Abstract
In the present study, permeated yeast cells were used as the catalyst to synthesize glutathione. When waste cells of brewer’s yeast were incubated with the three precursor amino acids and glucose for 36 h, 899 mg/L of glutathione were produced. To release the feedback inhibition of γ-glutamylcysteine synthetase caused by glutathione, two-stage reaction was adopted. In the first stage, glycine was omitted from the reaction mixture and only γ-glutamylcysteine was formed. Glycine was then added in the second stage, and 1,569 mg/L of glutathione were produced. The conditions of the two-stage reaction were optimized using Plackett–Burman design and response surface methodology. Under the optimized condition, commercially available baker’s yeast produced 3,440 mg/L of glutathione in 30 h, and most of the produced glutathione was in the medium. The two-stage reaction could effectively reduce the feedback inhibition caused by glutathione, but degradation of glutathione was significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glutathione (γ-l-glutmyl-cysteinyl-glycine, GSH) is the most ubiquitous non-protein thiol in most living cells from microorganisms to man [1, 2]. Intracellularly, most GSH is kept in its thiol form by glutathione disulfide (GSSG) reductase, an NADPH-dependent enzyme [3]. GSH has important cellular functions. It is involved in metabolism to maintain the thiol moieties of proteins and low molecular weight compounds, such as cysteine and coenzyme A. GSH is also involved in the formation of deoxyribonucleotides. GSH reacts enzymatically (glutathione S-transferase family) or non-enzymatically with toxic compounds to form GSH conjugates. It also protects against oxidative damage caused by reactive oxygen species (ROS) that may be formed normally in metabolism.
Biosynthesis of GSH in cells involves two consecutive, ATP-dependent reactions catalyzed by γ-glutamylcysteine (γ-GC) synthetase (γ-GCS, EC 6.3.2.2, catalyzes the reaction to form γ-GC from l-glutamic acid and l-cysteine) and GSH synthetase (GS, EC 6.3.2.3, catalyzes the reaction to form GSH from γ-GC and glycine). In most organisms, the activity of γ-GCS is subjected to feedback inhibition by GSH to avoid over-accumulation of GSH, and it has been shown that formation of γ-GC is the rate-limiting step in the GSH biosynthesis pathway [4]. Cellular GSH can be degraded by γ-glutamyltranspeptidase (γ-GT, EC2.3.2.2), which catalyzes the reaction to transfer the γ-glutamyl moiety of GSH and other γ-glutamyl compounds to amino acids, and hydrolytically releases l-glutamate [5].
GSH can be produced mainly by four ways: extraction from natural biomass, chemical synthesis, enzymatic synthesis, and fermentation. Since GSH is widely distributed in living organisms, extraction of GSH from animal or plant tissues was the main approach in early times when it was discovered. Later on, Harington et al. [6] established a chemical synthesis approach in 1935. However, the high cost to extract GSH with a solvent and formation of physiologically inactive d-isomer blocked the development of these two approaches.
At present, GSH is produced mainly by fermentation and enzymatic synthesis. For a fermentation process, the main approaches to improve GSH production are to increase the cell density and cellular content of GSH. Some fermentation control schemes, such as dissolved oxygen (DO)-stat [7] and pH-stat [8], have been applied for enhancing GSH production by increasing cell density. Fuzzy control has also been used in fed-batch culture of yeast to reduce ethanol concentration and to enhance GSH production [9]. Supplementation of the precursor amino acids or related amino acid (serine, arginine, or methionine) during fermentation can effectively increase intracellular GSH content [10, 11]. However, as an intracellular product, GSH production is limited by feedback inhibition. At low pH, GSH can be secreted into the medium and thus the feedback inhibition by intracellular GSH on γ-GCS activity can be alleviated [12]. Genetic engineering was applied to improve the activities of γ-GCS and GS in the past few decades [13–15]. Although the levels of γ-GCS and GS were improved significantly, the intracellular GSH content was limitedly improved due to inhibition of γ-GCS by GSH [16]. Therefore, release of the feedback inhibition caused by GSH becomes important for efficient production of GSH.
