Abstract
Fishmeal wastewater, a seafood processing waste, was utilized for production of lactic acid and fungal biomass by Rhizopus oryzae AS 3.254 with the addition of sugars. The 30 g/l exogenous glucose in fishmeal wastewater was superior to starch in view of productivities of lactic acid and fungal biomass, and COD reduction. Fishmeal wastewater can be a replacement for peptone which was the most suitable nitrogen source for lactic acid production among the tested organic or inorganic nitrogen sources. Exogenous NaCl (12 g/l) completely inhibited the production of lactic acid and fungal growth. In the medium of COD 5,000 mg/l fishmeal wastewater with the addition of 30 g/l glucose, the maximum productivity of lactic acid was 0.723 g/l h corresponding to productivity of fungal biomass 0.0925 g/l h, COD reduction 84.9% and total nitrogen removal 50.3% at a fermentation time of 30 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lactic acid, a naturally occurring multifunctional organic acid, has long been of use in the pharmaceutical, chemical and food industries. Recently, new applications of lactic acid, as a monomer in biodegradable and biocompatible polylactate polymers, an environment-friendly alternative to biodegradable plastics or as an intermediate in the synthesis of high-volume oxygen-containing chemicals, have the potential to greatly expand the market for lactic acid, if more economical production processes could be developed [1–3]. The efficiency and economics of the ultimate lactic acid fermentation is however still a problem from many points of view and media composition play a vital role in the improvement of such a process [4]. Much research has consequently focused on finding cheaper supplements such as whey, molasses, starch, beet- and cane-sugar and other carbohydrate-rich materials for the lactic acid production [1–3, 5].
Nitrogen source is usually the most expensive component of microbial growth substrates [6, 7]. Fishmeal wastewater containing soluble proteins is produced in large amounts as a waste byproduct of the fishmeal processing plants. In spite of a high efficiency reached in anaerobic treatment of this wastewater, an organic load accounting for about 20% of the initial COD remains in it. The nitrification percentage for the further treatment is about 20–65% [8]. On the other hand, increasing concern is being focused on converting these renewable materials into commercially valuable products. Although the use of fish protein hydrolysates for maintaining the growth of different microorganisms have received a great deal of attention [6, 9, 10], only limited number of reports have been published about the application of this substrate to metabolite production such as lysine, lipase and bio-fluccant [7, 11, 12]. This is the first time that the fishmeal wastewater has been tested as one substrate for lactic acid production.
In the present work, we tested the potential use of fishmeal wastewater as one substrate for the fermentative production of lactic acid by Rhizopus oryzae, one important mold that metabolizes L(+)-lactic acid [1–4, 13, 14] in order to recycle this abundant waste and develop a new process for lactic acid production integrated with fishmeal wastewater treatment.
Materials and methods
Characterization of fishmeal wastewater
The fishmeal wastewater used in this investigation was effluent coming from Longyuan Food and Can Pty Ltd, Dalian, China and arose from the commercial production of fish can. The pH of the fishmeal wastewater was 6.5–7.1. Other characteristic parameters (g/l) included total suspended solid, 81–89; volatile suspended solid, 54–57; COD, 48–56; total Kjeldahl-N, 8.0–12.7; protein, 50–79 and Cl−, 7.0–10.
Microorganisms
The strain of Rhizopus oryzae AS 3.254 was purchased from Culture Collection, Institute of Microbiology, Chinese Academy of Sciences. This strain was propagated and maintained on potato dextrose agar (PDA) slants at 4 °C.
Culture and fermentation medium
The composition of the pre-culture medium (g/l) was: soluble starch, 10; peptone, 5.0; yeast extract, 5.0; KH2PO4, 0.2; and MgSO4·7H2O, 0.2. This medium was autoclaved at 121 °C for 20 min.
Experimental set-up
Spores grown on PDA slants at 30 °C for 7 days were collected using a platinum loop and suspended in sterilized distilled water. A 250 ml flask containing 100 ml of the culture medium was inoculated with a final concentration of 105 spores/ml and incubated on a rotary shaker at 30 °C for 12 h. The overnight culture, as seed culture, was used to initiate growth in the fishmeal wastewater media.
