Abstract
This work was aimed at producing inulinase by solid-state fermentation of sugarcane bagasse, using factorial design to identify the effect of corn steep liquor (CSL) and soybean bran concentration, particle size of bagasse and size of inoculum. Maximum inulinase activity achieved was 250 U per g of dry substrate (gds) at 20% (w/w) of CSL, 5% (w/w) of soybean bran, 1 × 1010 cells mL−1 and particle size of bagasse in the range 9/32 mesh. The use of soybean bran decreased the time to reach maximum activity from 96 to 24 h and the maximum productivity achieved was 8.87 U gds−1 h−1. The maximum activity was obtained at pH 5.0 and 55.0°C. Within the investigated range, the enzyme extract was more thermostable at 50.0°C, showing a D-value of 123.1 h and deactivation energy of 343.9 kJ gmol−1. The extract showed highest stability from pH 4.5 to 4.8. Apparent K m and V max are 7.1 mM and 17.79 M min−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the use of solid-state fermentation (SSF) has been drawing much attention in the development of some bioprocesses and bioproducts, since it involves the use of agro-industrial residues of low cost [1–5]. Many factors may influence SSF performance, such as the selection of a good microorganism, choice of a suitable substrate and control of operational parameters (physical, chemical and biochemical). Such variables can be manipulated to improve the yield of the fermentative process [2–4].
The selection of a good solid material that supports the development of microorganism is a key aspect in SSF. This material can act as a source of nutrients and/or as support to microbial growth. There are two considerations regarding the selection of a good substrate: first, it may combine low water activity with high rate of oxygen transfer; second, the substrate should be suitable to produce the desired products [6]. The particle size of the substrate is a variable that presents a strong effect on fermentative process. Small particles increase the contact area between the microorganism and the gas phase. However, small particles can compact the medium, making the transfer of oxygen, water and nutrients difficult [7].
The enzyme world market is around one billion dollars per year, with an enhancement on amount of patents and research in this area. Many enzymes are produced by submerged fermentation and are considered commodities, since their production and recovery costs are inversely proportional to their concentration in the fermented broth [8].
Microbial inulinase can be produced by several microorganisms, including fungi, yeast and bacteria. The genus Kluyveromyces sp. is reported to be a good inulinase producer [9, 10]. The inulinases may be used to produce high fructose syrups (HFS) by enzymatic hydrolysis of inulin, yielding up to 95% of HFS. Inulinases also act on sucrose, yielding 45% of HFS [2, 6, 11]. Most of the investigations of inulinase production are based on submerged fermentations.
The knowledge of temperature and pH influence on the activity of enzyme preparations is very important in food processes. The determination of optimum temperature and pH rate is particularly essential in enzymatic processes. High temperature and low pH may decrease the risk of contamination, improve the solubility of some substrates, including inulin, and can reduce the color formation in some syrups [12]. The operational conditions are determined based on the D-value and first-order deactivation rate constant, k, of the enzymes used in the process [13].
So far, no previous studies that evaluate the effect of process parameters on production of inulinase by SSF could be found in literature. Investigations on characterization of inulinase obtained by SSF also could not be found elsewhere. Assessment of fermentation conditions for inulinase production is of relevance since many fermentation parameters may significantly affect productivity of the enzyme, and thus, production costs. Characterization of enzyme is of great importance, given that the conditions chosen for the enzyme reaction should be optimized for productivity of the desired product without causing loss of enzyme activity. In this context, this work aimed to evaluate the influence of size of inoculum, substrate supplementation (soybean bran and corn steep liquor, CSL) and particle size of substrate on inulinase production, as well as to partially characterize the crude enzyme extract.
Experimental
Agroindustrial residues
Sugar cane bagasse was obtained from COTREL Ltd (Erechim, RS, Brazil), CSL from Corn Products Brazil (Mogi Guaçu, SP, Brazil) and soybean bran from Olfar S.A. (Erechim, RS, Brazil). Sugar cane bagasse was collected and dried at 60°C for 24 h; manually crushed and stored at −15°C. The samples of sugar cane bagasse were classified between 0/9, 9/32 and 32/60 mesh. The soybean bran was classified between 9/32 mesh.
Microorganism and medium
The strain of Kluyveromyces marxianus NRRL Y-7571 was used for inulinase production. The microorganism was maintained on YM agar medium (g L−1): yeast extract 3.0, malt extract 3.0, peptone 5.0, glucose 10.0, agar 20.0 and subcultured every 3 weeks.
