Abstract
Rhodobacter capsulatus was used for the phototrophic hydrogen production on effluent solution derived from the thermophilic fermentation of Miscanthus hydrolysate by Thermotoga neapolitana. Pretreatments such as centrifugation, dilution, buffer addition, pH adjustment and sterilization were suggested for the effluent before being fed to the photofermentation. Batch-wise experiments showed that R. capsulatus grows and produces hydrogen on the pretreated effluent solution. Moreover, it was found that the hydrogen yield increased from 0.3 to 1.0 L/Lculture by addition of iron to the effluent solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The combination of photosynthetic bacteria with anaerobic bacteria can provide a system to produce hydrogen from residual carbohydrates. In such a system, fermentative bacteria produce hydrogen with the degradation of carbohydrates to organic acids without using light. Complete degradation of glucose to hydrogen and carbon dioxide is impossible by anaerobic digestion. However, resulting organic acids could be sources for photosynthetic bacteria to produce hydrogen since photosynthetic bacteria could use light energy to overcome the thermodynamic barrier of this reaction. The combination of both the kinds of bacteria not only reduces the light energy demand of photosynthetic bacteria but also increases hydrogen yield from carbohydrates [1].
The co-cultivation of a chemotrophic and phototrophic bacterial strain in one single reactor suggested by Zhu et al. [2] failed due to the strongly deviating conditions prefered by the different microorganisms. Thus, the complete microbial degradation of carbohydrates to hydrogen and CO2 has to be carried out in two separate stages.
It has been suggested that in such a two-stage bioprocess starting with a thermophilic fermentation of organic feedstock to hydrogen, CO2 and intermediates which is followed by a consecutive photo-heterotrophic fermentation, where all intermediates will be converted to more hydrogen and CO2, nearly 9 mol of hydrogen per mole of hexose could be obtained (corresponding to a conversion efficiency of 75%). Thus, the development of two-stage bioprocess is a scientific goal for the cost-effective production of pure hydrogen from multiple biomass feedstocks [3].
In a recent study, Tao et al. [4] employed photofermentation with Rhodobacter sphaeroides SH2C to convert the fatty acids produced during dark fermentation into hydrogen and increased the total hydrogen yield from sucrose from 3.7 mol H2/mol sucrose in dark fermentation to 6.6 molH2/mol sucrose by using the two-step process.
Similarly, Nath et al. [5] combined dark and photofermentation to study the feasibility of biological hydrogen production. In dark fermentation, they employed Enterobacter cloacae strain DM11 using glucose as substrate. This was followed by a photofermentation process where the spent medium from the dark process underwent photofermentation by Rhodobacter sphaeroides O.U.001 in a column photobioreactor. The overall yield of hydrogen in the combined process, considering glucose as the preliminary substrate, was found to be higher than that in a single process.
In the present study, the effluent solution derived from a thermophilic dark fermentation process based on material of real biological origin was used as a substrate for hydrogen production in photofermentation by purple non-sulphur bacteria. The need for pretreatments and the effects of the external microelements such as iron and vitamins on hydrogen yield were also examined. To the best of our knowledge, there is no research published on the utilization of thermophilic fermentation effluent for production of hydrogen in a subsequent photofermentation. Thus, the results obtained throughout this study bring a novel approach to biological hydrogen production processes.
Material and methods
Bacteria and culture
Rhodobacter capsulatus (DSM 155) was used in this study as a photofermentative bacterium. The inoculum was prepared by growing cells in the modified medium of Biebl and Pfennig [6] to contain sodium glutamate (2 mM) as the nitrogen source, acetate (40 mM) and lactate (7.5 mM) as the carbon sources; 10% inoculation by volume of the fresh medium was made into the bioreactors.
Dark fermentation effluent was obtained from Agrotechnology and Food Sciences Group, Wageningen University, Netherlands. Miscanthus hydrolysate containing 10 g/l monosaccharides was fermented by Thermotoga neapolitana at 80 °C. The resulting fermentation effluent was analyzed by HPLC to identify the organic acids and sugars. It was determined that the effluent contained 94 mM of acetate, 8.9 mM of lactate, 2 mM of fructose and less than 0.2 mM of formate as the carbon sources. The pH of the effluent was 7.53.
Photobioreactors
Sealed glass bottles with 12–105 ml culture volume were used as photobioreactors. Argon gas was used to create anaerobic conditions. The bioreactors were connected to graded cylinders initially filled with water by capillary tubing made of steel. The produced gas amount was measured from the displaced water, escaping at the bottom of the gas collectors.
Operating conditions
The photobioreactors were maintained at 30–33 °C. The illumination was provided by a 150 W halogen lamp. Uniform light intensity of 4,000 lux at the surface of photobioreactors was attained; 4,000 lux was found to be equivalent to 1,370 μmol photons/m2/s (PAR). The initial pH in photobioreactors was 6.6–6.8.