The lactic acid production by Rhizopus oryzae AS 3.254 were conducted in 250 ml flasks containing 100 ml final volume of the wastewater media inoculated with 5 ml of the seed culture. In order to avoid pH decrease due to lactic acid production, the addition of sterile CaCO3 (5%, v/v) in powder form to each flask after inoculation was carried out. Unless otherwise stated, the agitation rate and incubation temperature were 150 rpm and 30 °C, respectively.
Sample preparation and analytical methods
The culture broth was centrifuged and the resulting supernatant was used for measurement of glucose, COD and total Kjeldahl-N. The glucose was estimated by the dinitrosalicylic acid method [15]. Solids, COD and total Kjeldahl-N were measured according to procedures described in Standard Methods for the Examination of Water and Wastewater [16]. Lactic acid and dry cell weight were determined as described previously [2]. All experiments were carried out in duplicate and repeated at least twice. Appropriate tests of significance, analysis derivations (ANOVA) and confidence difference at 5% level were used in the data evaluations.
Results and discussion
The use of fishmeal wastewater
Lactic acid fermentation was investigated using fishmeal wastewater under different strategies. In the first assay, the fermentation medium contained only fishmeal and water. No lactic acid production and fungal growth were observed in this medium (data was not shown). These results revealed that although fishmeal wastewater contained large amount of organic protein, the fungal cells could not metabolize them in this form. In another experiment, it was thought that the addition of an easily metabolized sugar to the fermentation medium containing only fishmeal wastewater and water, might improve lactic acid production. Therefore, the diluted fishmeal wastewater was mixed with 40 g/l glucose and the results showed that the exogenous glucose had great beneficial effect on lactic acid production and fungal growth. Fishmeal wastewater with COD ranging from 2,500–10,000 mg/l had a comparatively high productivity of lactic acid and fungal biomass. The maximum productivity of lactic acid and biomass were about 0.311 g/l h at COD 2,500 mg/l and 0.062 g/l h at COD 10,000 mg/l, respectively. Otherwise stated, fishmeal wastewater with COD 5,000 mg/l was chosen for the following investigation.
Effect of substrates
In order to identify the fermentation capacity of R. oryzae AS 3.254 and the effect of two common carbon sources, glucose and starch, the fungi was cultivated in the fishmeal wastewater with a glucose or starch concentration of 30 g/l. The results in Table 1 showed this Rhizopus fungus performed at a higher COD reduction, productivity of lactic acid and biomass in glucose medium. These phenomena may be explained by an easy access of glucose by the fungal whereas it needs to access monosaccharide by secreting amylolytic enzymes for the degradation of starch substrates. During the 24 h of cultivation, lactic acid had a volumetric concentration of 15.9 g/l in glucose medium, while only 0.818 g/l lactic acid was measured in the starch medium. Additionally, R. oryzae AS 3.254 grew better in glucose than starch and the biomass volumetric production in glucose and starch medium was 2.22 and 1.24 g/l, respectively. As expected, the addition of glucose in fishmeal wastewater has an evidently higher COD reduction than starch supplementation during the 24-h incubation period. With the prolonged incubation time from 24 to 48 h, the difference between glucose and starch in terms of lactic acid and biomass productivities, and COD reduction became less although glucose led to a high productivity of lactic acid and a fast cell growth termed as biomass productivity.
From the lactic acid yield point of view, at 48 h incubation time, fishmeal wastewater with a glucose concentration of 30 g/l achieved a yield of 0.818 g COD/g COD, higher than the 0.673 g COD/g COD in pure glucose medium. Comparatively, fishmeal wastewater containing 30 g/l starch only had a yield of 0.220 g COD/g COD which was also higher than the 0.132 g COD/g COD in the pure starch medium. These results showed comparatively higher fermentation efficiencies of fishmeal wastewater than pure glucose or starch medium.
Effect of glucose concentration
Productivities of lactic acid and biomass, and COD reduction were enhanced with increasing glucose concentration with an optimum at 30 g/l (Fig. 1). Any further increase in glucose level resulted in a decrease in data of the three parameters. This decrease-trend at high initial glucose concentrations could be partly attributed to be a result of repressive effect of glucose on R. oryzae AS 3.254 growth, and consequently led to a lower lactic acid and biomass productivities and COD reduction. This could also be due to the increased accumulation of lactic acid [1, 2, 4].