Cell production for pre-inoculum was carried out in 50-mL test tubes with 10 mL of liquid YM medium. The medium was inoculated with a loopful of stock culture and incubated at 30°C for 24 h. Medium for pre-inoculum contained (g L−1): sucrose 20.0, yeast extract 5.0, K2HPO4 5.0, NH4Cl 1.5, KCl 1.15 and MgSO4·7H2O 0.65. Each test tube with YM medium was transferred to a 500 mL Erlenmeyer flask with 100 mL of medium and incubated at 30°C and 150 rpm for 24 h.
SSF technique
Fermentations were carried out in batch tray reactors with 5 g of dry bagasse (3 cm), supplemented with CSL and soybean bran. Each flask was covered with hydrophobic fabric and sterilized at 121°C for 20 min. The flasks were inoculated with a cell suspension and incubated for 96 h in a chamber with temperature and humidity control. The inulinase activity was determined in 24, 48, 72 and 96 h for all the experiments. The concentrations of supplements and inoculum were defined by the factorial design. After fermentation, the enzyme was extracted by adding 50 mL of sodium acetate buffer 0.1 mol L−1 pH 4.8 to the fermented medium, following incubation at 30°C and 150 rpm for 30 min. Inulinase activity was assayed in the supernatant after solid filtration.
Experimental design
The Plackett–Burman experimental design assumes that there are no interactions between the different variables, x i , in the range under consideration. A linear approach is considered to be sufficient for screening. \( Y = \beta _{0} + {\sum {\beta _{i} x_{i} } }\quad (i = 1, \ldots ,k) \) where Y is the estimated target function and β i are the regression coefficients. The Plackett–Burman experimental design is a fractional factorial design and the main effects of such a design may be simply calculated as the difference between the average of measurements made at the high level (+1) of the factor and the average of measurements at the low level (−1) [14].
In the present work, four variables were screened in 11 trials, with three central points. The effect of CSL and soybean bran concentration, particle size of sugar cane bagasse and size of inoculum were studied. The experimental design is showed in Table 1. All trials were performed in duplicate and the average results are presented.
Inulinase assay
The inulinase activity in the clarified samples was measured by incubating 0.5 mL of appropriately diluted enzyme source with 4.5 mL of 2% (w/v) sucrose solution at 50°C in sodium acetate buffer (0.1 mol L−1 pH 4.8). Reducing sugars released were measured by the 3,5-dinitrosalicylic acid method [15]. A separate blank was set up for each sample to correct the non-enzymatic release of sugars. One unit of inulinase activity was defined as the amount of enzyme that released 1 μmol of reducing sugars as glucose per minute under the standard assay conditions.
Preliminary characterization of enzyme extract
The kinetic study with crude enzyme extracts was carried out in terms of optimum temperature, thermostability, optimum pH and pH stability. The enzyme extract was also studied for apparent Michaelis–Menten constants, K m and V max. The enzyme extract used was obtained using the conditions of the central point of the experimental design.
Effect of temperature and pH on inulinase activity
The effect of temperature and pH was assessed by a 22 full factorial design, with three central points. Table 1 shows the range of factors investigated. All the experiments were carried out in duplicate and the average results are presented. The inulinase activity was determined as described above.
Thermal inactivation of inulinase
Isothermal inactivation treatment was performed in a water bath, in the range of 50–65°C. The residual inulinase activity was determined as described above.
Enzyme inactivation often follows the first-order kinetics [13]. Under isobaric–isothermal conditions, the decrease of enzyme activity as function of treatment time can be described by:
where k is inactivation rate constant; A 0 and A are the initial enzyme activity and the remaining activity after heating for time t, respectively.
In food processing, it is common to express first-order reactions in terms of D-values [13]. The decimal reduction time, or D-value, is defined as the time, at constant temperature and pressure, needed for a 90% reduction of the initial activity. For first-order reactions, the D-value is inversely proportional to the inactivation rate constant:
Activation energies were calculated based on Arrhenius plots.
Effect of pH on enzyme activity
The effect of pH on inulinase activity was investigated in the range of 3.5–5.5, at 50°C. The residual inulinase activity was determined as described above.