Enzymatic synthesis of GSH has the advantages of higher concentration of product, shorter production cycle, less byproducts, etc., compared to the fermentation method. In this study, permeated yeast cells were used as the catalyst to synthesize GSH from the precursor amino acids, and glucose was used to provide ATP by the cellular activities in the glycolytic pathway. To alleviate feedback inhibition on γ-GCS, we proposed a convenient way. The whole process was divided into two stages. In the first stage, glycine was omitted from the reaction mixture and only γ-GC was formed, then in the second stage, glycine was added to the reaction mixture for production of GSH. In such a way, inhibition of γ-GCS by GSH could be effectively reduced and production of GSH was significantly enhanced.
Materials and methods
Microorganisms
Waste Saccharomyces cerevisiae cells were kindly provided by Asia-Pacific Brewery Co. Ltd., Shanghai, China. Active dry baker’s yeast was produced by Angel Yeast Co. Ltd., (Hubei, China), and was purchased from a supermarket. Candida tropicalis and another strain of S. cerevisiae were from our laboratory stock.
Cell pretreatment
The waste brewer’s yeast cells were harvested by centrifugation of the yeast slurry at 1,050×g and 4°C for 5 min. The pelleted cells were washed three times with 0.02 M phosphate buffer (pH 7.0) and centrifuged. The active dry baker’s yeast (10 g) was suspended in 50 ml phosphate buffer (0.02 M, pH 7.0) for 30 min, and then centrifuged and washed under the same conditions to obtain the cell pellet. The laboratory stocked S. cerevisiae and C. tropicalis were cultured at 30°C in the medium containing (per liter): glucose 40 g, urea 2 g, K2HPO4 1.5 g, MgSO4·7H2O 0.5 g, FeSO4·7H2O 3 mg, MnCl2·4H2O 0.3 mg, ZnSO4·7H2O 4 mg, CaCl2·2H2O 1 mg, CuSO4·5H2O 0.5 mg, and biotin 0.05 mg (pH 6.0). Shake flask cultures were carried out in 250-ml flasks containing 30 ml medium. After inoculation, the flasks were incubated at 30°C and 220 rpm for 30 h. Then, the cells were centrifuged and washed three times with the same phosphate buffer. The harvested wet yeast cells were frozen at −20°C and thawed at 37°C three times for permeation.
Enzymatic synthesis of GSH
10 g (wet weight) permeated yeast cells were suspended in 100 ml reaction mixture containing 0.2 M phosphate buffer (pH 7.0), 20 mM MgCl2·6H2O, 10 mM CaCl2·2H2O, 0.4 M glucose, 10 mM l-glutamic acid, 10 mM l-cysteine, and 10 mM glycine. The amino acids were of technical grade and l-cysteine was kindly supplied by Chuyuan Chemicals Co., Hubei, China. The reaction was carried out at 37°C in flasks immersed in a thermostat water bath, or in a 500-ml glass reactor with a magnetically driven rotator and a water jacket through which thermostat water was circulated.
GSH degradation
Aliquots of 1-g baker’s yeast cells (wet weight) were suspended in 10 ml 0.2 M potassium phosphate buffer (pH7.0) containing 5 mM GSH. Different concentrations of the constituent amino acids and glucose were added to the reaction medium. The reaction was carried out at 37°C for 10 h and the residual GSH concentrations were measured.
Assay
Reduced GSH was measured with the DTNB [5,5′-Dithio-bis (2-nitrobenzoic acid), Sigma-Aldrich, St Louis, MO, USA] method [17]. The sample of reaction mixture was centrifuged at 9,400×g for 10 min at 4°C and the supernatant was used to determine the GSH in the reaction medium. The GSH inside the yeast cells was extracted with 10 volume of boiling water for 3 min, then the cell suspension was cooled immediately to 25°C and centrifuged at 9,400×g and 4°C for 10 min, and GSH in the supernatant was determined.
