable PVDF membrane normally used for wastewater treatment is useful for the improvement of productivity during fermentative production.
This study demonstrated continuous cultivation without fouling using yeast, which has a large enough cell size compared with the pore size of the PVDF membrane. According to preliminary critical flux tests using bacteria with small cell sizes, the critical flux was approximately equal to that of yeast T. glabarata (data not shown). This shows that factors other than the cell size (i.e. the structure of the cell surface) may affect PVDF membrane fouling. Further research is necessary to confirm this hypothesis.
The results of continuous production of pyruvic acid using the MFR system clearly demonstrated that the system could be run for long periods (>300 h) with high productivity (3.5–4.2 g/l/h) and no membrane fouling, when the filtration rate was below the critical flux value (around 0.4 m/day). The yield of pyruvic acid during continuous fermentation (0.39 g/g-glucose) was slightly below that from batch fermentation (0.43 g/g-glucose). It has been shown that the yield of pyruvic acid during fermentation is affected by the dissolved oxygen concentration [12]. Although the dissolved oxygen concentration during the continuous fermentation remained between 0.2 and 0.5 mg/l, the oxygen supply was thought to be insufficient for promoting pyruvic acid production.
The PVDF membrane used here was shown to be autoclavable, thereby eliminating contamination, a major problem of fermentation. Hollow fiber PVDF membranes are also available and have been shown to be useful for practical wastewater treatment [13]. These may also be useful for the MFR system.
In conclusion, the MFR system developed in this study is simple, highly efficient and requires no special equipment. The system is expected to be applicable to many other fermentative production processes.
References
Chang HN, Yoo IK, Kim BS (1994) High density cell culture by membrane-based cell recycle. Biotechnol Adv 12:467–487
Charcosset C (2006) Membrane processes in biotechnology: an overview. Biotechnol Adv 24:482–492
Stephenson T, Judd S, Jefferson B, Brindle K (2000) Membrane bioreactors for wastewater treatment. IWA Publishing, London
Yamamoto K, Hiasa M, Mahmood T, Matsuno T (1989) Direct solid–liquid separation using hollow fiber membrane in an activated-sludge aeration tank. Water Sci Technol 21:43–54
Meng F, Chae SR, Drews A, Kraume M, Shin HS, Yang F (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43:1489–1512
Yonehara T, Miyata R (1994) Fermentative production of pyruvate from glucose by Torulopsis glabrata. J Ferm Bioeng 78:155–159
Miyata R, Yonehara T (2000) Improvement of fermentative production of pyruvate from glucose by Torulopsis glabrata. J Ferm Bioeng 82:475–479
Henmi M, Fusaoka Y, Matsuka N, Kurihara M (2003) Development of flat sheet immersed membrane module and operation performance in MBR International Desalination Association, World Congress on Desalination and Water Reuse September 28–October 3, 2003, Bahama
Yonehara T, Miyata R, Matsuno H, Goto M, Yahanda S (2000) Development of fermentative production of pyruvate by metabolic control. Seibutsu Kogakkaishi 78:56–62
Clech PL, Chen V, Fane T (2006) Fouling in membrane bioreactors used for wastewater treatment—a review. J Memb Sci 284:17–53
Ognier S, Wisniewski C, Grasmick A (2002) Characterization and modeling of fouling in membrane bioreactor. Desalination 146:141–147
Hua Q, Yang C, Shimizu K (1999) Metabolic flux analysis for efficient pyruvate fermentation using vitamin-autotrophic yeast of Torulopsis grabarata. J Biosci Bioeng 87:206–213
Minegishi S, Matsuka N (2007) Advanced fouling resistant PVDF hollow fiber membrane modules “Torayfil HFM, HFS, HFU”. Membrane 32:311–314
Acknowledgments
This work was partially supported by a grant from the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawai, H., Mimitsuka, T., Minegishi, Si. et al. A novel membrane-integrated fermentation reactor system: application to pyruvic acid production in continuous culture by Torulopsis glabrata . Bioprocess Biosyst Eng 34, 721–725 (2011). https://doi.org/10.1007/s00449-011-0521-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0521-3