Determination of Michaelis–Menten constants
Enzyme assays with 0.5 mL of crude enzyme extract were performed in sodium acetate buffer 0.1 M pH 4.8 at 50°C with concentrations of sucrose from 0.5 to 35 g L−1. The K m and V m were determined by Lineweaver–Burk plots.
Results and discussion
Inulinase production
Table 2 shows the results for 96 h of fermentation. Maximum inulinase activity achieved was 241 U per g of dry substrate (gds) in central point [5% (w/w) of soybean bran, 20% (w/w) CSL, 4 mL inoculum suspension and particle size of 9/32 mesh]. In a study carried out using one-factor-at-a-time experiments, Selvakumar and Pandey [9] obtained a maximum inulinase activity of 122.9 U gds−1 in wheat bran, which is almost half of the maximum activity obtained in the present work. These authors investigated the effect of incubation temperature, heat treatment of solid substrate, size of inoculum and moisture content, using K. marxianus ATCC 52466. Other typical results of inulinase activity are only reported in literature for submerged fermentation (up to 176 U mL−1) [16]. However, comparison of results of submerged and SSF is difficult due to the great differences in these fermentation systems.
Figure 1a, b show the kinetic behavior of inulinase production for all trials. In a previous study we showed that maximum production could be achieved in 96 h of fermentation with K. marxianus NRRL Y-7571 using sugar cane bagasse and CSL as substrate [17]. In the present work it is clearly shown (Fig. 1) that the addition of soybean bran lowers the time to reach maximum inulinase activity. This behavior might be related to the increase in nitrogen content due to the addition of soybean bran.
Kinetics of inulinase production for all the experiments of the Plackett–Burman experimental design presented in Table 2. a Experimental runs 2, 3, 4, and 6 were carried out with 10% of soybean bran. b Experimental runs 1, 5, 7 and 8 were carried out without supplementation with soybean bran. In both figures experimental run 9 was included, which refers to the central point of the experimental design and is the average of three independent experiments. Inulinase activity is expressed in units per gram of dry substrate (U gds−1)
The control of process variables (temperature, moisture), the selection of substrate and microorganism are some parameters that can greatly affect the maximum inulinase production by SSF. This result is similar to those obtained in trials 1 and 5, where the substrate was sugar cane bagasse supplemented with CSL (Fig. 1b). Selvakumar and Pandey found a maximum production at 72 h for K. marxianus CDBB-L-278 and 48 h for Staphylococcus sp. using several agroindustrial residues [9].
The results of Fig. 1 were statistically analyzed for evaluation of the main effects of the studied factors. The effects were then represented by a Pareto chart presented in Fig. 2. This figure shows that the factors that significantly (p < 0.1) affect the inulinase production in SSF were the soybean bran concentration, particle size of substrate and CSL concentration. Soybean bran concentration presented a positive effect on production, while CSL and particle size showed negative effects on the response.
Effect of independent factors on inulinase production (48 h), plotted as a Pareto chart of effects. The horizontal bars represent the magnitude of the effect estimates divided by the standard error (absolute value of the effect). Bars that cross the dotted line stand for statistically significant effects with 90% of confidence
Inoculum size did not significantly influence the production of inulinase. Usually the size of inoculum plays a significant role in the production of metabolites under SSF [6, 7]. It is important to provide an optimal size of inoculum in fermentation process, since a lower inoculum size may give insufficient biomass and permit the growth of undesirable organisms, whereas a higher inoculum size may produce too much biomass and deplete the substrate of nutrients necessary for product formation [9]. However, some authors also report that this factor did not affect enzyme production in SSF [9, 18, 19].
Optimal particle size of substrate may be determined to obtain high yield [6, 7]. The increment in particle size of sugar cane bagasse showed a negative effect on inulinase production. This negative effect can be explained by the fact that the substrate acts both as nutrient source and support for microbial growth. Higher particles render mass transfer difficult, due to the decrease in superficial area, and then in the availability of nutrients.
Soybean bran and CSL may be used as nitrogen supplements, because the sugar cane bagasse is a very poor nitrogen source [3]. However, the soybean bran and CSL are also source of minerals, vitamins, amino acids and other essential nutrients to growth. In this work, CSL showed a negative effect on inulinase production and soybean bran showed a positive effect. This behavior may be related to inhibition due to excess of some nutrients that are present in CSL. In fact, when the composition of these two substrates is compared, it is possible to verify that CSL presents lower concentrations of most nutrients (carbohydrates, nitrogen, fat, fibers, amino acids, vitamins and some minerals like Ca, Na, Mg, K, P, Fe and Mn) than soybean bran. However, CSL shows higher content of Cu (1.5 mg 100 g−1), Se (0.03 mg 100 g−1) and Zn (6.0 mg 100 g−1) when compared to soybean bran (1.5 × 10−6 mg 100 g−1, 0.016 mg 100 g−1 and 5.0 mg 100 g−1, respectively). Possibly the excess of such minerals might be causing the negative effect of the concentration of CSL on inulinase production.