When the fungus is grown in excess nitrogen and growth is limited by the carbon source, the protein component of the cell is degraded most rapidly. Conversely, if excess carbon is present in the media, autolysis raises the amount of reducing substances [17]. Suitable C/N ratio is consequently very important for microorganism metabolism. The 30 g/l exogenous glucose could keep a suitable C/N ration in fishmeal wastewater for R. oryzae AS 3.254 and it could keep higher levels of lactic acid production, fungal growth, COD and total nitrogen reduction in the medium in every case of our study.
Effect of NaCl concentration
The fishmeal wastewater contained high salt and it was necessary to investigate the effect of salts termed as NaCl on growth and lactic acid production of R. oryzae AS 3.254. This exploration was carried out in COD 5,000 mg/l fishmeal wastewater medium with addition of 30 g/l glucose and the incubation time was 48 h. The relationship between NaCl concentration, glucose change, productivity of fungal biomass and lactic acid was shown in Fig. 2. The productivity of lactic acid and fungal biomass were greatly affected by NaCl concentration which decreased to 98.5 and 95.1% from 0.408 and 0.0479 g/l h in the no exogenous NaCl medium to 0.00600 and 0.00235 g/l h at 12 g/l exogenous NaCl, respectively. Accordingly, glucose in exogenous NaCl below 8.0 g/l could be easily metabolized and the consumption was completely stopped in NaCl concentration above 12.0 g/l.
Cells under salt stress initially accumulate salts as free osmotica; however, a toxic specific ion effect appears once a certain threshold level of Na and/or Cl accumulation has been reached. An excess of these ions may alter membrane integrity, enzymatic activity, and protein and nucleic metabolism [18]. Stress condition by salt is also reported to enhance membrane fluidity by desaturation of fatty acids and further result in the change of organic acid productivity, as found in biochemical studies [19]. For the current fungi R. oryzae AS 3.254, 12.0 g/l exogenous NaCl (total salt concentration in the medium, termed as NaCl, was 13.2 g/l) may be above the threshold level and lead to the great composition change of fatty acids in the membrane of R. oryzae AS 3.254, and further restrains its lactic acid production. Also, compared with R. oryzae IFO 4707 reported in the literature, R. oryzae AS 3.254 was more sensitive to NaCl whereas the former had no virtual change in fatty acid composition in 20.0 g/l NaCl medium [20].
Evaluation of nitrogenous substrates from fishmeal wastewater
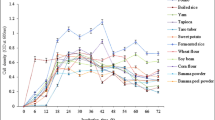
People working in the fermentation industry or, more generally, in biotechnology are used to comparing nitrogenous substrates on a weight basis, whatever the water content and the nitrogen content of the tested samples. So, R. oryzae AS 3.254 was grown in the same culture medium containing 0.2% (wt/vol or vol/vol) organic or inorganic nitrogen sources. The medium were set at (g/l): glucose 30.0; KH2PO4, 0.2; MgSO4·7H2O, 0.2; NaCl, 0.1; FeSO4·7H2O, 0.01; CaCl2·2H2O, 0.01; ZnSO4, 0.001; MnSO4·4H2O, 0.001; CuSO4·5H2O, 0.0002; Thiamine HCl, 0.0002; D-Biotin 0.00002 in addition to 2.0 g/l of ammonium sulfate, ammonium chloride, urea, yeast extract or peptone. Samples with fishmeal wastewater were diluted ten times as nitrogen source before 30.0 g/l glucose was added and no other nutrient was supplemented.
The results summarized in Fig. 3 revealed that R. oryzae utilized this fishmeal wastewater and other organic or inorganic nitrogen sources for stimulating fungal cell growth and lactic acid production. In the peptone media, the most suitable nitrogen source for lactic acid production among the tested samples, the productivity of lactic acid and fungal biomass were 0.509 and 0.0463 g/l h, respectively. Compared with peptone, a similar amount of lactic acid productivity 0.500 g/l h and fungal biomass 0.0438 g/l h were obtained in the fishmeal wastewater. These results showed, in view of the nitrogen source, fishmeal wastewater can be a suitable substitute for high cost peptone for lactic acid production and fungal biomass growth. Yeast extract and urea appeared to be a less favored nitrogen source for lactic acid production, whereas more nutrients in yeast extract media was transformed into fungal biomass.