Analytical methods
Light intensity measurements were made by a luxmeter (Mavolux 5032C). Evolved gas was analyzed by gas chromatography (Hewlett Packard 5200 HP). The bacterial cell concentration was determined in terms of gram dry weight/liter culture by centrifuging the samples and weighing after drying overnight. High pressure liquid chromatography (Agilent 1100 Series) was applied to determine the composition of the initial substrates. Reactor surface temperatures were frequently checked by means of an infrared thermometer (Testo 830-T1). A pH-meter (Portamess 911pH) was used to measure the pH of the liquids.
Pretreatment of the dark fermentation effluent
Pretreatment of the dark fermentation effluent was considered before feeding into the photofermentation. The pretreatments applied were centrifugation, dilution, buffer addition, pH adjustment and sterilization.
Centrifugation and filtering
Photofermentation depends on the light penetration into the media, reducing the color is important in case of dark colored effluents. Centrifugation or filtering not only removes the colloidal particles (i.e., bacteria) from the media but also helps reducing the color of the effluent. In this study, the fermenter effluent was centrifuged after the dark fermentation process; the resulting liquid was visually clear and free from colloidal particles, and further filtering was not applied.
Dilution
The organic acid concentration in the media has a strong effect on the hydrogen production of the bacteria [7]. In order to screen for limitations in the growth and to determine the minimum dilution rate of the effluent, tests were performed in defined media with fixed initial lactate concentration (5 mM) and varying initial acetate concentrations (11–55 mM). It was found that the concentration of acetate in the medium has a significant impact on the duration of the growth lag phase of R. capsulatus; the average growth lag times were found to be 1.0, 1.0, 1.3, 3.0, 4.0 and 7.0 days (average of three runs) in media containing 11, 22, 33, 44, 55 and 66 mM of acetate. Hydrogen production lag times were slightly longer (0.5–1.0 day) than those values. These findings state that acetate concentration higher than 33 mM slows down the growth of R. capsulatus noticeably and more than 55 mM of acetate results in a tremendously long and unacceptable lag time for a continuously operated technical process.
Based on these results, the fermenter effluent which contained 94 mM of acetate was diluted in 1:1 ratio with distilled water and the acetate concentration was halved to 47 mM.
Buffer addition
Fang et al. [8] investigated hydrogen production of photofermentative bacteria in media containing acetate at various pH levels. They found that the favorable pH for the highest hydrogen production rate was in the range of 6.0–8.0.
In order to keep pH stable at the desired level (6.5–7.5), 20 mM of KH2PO4 was added into the filtered and diluted media as buffer and the initial pH was adjusted to 6.5 by addition of NaOH or HCl into the media.
Sterilization
The final effluent solution was sterilized by heat at 121 °C for 15 min. However, the requirement of sterilization might be argued since thermophilic bacteria employed in dark fermentation are not able to grow at photofermentation temperatures (30–35 °C).
Cooling
The temperature of the thermophilic fermentation effluent needs to be decreased from 80 to 30–35 °C so that the mesophilic photofermentative microorganisms survive and carry out the hydrogen production. The dilution of the media accomplishes partial cooling. By allowing the remaining heat to be lost to the surroundings entailed an energy waste in the process. The wasted heat, which would be even bigger in non-diluted cases, could be used for preheating the media to be fed to the thermophilic fermentation stage.
Results and discussion
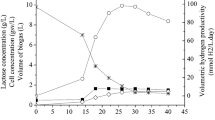
Studies were carried out to investigate hydrogen production of R. capsulatus in the effluent media (pretreated dark fermentation effluent) with or without the addition of other minor nutrients such as vitamins, iron and trace elements. Five bioreactors were run in parallel. The experiments were repeated four times, and the average hydrogen production results are given in Fig. 1.
Effect of external microelements on the hydrogen production of R. capsulatus in bioreactors containing effluent media. Media: pretreated dark fermentation effluent, Vitamins: biotin (0.015 mg/l), niacin (0.5 mg/l) and thiamine (0.5 mg/l), Iron: iron(III)citrate (5 mg/l), Trace elements: ZnCl2 70 μg/l, MnCl2·4H2O 100 μg/l, H3BO3 60 μg/l, CoCl2·6H2O 200 μg/l, CuCl2·2H2O 20 μg/l, NiCl2·6H2O 20 μg/l, NaMoO4·2H2O 40 μg/l
The final pH for all of the runs was in the range of 6.55–7.57. The average final biomass obtained in the runs containing media supplemented with iron and vitamins was 0.48 g/l and the average composition of the gas obtained was 86% H2 and 14% CO2. In theory, the stoichiometric conversion of the substrates should yield 67% H2 and 33% CO2 according to the following hypothetical reactions [9]:
However, it should also be noted that some of the CO2 produced remains in the media as bicarbonate (HCO3 −) and the bacteria are able to utilize it back as a C source, thus those stoichiometric equations do not reflect the actual gas output compositions.
It is clearly observed from Fig. 1 that iron increased gas productivity drastically, from 0.3 LH2/Lculture to 1.0 LH2/Lculture. Therefore, it is concluded that the iron addition is necessary into the media before photofermentation. On the other hand, addition of vitamins and trace elements did not enhance the hydrogen production at all; hydrogen productions were within the same range.