The performance of a fermentative process may be determined by productivity. Figure 3 presents the kinetic behavior of inulinase productivity. Maximum inulinase productivity achieved was 8.87 U gds−1 h−1 at the conditions of the central point of the Plackett–Burman Design. This value is almost five times higher than those already reported for inulinase production using SSF in wheat bran (1.7 U gds−1 h−1) [9], and more than the productivity obtained in previous works of our group (5.44–6.18 U gds−1 h−1) [17, 20]. Trials carried out with addition of soybean bran achieved maximum productivity within the first 24 h of fermentation. In experiments without this supplement, maximum production occurred around 96 h, which decreased productivity. The statistical analysis of productivity results yielded the same tendencies for inulinase activity. These results are important regarding inulinase production on industrial scale, because faster fermentations yield higher productivity. A short fermentation time is also important because it decreases the risk of contamination [21].
Productivity of inulinase for all the experiments of the Plackett–Burman experimental design presented in Table 2. Experimental run 9 refers to the central point of the experimental design and the curve presented is the average of three independent experiments. Inulinase productivity is expressed in units per gram of dry substrate per hour (U gds−1 h−1)
Figure 4a, b shows the behavior of moisture level during the fermentative process. Decrease in moisture, is due to water vaporization and to the water used by the microorganism to maintain its vital functions. It is possible to note the large decrease in moisture after 96 h of fermentation in most of the trials. Fermentation without addition of soybean bran seems to present a lower loss of moisture when compared to the trials run with the addition of this supplement. This may be related, as stated earlier, to the increase in nitrogen content of the medium, which could have increased metabolic activity and water uptake. Water activity (a w) was also monitored during the experiments. It is worth noting that even when the moisture level was at its minimum (45%), water activity was still high. The minimum a w was 0.90, which is still suitable for yeast growth.
Evolution of moisture for all the experiments of the Plackett–Burman experimental design presented in Table 2. a Experimental runs 2, 5, 6, and 8. b Experimental runs 1, 3, 4 and 7. In both figures experimental run 9 was included, which refers to the central point of the experimental design and is the average of three independent experiments
Preliminary characterization of enzyme extract
Effect of temperature and pH on inulinase activity
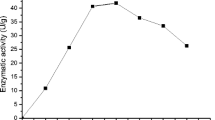
Table 3 presents the matrix of the complete experimental design with central points and inulinase activity as response. The results show that the pH and temperature that yield the maximum inulinase activity was 5.0 and 55°C, respectively, which were obtained at the central point.
Statistical analysis of the data presented in Table 3 resulted in an empirical coded model for inulinase activity in function of pH and temperature. This model is useful for prediction of inulinase activity in the studied range. Linear and quadratic parameters for the pH and quadratic parameters for temperature were statistically significant (p < 0.05). The coded model for inulinase activity was validated by the analysis of variance. A high correlation coefficient (R = 0.93) and a good performance of the F-test for regression (calculated value about 3.5 times the listed one) were obtained. Therefore, Eq. 4 is predictive of inulinase activity in the investigated range of factors, and consists in a second-order function of pH and temperature:
There are several reports in literature regarding optimal pH and temperature for inulinase activity obtained by submerged fermentation, but there is none about characterization of inulinase obtained by SSF. The optimum activity pH and temperature change among the works are dependent on the microorganism, substrate, control of process variables, etc. Pessoa and Vitolo [22] characterized an inulinase produced by K. marxianus ATCC 52466 and found the optimal pH and temperature of 5.0 and 50°C, respectively. Cazetta et al. [23] characterized an inulinase produced by K. marxianus var. bulgaricus and found the optimal pH and temperature of 3.5 and 60°C, respectively. Using Rhizopus sp. for inulinase production, Otha et al. [11] found an optimal pH and temperature of 5.5 and 40°C, respectively.