Although R. oryzae has the advantage of synthesizing lactic acid in a minimal medium containing ammonium salts as the sole nitrogen source [21], R. oryzae AS 3.254 can have a comparatively higher production of lactic acid and fungal biomass in fishmeal wastewater or peptone medium than the tested non-organic ammonium salts medium in this study. In view of the “waste”, it is feasible that fishmeal wastewater was recycled for lactic acid production by R. oryzae AS 3.254. On the other hand, taking the fungal ability of lactic acid production into consideration, the value of fishmeal wastewater medium may be even equal to peptone medium and this supported previous report in that case peptone from fish can give overall comparably results with the casein peptone [6].
Kinetic characteristics of metabolic parameters
The results depicted in Fig. 4 showed R. oryzae AS 3.254 had a high capacity to assimilate glucose and other nutrients in fishmeal wastewater. Glucose was utilized immediately after cultivation started. Simultaneously, the total nitrogen removal was 48.1% at 24 h incubation. Theoretically, at 24 h incubation time, the disappeared glucose contribution to COD was 11.3 g/l, lower than the total disappeared COD 30.8 g/l in the culture. This phenomena indicated that the carbon and nitrogen sources in fishmeal wastewater were concomitantly utilized with the exogenous glucose by R. oryzae AS 3.254 and resulted in lactic acid production and fungal growth. The trends of lactic acid production increase and glucose decrease were evident between 24 and 48 h. Comparatively, the increase of COD reduction, fungal biomass and total nitrogen reduction was rather slow during this period. Stationary phases for total nitrogen reduction and cell growth occurred after 24 and 48 h. The fermentation broth was clearly free from suspended solids after 24 h, after which the biomass could be separated by simple filtration. The highest lactic acid production and yield were 31.1 g/l and 0.897 g COD/g COD, respectively, at a fermentation time 66 h. In terms of productivity, however, a period of 30 h fermentation may be selected for a batch process.
The addition of glucose or starch to the fishmeal wastewater can realize lactic acid production integrated with fishmeal wastewater treatment. However, this process may bring in an extra cost. Cheap raw materials, such as molasses, beet-sugar, potato starch and other carbohydrate-rich materials have been used for lactic acid production by Rhizopus oryzae [1–3, 5]. Therefore, the replacement of glucose by cheap carbohydrate-rich materials may reduce this process cost. On the other hand, although the fermentation broth was clearly free from suspended solids after 24 h, nearly 50% total nitrogen was still in this medium. According to the available literature, extracellular proteases can play a key role in the physiology of some fungi [10, 22]. Consequently, the 50% nitrogen left in the medium may be partly attributed to the extracellular proteases secreted by Rhizopus oryzae. The above possibilities will be the subject of further studies.
Conclusion
Lactic acid production integrated with fishmeal wastewater treatment by Rhizopus oryzae was investigated in this study. Glucose was one superior additive to starch in view of productivities of lactic acid and fungal biomass, and COD reduction. Fishmeal wastewater can be a replacement for peptone which was the most suitable nitrogen source for lactic acid production among the tested organic or inorganic nitrogen sources. Exogenous NaCl (12 g/l) completely inhibited the production of lactic acid and fungal growth. Although total nitrogen removal was not high, several interesting facts termed as 0.723 g/l h productivity of lactic acid corresponding to 0.0925 g/l h fungal biomass and 84.9% COD reduction were observed at fermentation time 30 h. Additionally, the highest lactic acid production 31.1 g/l and yield 0.897 g COD/ g COD at fermentation time 66 h were also achieved in this study. This process of lactic acid production integrated with fishmeal wastewater treatment helps to utilize wastes like scales which are environmental problems to fish processing industries due to gradual increase in production of fishes around the world.