Glucose was determined by an enzymatic assay kit (Kexin Biotech, Shanghai, China). The concentration of ethanol was determined by GC equipped with a 2-m column filled with Chromosorb 101 (Dikma, Lampoc, CA, USA) and a flame ion detector, at the temperatures of column, injector and detector of 160, 180 and 180°C, respectively.
Experimental design and optimization of GSH production
Plackett–Burman design [18] is an efficient means to screen important factors among a large number of variables with least experiments. Several variables involved in the components of the reaction medium and reaction conditions were chosen, and the levels of experimental variables under study are shown in Table 1.
The central composite rotatable design [19] was used to develop a mathematical model to relate the examined variables with GSH production. According to the experimental results of Plackett–Burman design, five variables, namely the concentrations of l-glutamate, l-cysteine, phosphate buffered saline (PBS), CaCl2 and pH, were chosen to correlate with GSH production. The five variables were further optimized by the response surface methodology (RSM). The central composite design (CCD) and the corresponding experimental data are shown in Table 2.
The response equation in which the parameters were fitted based on the data in Table 2 is a quadratic equation as follows:
where Y is the calculated response (GSH production); X i the actual variable to be optimized; b 0 a constant; b i the ith linear coefficient; b ii the squared coefficient; b ij the ijth cross-product coefficient. All the experimental design and data analysis were performed by using Minitab 15 (Minitab Inc., PA, USA).
Results and discussion
GSH synthesized by different yeasts
Four kinds of yeasts including baker’s yeast, waste brewer’s yeast, and two laboratory collected strains (S. cerevisiae and C. tropicalis) were used for synthesis of GSH. Before use, the yeast cells were permeated by repeated freeze and thaw to improve the transport of the precursor amino acids and formed GSH. The results are shown in Table 3. It can be seen that the waste brewer’s yeast and the baker’s yeast were much better than the laboratory stock strains. By incubation of 10-g wet yeast cells of the former two strains in shake flasks with the three precursor amino acids (each at 10 mM) and glucose (400 mM) for 36 h, the GSH concentrations reached 899 and 871 mg/L, respectively. The GSH produced was about twofold that produced by the laboratory strains under the same conditions, and the extracellular GSH accounted for 95% of the total produced GSH. These results indicated that the cellular activities in the glycolysis and GSH synthesis pathways functioned well and GSH could be synthesized even without addition of ATP. This was also verified by Murata et al. [20], who used intact and immobilized S. cerevisiae cells and the glutathione concentration reached nearly 460 and 610 mg/L, respectively.
Synthesis of GSH in a 500-ml agitated reactor
Synthesis of GSH using the waste brewer’s yeast cells was further carried out in a temperature-controlled 500-ml reactor mixed with a magnetically driven rotor. The initial volume was 250 ml. Figure 1 shows the time courses of GSH, glucose, and ethanol concentrations. The total GSH including those secreted to the medium and remained inside the cells reached 836 mg/L (2.72 mM) at 34 h and the molar yield was 27.2% based on added l-cysteine. Furthermore, most produced GSH (more than 96%) was in the reaction medium. During the reaction, glucose was consumed rapidly and the ATP generated was more than that actually required for GSH synthesis. According to the concentration of produced ethanol, the calculated ATP generated in the glycolic pathway was 675 mM. Therefore, only 0.4% of the generated ATP was used for synthesis of GSH, and the phenomenon was in agreement with what Murata et al. observed [20]. This was attributed to the high ATPase activity in brewing yeasts especially in industrially used fast fermentation yeasts. In such yeast cells, ATP formed in the glycolytic pathway is degraded quickly to maintain a low level of ATP, and glucose is metabolized quickly.