Thermal inactivation of inulinase
Fits of thermal inactivation data showed that the inactivation follows first-order kinetics, as described in Eq. 1. The crude enzyme extract showed higher stability at 50°C, maintained a residual activity of 33% after 55 h of exposure. Cazetta et al. [23] also found an inulinase stable until at 50°C. After calculation of the rate constant of inactivation k at several temperatures, an Arrhenius plot was constructed, yielding deactivation energy of 343.9 kJ mol−1.
Table 4 summarizes the apparent thermal parameters for enzyme extract, regarding rate constant k, D-values and deactivation energy. As expected, the rate constant k increases from 0.02 to 4.34 h−1 with the increase in temperature from 50 to 65°C. At 50°C the inulinase showed a D-value of 123.1 h−1. The increase in 5°C on temperature causes a drastic drop in D-value (17.8 h−1). At 60 and 65°C, the enzyme presents lower stability and D-values are 1.39 and 0.53 h−1, respectively. Cruz-Guerrero et al. characterized an inulinase produced by K. marxianus CDBB-L-278 in submerged fermentation and found a deactivation rate constant of 0.23 h−1 and a D-value of 10 h−1 at 50°C [24].
Effect of pH on stability
Figure 5 presents the residual activity of inulinase with pH ranging from 3.5 to 5.5 in sodium acetate buffer 0.1 M. Extremes of pH (3.5 and 5.5) cause a steep decrease in inulinase activity. At pH 3.5, after 2 h of reaction, a reduction of 76.3% in activity is observed. A reduction of 81.0% in activity occurred at pH 5.5 after 24 h. The highest stability was verified at pH 4.5 and 4.8, with a decrease of 67% in activity after 55 h. At pH 4.0 and 5.0, reductions of 81.4 and 74.7% were observed, respectively. The pH stability of inulinase preparation was comparable to that of recently reported K. marxianus var. bulgaricus, which was stable up to pH 4 [23].
Determination of Michaelis–Menten constants
The value of K m was 7.10 mM and V max was 17.79 M min−1. The K m value determined in this work was lower than those found in some reports in literature for sucrose hydrolysis. Catana et al. [25] found a K m of 82 mM for free enzyme and 184 mM for immobilized enzyme. Gill et al. [26] determined a K m of 3.33 mM for sucrose hydrolysis with an inulinase produced by Aspergillus fumigatus in submerged fermentation. It is worth noting that the K m found in the present work is within the range of most of industrially used enzymes (0.01–100 mM) [27].
Conclusions
In this work, production of inulinase by SSF and its preliminary characterization were carried out. Maximum inulinase production was 250 U gds−1 with 5% (w/w) soybean bran, 20% (w/w) CSL, 4 mL inoculum size and particle size of sugar cane bagasse in the range 9/32 mesh. The time for maximum production was reduced from 96 to 24 h, when soybean bran was added, yielding a maximum productivity of 8.87 U g−1 h−1.
The temperature and pH for maximum inulinase activity were 5.0 and 55°C, respectively. The crude enzyme extract showed highest stability at 50°C, with a D-value of 123.1 h and apparent deactivation energy of 343.86 kJ mol−1. Highest pH stability was found at pH 4.5 and 4.8, maintaining a residual activity of 33% after 55 h of exposure. Apparent Michaelis–Menten parameters K m and V max for sucrose hydrolysis were 7.1 mM and 17.79 M min−1, respectively.