References
Huang LP, Jin B, Paul L, Zhou JT (2003) Biotechnological production of lactic acid integrated with potato wastewater treatment by Rhizopus arrhizus. J Chem Technol Biotechnol 78:899–906
Huang LP, Jin B, Paul L (2005) Direct fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Bioprocess Biosyst Eng 27:229–238
Jin B, Yin PH, Ma YH, Zhao L (2005) Production of lactic acid and fungal biomass by Rhizopus fungi from food processing waste streams. J Ind Microbiol Biotechnol 32:678–686
Bulut S, Elibol M, Ozer D (2004) Effect of different sources on L(+)-lactic acid production by Rhizopus oryzae. Biochem Eng J 21:33–37
Ueno T, Ozawa Y, Ishikawa M, Nakanishi K, Kimura T (2003) Lactic acid production using two food processing wastes, canned pineapple syrup and grape invertase, as substrate and enzyme. Biotechnol Lett 25:573–577
Dufosse L, De La Broise D, Guérard F (2001) Evaluation of nitrogenous substrates such as peptones from fish: a new method based on gompertz modeling of microbial growth. Curr Microbiol 42:32–38
Ghorbel S, Souissi N, Triki-Ellouz Y, Dufossé L, Guérard F, Nasri M (2005) Preparation and testing of Sardinella protein hydrolysates as nitrogen source for extracellular lipase production by Rhizopus oryzae. World J Microbiol Biotechnol 21:33–38
Garrido JM, Guerrero L, Méndez R, Lema JM (1998) Nitrification of waste waters from fish-meal factoties. Water SA 24(3):245–250
De la Broise D, Dauer G, Gildberg A, Guérard F (1998) Evidence of positive effect of peptone hydrolysis rate on Escherichia coli culture kinetics. J Marine Biotechnol 6:111–115
Basu B, Das M, Banik AK (2005) Biodegradation of fish scale by Aspergillus niger: an enzymatic and scanning electron microscopic study. J Food Sci Technol 42(5):387–391
Coello N, Brito L, Nonus M (2000) Biosynthesis of L-lysine by Corynebacterium glutamicum grown on fish silage. Bioresource Technol 73:221–225
Zhou X, Huang LP, Wang J, Zhou JT, Zhang JS (2003) Production and properties of bioflocculant by Pseudomonas sp. GX4-1 using fish meal wastewater. Chin J Appl Environ Biol 9(4):436–438
Bai DM, Jia MZ, Zhao XM, Ban R, Shen F, Li XG, Xu SM (2003) L(+)-lactic acid production by pellet-form Rhizopus oryzae R1021 in a stirred tank fermentor. Chem Eng Sci 58:785–791
Bai DM, Zhao XM, Li XG, Xu SM (2004) Strain improvement of Rhizopus oryzae for over-production of L(+)-lactic acid and metabolic flux analysis of mutants. Biochem Eng J 18:41–48
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
APHA (1992) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association, Washington
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Bois G, Bertrand A, Piché Y, Fung M, Khasa DP (2006) Growth, compatible solute and salt accumulation of five mycorrhizal fungal species grown over a range of NaCl concentrations. Mycorrhiza 16:99–109
Guerzoni ME, Lanciotti R, Cocconcelli PS (2001) Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiol 147:2255–2264
Oda Y, Yajima Y, Kinoshita M, Ohnishi M (2003) Differences of Rhizopus oryzae strains in organic acid synthesis and fatty acid composition. Food Microbiol 20:371–375
Hang YD, Hamamci H, Woodams E (1989) Production of L(+)-lactic acid by Rhizopus oryzae immobilized in calcium alginate gels. Biotechnol Lett 11:119–120
Damare S, Raghukumar C, Muraleedharan UD, Raghukumar S (2006) Deep-sea fungi as a source of alkaline and cold-tolerant proteases. Enzyme Microbial Technol 39:172–181
Acknowledgments
This study was funded by SRF for ROCS, SEM of China and Key Laboratory of Industrial Ecology and Environmental Engineering, Ministry of China Education. We would like to thank Longyuan Food and Can Pty Ltd, Dalian, China for supplying fishmeal wastewater samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, L.P., Dong, T., Chen, J.W. et al. Biotechnological production of lactic acid integrated with fishmeal wastewater treatment by Rhizopus oryzae . Bioprocess Biosyst Eng 30, 135–140 (2007). https://doi.org/10.1007/s00449-006-0110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-006-0110-z