Feedback inhibition by GSH
It is reported that γ-GCS is inhibited by reduced GSH in most eukaryotic and prokaryotic cells [5]. To examine the effects of feedback inhibition caused by GSH, the reaction was initiated at different GSH concentrations and the GSH formed was measured in an hour. The results are shown in Fig. 2. It can be seen that GSH strongly inhibited the GSH production. The synthesis of GSH was almost completely inhibited at the initial GSH concentration of 2 mM. In order to get more GSH, the feedback inhibition should be reduced as much as possible.
Two-stage reaction
To release the feedback inhibition of γ-GCS caused by GSH, the whole reaction was divided into two stages. In the first stage, glycine was omitted from the reaction mixture and only γ-GC was formed. Because no GSH was produced in this stage, the γ-GCS activity could be maintained, and more γ-GC could be produced. Then, glycine was added to the reaction mixture at 7.5 h to start the second stage for production of GSH. The result is shown in Fig. 3. Under this condition, the GSH produced by the waste brewer’s cells reached 1,569 mg/L in 31 h, 1.88 times as high as that obtained when the three amino acids were added at the same time. In addition, 1,430 mg/L GSH was in the reaction medium due to cell permeation. The glutathione produced by intact cells was accumulated in the cells and was not excreted [20]. Therefore, the two-stage reaction using permeated cells could effectively reduce the feedback inhibition caused by GSH, thus enhanced GSH production. According to the rate of GSH production, the second stage could be further divided into three sections after glycine addition. During the initial 3 h, GSH was produced at an average rate of 0.72 mmol/(l h), which was consistent with fast glucose consumption. Then GSH production was slowed down. This was assumed to be caused by two reasons. First, the reduction of the concentration of intermediate substrate γ-GC caused GSH production to slow down. Second, glucose was consumed slower than in the first section. The second section continued until glucose was consumed very slowly at 23.5 h. In the third section, little glucose was used and GSH was hardly formed. The decreased synthesis of GSH in the later period of reaction was attributed to leakage of enzymes due to permeation by freeze and thaw, because the protein concentration in the reaction medium was trebled in this period (data not shown).
The profiles of total glutathione, glucose, and ethanol in two-stage reaction catalyzed by waste brewer’s yeast cells. The initial concentrations of both l-glutamate and l-cysteine were 10 mM. Glycine (20 mM) was added to the reaction mixture at 7.5 h. The arrow shows the supplementation of glucose. Multiplicate experiments indicated the average final GSH concentration of 5.11 ± 0.01 mM
Optimizing GSH production using statistical experimental methods
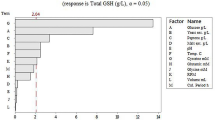
Because the quality of waste brewer’s yeast varied to a large extent, in the following experiments, the commercially available baker’s yeast was used. Preliminary experiments on single factor affecting GSH production were performed in the two-stage reaction mode (not shown), and 11 factors were chosen (Table 1). Benzoic acid was added to examine whether it would be useful to better use of ATP [21]. The effects of these variables were studied using Plackett–Burman design, and the significance was estimated by the P-value (Table 4). A P-value less than 0.05 indicated influence of the variable was significant. From the experimental data, five significant variables including pH and the concentrations of cysteine, glutamic acid, CaCl2, and PBS were identified. The effect of the time to add glycine was not significant, suggesting less change in the production of γ-GC during the examined period.
The five variables were further optimized by RSM. Except for the examined variables, the reaction conditions were the same as that described in “Materials and methods”. The whole reaction was carried out for 30 h, and glycine was added at 5 h. The levels of these variables in the CCD design and the experimental results are shown in Table 2. The experimental data were analyzed with MINITAB 15, and the analysis of variance (ANOVA) is listed in Table 5. The parameters in Eq. 1 describing the relationship between GSH production (Y) and tested variables were obtained and are shown in Eq. 2:
The R 2 of the model was 96.16%, and the adjusted R 2 was 89.17%, indicating a very high correlation.