References
Nigam P, Singh D (1994) Solid state (substrate) fermentation systems and their application in biotechnology. J Basic Microbiol 34:404–423
Pandey A, Soccol CR, Mitchell DA (2000) New development in solid state fermentation: I—bioprocesses and bioproducts. Process Biochem 35:1153–1169
Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues: I sugarcane bagasse. Bioresour Technol 74:81–87
Pandey A (1992) Recent developments in solid state fermentation. Process Biochem 27:109–117
Robinson T, Nigam P (2003) Bioreactor design for protein enrichment of agricultural residues by solid state fermentation. Biochem Eng J 13:197–203
Pandey A (2003) Solid state fermentation. Biochem Eng J 13:81–84
Barrios-González J, González H, Mejía A (1993) Effect of size particle, packing density and agitation on penicillin production in solid state fermentation. Biotechnol Adv 11:539–547
Dyaz-Godynez G, Soriano-Santos J, Augur C, Viniegra-Gonzalez G (2001) Exopectinases produced by Aspergillus niger in solid state and submerged fermentation: a comparative study. J Ind Microbiol Biotechnol 26:271–275
Selvakumar P, Pandey A (1999) Solid state fermentation for the synthesis of inulinase from Staphylococcus sp. and Kluyveromyces marxianus. Process Biochem 34:851–858
Zhang L, Zhao C, Zhu D, Ohta Y, Wanga Y (2004) Purification and characterization of inulinase from Aspergillus niger AF10 expressed in Pichia pastoris. Protein Expr Purif 35:272–275
Otha K, Suetsugu N, Nakamura T (2002) Purification and properties of an extracellular inulinase from Rhizopus sp. strain TN-96. J Biosci Bioeng 94:78–80
Vandamme EJ, Derycke DG (1983) Microbial inulinases: fermentation process, properties and application. Adv Appl Microbiol 29:139–176
Fachin D, Loey AMV, Nguyen BL, Verlent I, Indrawati I, Hedricks ME (2002) Comparative study of the inactivation kinetics of pectinmethylesterase in tomato juice and purified form. Biotechnol Prog 18:739–744
Rodrigues MI, Iemma AF (2005) Planejamento de Experimentos e Otimização de Processos: Uma estratégia seqüencial de planejamentos. Casa do Pão, Campinas, Brazil
Miller GL (1959) Use of dinitrosalisylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Santisteban-Silva BOY, Maugeri F (2005) Agitation, aeration and shear stress as key factors in inulinase production by Kluyveromyces marxianus. Enzyme Microb Tech 36:717–724
Mazutti M, Bender JP, Treichel H, Di Luccio M (2006) Optimization on inulinase production by solid state fermentation using sugar cane as substrate. Enzyme Microb Technol 39:56–59
Ikasari L, Mitchell DA (1994) Protease production by Rhizopus oligosporus in solid-state fermentation. World J Microbiol Biotechnol 10:320–324
Saxena S, Saxena RK (2004) Statistical optimization of tannase production from Penicillium variable using fruits (chebulic myrobalan) of Terminalia chebula. Biotechnol Appl Biochem 39:99–106
Bender JP, Mazutti MA, Oliveira D, Treichel H, Di Luccio M (2006) Inulinase production by Kluyveromyces marxianus NRRL Y-7571 using solid state fermentation. Appl Biochem Biotech 32:951–958
Brand D, Pandey A, Rodriguez-Leon JA, Roussos S, Brand I, Soccol CR (2001) Packed bed column fermenter and kinetic modelling for upgrading the nutritional quality of coffee husk in solid state fermentation. Biotechnol Prog 17:1065–1070
Pessoa A, Vitolo M (1999) Inulinase from Kluyveromyces marxianus: culture medium composition and enzyme extraction. Braz J Chem Eng 16:237–245
Cazetta ML, Martins PMM, Monti R, Contiero J (2005) Yacon (Polymnia sanchifolia) extract as a substrate to produce inulinase by Kluyveromyces marxianus var. bulgaricus. J Food Eng 66:301–305
Cruz-Guerrero A, Garcia-Peña I, Barzana E, Racia-Garibay M, Gómez-Ruiz L (1995) Kluyveromyces marxianus CDBB-L-278: a wild inulinase hyperproducing strain. J Ferment Bioeng 80:159–163
Catana R, Ferreira BS, Cabral JM S, Fernandes P (2005) Immobilization of inulinase for sucrose hydrolysis. Food Chem 91:517–520
Gill PK, Manhas RK, Singh P (2006) Comparative analysis of thermostability of extracellular inulinase activity from Aspergillus fumigatus with commercially available (Novozyme) inulinase. Bioresour Technol 97:355–358
Fullbrook PD (1996) Practical applied kinetics. In: Godfrey T, West S (eds) Industrial enzymology, 2nd edn. Stockholm Press, New York, pp 483–540
Acknowledgments
The authors thank FAPERGS for the financial support for this work and scholarships and Laboratório de Engenharia de Bioprocessos (FEA/UNICAMP) for providing the strain used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazutti, M., Ceni, G., Luccio, M.D. et al. Production of inulinase by solid-state fermentation: effect of process parameters on production and preliminary characterization of enzyme preparations. Bioprocess Biosyst Eng 30, 297–304 (2007). https://doi.org/10.1007/s00449-006-0096-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-006-0096-6