This result is supported by the findings of Kars et al. [10] who utilized R. sphaeroides O.U.001 and reported that there was a growth delay and almost no hydrogen production in the defined medium which had no iron, showing that iron is vital for cellular functions and the hydrogen production. They suggested 0.1 mM of ferric citrate as optimum concentration for hydrogen production. Similarly, Zhu et al. [11] studied the effect of ferrous ion (0–57 μM) on photo heterotrophic hydrogen production by R. sphaeroides and reported that hydrogen production was significantly suppressed when Fe2+ was limited. They found that the hydrogen production increased linearly with an increase in Fe2+ concentration in the range of 0–29 μM; reaching a maximum at 43 μM.
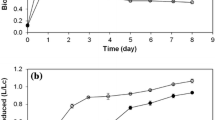
Figure 2 shows the cumulative hydrogen production with respect to time for a run containing effluent media supplemented with iron and vitamins.
At the end of this run, 1.37 LH2/Lculture was obtained. The maximum hydrogen production rate was calculated as 19 ml H2/Lculture.h and the final cell concentration was determined to be 0.46 g/l. These results are comparable to the results obtained in defined media with similar C content: 40 mM of acetate and 7.5 mM of lactate were used as the carbon sources and 1.19 LH2/Lculture was obtained at the end of a batch run at a maximum hydrogen production rate of 17 ml H2/Lculture.h, the final cell concentration was 0.52 g/l (unpublished data).
Conclusion
Rhodobacter capsulatus grew on fermenter effluent and produced hydrogen. The results suggest that R. capsulatus is a good candidate to employ in the photofermentation step of a combined dark- and photofermentation process.
Acetate concentration higher than 60 mM is not acceptable in the media due to growth inhibition of R. capsulatus.
Centrifugation and filtering to increase the transparency of the effluent media, dilution with water to reduce the organic acid concentration, buffer addition to keep pH stable during photofermentation and sterilization to prevent contamination were suggested as the pretreatment processes to the dark fermenter effluent.
It was also found that among the external micronutrients tested, iron addition increased the hydrogen production the most whereas vitamins and other trace elements were not that effective.
References
Das D, Veziroglu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energy 26:13–28
Zhu H, Wakayama T, Asada Y, Miyake J (2001) Hydrogen production by four cultures with participation by anoxygenic phototrophic bacterium and anaerobic bacterium in the presence of NH4 +. Int J Hydrogen Energy 26:1149–1154
Claassen PAM, deVrije T (2006) Non-thermal production of pure hydrogen from biomass: HYVOLUTION. Int J Hydrogen Energy 31:1416–1423
Tao Y, Chen Y, Wu Y, He Y, Zhou Z (2007) High hydrogen yield from a two-step process of dark- and photo-fermentation of sucrose. Int J Hydrogen Energy 32:200–206
Nath K, Muthukumar M, Kumar A, Das D (2008) Kinetics of two-stage fermentation process for the production of hydrogen. Int J Hydrogen Energy 33:1195–1203
Biebl H, Pfennig N (1981) Isolation of members of the family Rhodosprillaceae. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes, vol 1. Springer, New York, pp 267–273
Asada Y, Ohsawa M, Nagai Y, Ishimi K, Fukatsu M, Hideno A, Wakayama T, Miyake J (2008) Re-evaluation of hydrogen productivity from acetate by some photosynthetic bacteria. Int J Hydrogen Energy 33:5147–5150
Fang HHP, Liu H, Zhang T (2005) Phototrophic hydrogen production from acetate and butyrate in wastewater. Int J Hydrogen Energy 30:785–793
Koku H, Eroglu I, Gündüz U, Yücel M, Türker L (2002) Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides O.U. 001. Int J Hydrogen Energy 27:1315–1329
Kars G, Gündüz U, Yücel M, Türker L, Eroglu I (2006) Hydrogen production and transcriptional analysis of nifD, nifK and hupS genes in Rhodobacter sphaeroides O.U.001 grown in media with different concentrations of molybdenum and iron. Int J Hydrogen Energy 31:1536–1544
Zhu H, Fang HHP, Zhang T, Beaudette LA (2007) Effect of ferrous ion on photo heterotrophic hydrogen production by Rhodobacter sphaeroides. Int J Hydrogen Energy 32:4112–4118
Acknowledgments
This study was financially supported by EU 6th framework project “HYVOLUTION” and by Middle East Technical University, Research Fund with the project number of BAP-08-11-DPT.2002K120510 (OYP-FBE-BTEK1). The authors thank Dr. Truus de Vrije from Agrotechnology and Food Sciences Group, Wageningen University, Netherlands for kindly providing the dark fermentation effluent samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uyar, B., Schumacher, M., Gebicki, J. et al. Photoproduction of hydrogen by Rhodobacter capsulatus from thermophilic fermentation effluent. Bioprocess Biosyst Eng 32, 603–606 (2009). https://doi.org/10.1007/s00449-008-0282-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-008-0282-9