According to Eq. 2, the predicted highest yield of GSH based on cysteine was 0.84 mol/mol with a GSH concentration of 4.12 mM, and the highest GSH concentration of 12.1 mM with a yield of 0.48 mol/mol. To achieve reasonable GSH concentration and yield, the optimal conditions were obtained by weighting the GSH yield by 0.6 and GSH concentration by 0.4, and the optimal conditions were: pH 6.8, cysteine 23 mM, glutamic acid 25 mM, CaCl2 4 mM, and PBS 0.65 M. Two-stage reaction was carried out under the optimized conditions, and the baker’s yeast produced 3,440 mg/L (11.2 mM) of GSH in 30 h. The time courses in the reaction for GSH production are shown in Fig. 4. It can be seen that the course of GSH production by baker’s yeast was rather similar to the waste brewer’s yeast except that baker’s yeast consumed glucose even faster than the waste brewer’s yeast. In addition, the pH dropped slower than in the previous experiments due to the higher concentration of PBS to provide better buffering capability. After 19 h, the added glucose was all consumed, but ethanol and GSH were still produced at a slower rate. This could be attributed to the degradation of intermediate products such as fructose-1, 6-diphosphate to generate ATP [20].
The time courses of total glutathione, glucose, and ethanol in two-stage reaction catalyzed by baker’s yeast cells. The initial concentrations of l-glutamate and l-cysteine were 25 and 23 mM, respectively. Glycine (50 mM) was added to the reaction mixture at 5 h. The concentration of potassium phosphate buffer was 0.65 M. Multiplicate experiments indicated the average final GSH concentration of 11.2 ± 0.05 mM
Degradation of GSH
In yeast cells γ-glutamyltranspeptidase that catalyzes degradation of GSH exists [22]. In the two-stage reaction, feedback inhibition caused by GSH could be effectively reduced. However, the molar GSH yield based on added cysteine was only around 50%, far below the theoretical yield. The residual cysteine existed in the reaction medium was very low at the end of reaction (data not shown). Thereby, it was supposed that GSH degradation was the main reason to reduce the GSH yield. GSH degradation was examined under different conditions: A, GSH plus glucose, cysteine and glycine; B, GSH plus cysteine and glycine; C, GSH alone. The results are shown in Fig. 5. When glucose was present, GSH degraded more slowly compared with the other experiments (B or C) where glucose was missing. This could be due to the resynthesis of GSH, but the rate of degradation was faster than that of synthesis. Comparison of the experiment C where the reaction mixture contained nothing but GSH, with B where cysteine and glycine were added, the degradation of GSH was at the similar level. Therefore, the concentration of the precursor amino acids seemed to have no effect on GSH degradation.
GSH degradation under different conditions. 1-g baker’s yeast cells (wet weight) was suspended in 10 ml 0.2 M potassium phosphate buffer (pH 7.0) containing 5 mM GSH. The mixture was supplied with different amounts of amino acids and glucose, and incubated with gentle shaking for 10 h at 37°C. A 320 mM glucose, 10 mM l-cysteine, and 10 mM glycine; B 10 mM l-cysteine and 10 mM glycine; C neither amino acid nor glucose was added
Conclusions
The waste brewer’s yeast and commercial baker’s yeast can convert the precursor amino acids to GSH in the presence of glucose. To reduce the feedback inhibition of γ-GCS caused by GSH and to improve GSH production, the reaction was divided into two stages. In the first stage, glycine was omitted to synthesize γ-GC only. Then glycine was added to synthesize GSH which achieved 1,569 mg/L, 1.88 times that obtained in the conventional one-stage reaction. The optimal conditions were established by using Plackett–Burman design and RSM, and the GSH concentration reached 3,440 mg/L in 30 h. Most of the produced GSH was in the medium. GSH degradation was also found to be responsible to the low GSH yield, and presence of glucose decreased degradation of GSH.
References
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Penninckx MJ, Elskens M (1993) Metabolism and functions of glutathione in micro-organisms. Adv Microb Physiol 34:239–301
Carmel-Harel O, Storz GR (2000) Roles of the glutathione and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol 54:439–501
Richman PG, Meister A (1975) Regulation of γ-glutamycysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem 250:1422–1426
Tate S, Meister A (1981) γ-Glutamyltranspeptidase: catalytical, structural and functional aspects. Mol Cell Biochem 39:357–368
Harington CR, Mead TH (1935) Synthesis of glutathione. Biochem J 29:1602–1611
Liang GB, Du GC, Chen J (2008) A novel strategy of enhanced glutathione production in high cell density cultivation of Candida utilis—cysteine addition combined with dissolved oxygen controlling. Enzyme Microb Technol 42:284–289
Tong Q, Wang G, Du GC, Chen J (2003) Effect of feeding method on production of glutathione by Saccharomyces cerevisiae. J Ind Microbiol 33:19–22
Alfafara CG, Kanda A, Shioi T, Shimizu H, Shioya S, Suga K, Suzuki K (1993) Fuzzy control of ethanol concentration and its application to maximum glutathione production in yeast fed-batch culture. Biotechnol Bioeng 41:493–501
Alfafara CG, Kanda A, Shioi T, Shimizu H, Shioya S, Suga K (1992) Effect of amino acids on glutathione production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 36:538–540
Alfafara CG, Miura K, Shimizu H, Shioya S, Suga K (1992) Cysteine addition strategy for maximum glutathione production in fed-batch culture of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 37:141–146
Liang G, Du GC, Chen J (2008) Enhanced glutathione production by using low-pH stress coupled with cysteine addition in the treatment of high cell density culture of Candida utilis. Lett Appl Microbiol 46:507–512
Gushima H, Miya T, Murata K, Kimura A (1983) Construction of glutathione-producing strains of Escherichia coli B by recombinant DNA techniques. J Appl Biochem 5:43–52
Ohtake Y, Watanabe K, Tezuka H, Ogata T, Yabuuchi S, Murata K, Kimura A (1988) The expression of γ-glutamylcysteine synthetase gene of Escherichia coli in Saccharomyces cerevisiae. Agric Biol Chem 52:2753–2762
Ohtake Y, Watanabe K, Tezuka H, Ogata T, Yabuuchi S, Murata K, Kimura A (1989) Expression of the glutathione synthetase gene of Escherichia coli B in Saccharomyces cerevisiae. J Ferment Bioeng 68:390–394
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Plackett RL, Burman JP (1946) The Design of optimum multifactorial experiments. Biometrika 33:305–325
Deming SN, Morgan SL (1987) Experimental design: a chemomatric approach. Elsevier, Oxford
Murata K, Tan K, Kato J, Chibata I (1981) Glutathione production by immobilized Saccharomyces cerevisiae cells containing an ATP regeneration system. Appl Microbiol Biotechnol 11:72–77
Larsson C, Nilsson A, Blomberg A, Gustafsson L (1997) Glycolytic flux is conditionally correlated with ATP concentration in Saccharomyces cerevisiae: a chemostat study under carbon- or nitrogen-limiting conditions. J Bacteriol 179:7243–7250
Penninckx MJ (2002) An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res 2:295–305
Acknowledgment
We thank Asia-Pacific Brewery Co., Shangahi, China, for providing the waste brewer’s yeast, and Chyuan Chemical Co., Hubei, China, for providing l-cysteine. This work was partly supported by the project of Shanghai Leading Academic Disciplines No. B505.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Li, Z. & Ye, Q. Enzymatic synthesis of glutathione using yeast cells in two-stage reaction. Bioprocess Biosyst Eng 33, 675–682 (2010). https://doi.org/10.1007/s00449-009-0361-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-009-0361-6