Abstract
With at least four eruptions over the last 20 years, Sirung is currently one of the more active volcanoes in Indonesia. However, due to its remoteness, very little is known about the volcano and its hyperacid crater lake. We report here on the first measurements of gas and heat emissions from the volcano. Notable is the substantial heat loss from the crater lake surface, amounting to 220 MW. In addition, ~17 Gg of SO2, representing 0.8% of Indonesian volcanic SO2 contribution into the atmosphere, ~11 Gg of H2S, ~17 Gg of CO2, and ~550 Gg of H2O are discharged into the atmosphere from the volcano annually. The volatiles degassed from Sirung magmas are subjected to hydrothermal fluid-rock interactions and sulfide depositions, initiated by the disproportionation of SO2. These processes lead to distinct gas compositions and changing lake water chemistry (in the sub-craters and the main crater lake). However, the occurrence of SO2-rich fluids and strong gas flux appear to highlight a rapid fluid transfer to surface, avoiding re-equilibration with lower temperature rocks/fluids in the conduits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
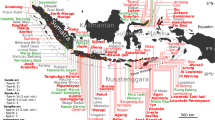
Indonesia hosts 127 active volcanoes spanning the 5000 km length of the archipelago (Fig. 1). However, many of them remain poorly studied, particularly those located in the far eastern part of the archipelago, reflecting their comparative isolation. This is true for Sirung volcano, located on the southern part of Pantar island, in the East Sunda arc (Fig. 1). Very few scientific expeditions have targeted this volcano, so little is known about its activity. The only recent work we are aware of is that of Poorter et al. (1989) who studied the hyperacidic lake on Sirung. This lake, of about 0.3 km2 in area, lies within a 2-km-diameter summit caldera, and sometimes engulfs smaller craters on its southwestern border. Bubbling mud pools are located in these subsidiary craters.
Sirung volcano, located on the southern part of Pantar island, east Indonesia. The volcano hosts within its caldera one of the largest hyperacid crater lake on earth. Major tectonic features in the eastern Sunda arc, including the major thrusts, accretionary wedges, and extent of continental crust are shown in the rectangle below with location of active volcanoes in red dots (adapted from Silver et al. 1983)
During our visit (in Aug. 2015), degassing occurred through at least four main fumarole zones (Fig. 2), mostly situated in the southwestern part of the caldera. A well-defined gas discharge site is the 100 m-wide sub-crater B, located 300 m southwest of the main crater lake (Fig. 2). According to Poorter et al. (1989), this sub-crater B occasionally hosts a small lake, but this was not the case during our survey. Instead there were fresh ash deposits around the crater after a recent phreatic eruption happened on July 8, 2015 (Table 1). We also observed two actively degassing mud pools in the sub-craters (Fig. 2) that contributed to the whole degassing.
The principal aim of our study was to characterize the degassing budget and the magmatic supply of this poorly known volcanic system of this little known active volcano using a combination of Multi-GAS, DOAS and IR Camera measurements, supplemented by rock and water sampling.
Sirung volcano: tectonic setting and volcanic activity
Sirung volcano is part of the East Sunda volcanic arc that extends from Bali to Romang (Fig. 1). The geodynamic of this region is controlled by the collision between Australia and the Sunda arc that began 3 million years ago (Bowin et al. 1980; Hamilton 1979; Chamalaun et al. 1976) and involves the forcing of continental crust beneath an accretionary wedge and associated uplift (Silver et al. 1983). The back-arc region of this East Sunda arc is complex: it includes both shallow (Bali) and deep (from Sumbawa to Flores) narrow basins, underlain by oceanic crust, as well as a series of shallow ridges between Flores and the South Banda basin. The continuous volcanic front from Java to Sumbawa is offset to the south of Flores due to the oblique trend of the subduction (Hamilton 1979) and NE-SW cross-arc faults (Silver et al. 1983). This latter direction is also evident on Sirung volcano where the craters are aligned NE-SW (Fig. 1). The old Delaki edifice on Pantar island is also aligned SW to Sirung.
At least 12 eruptions have occurred on Sirung since 1852 (Table 1; GVP 2013). All of them were classified as phreatic although no detailed studies are available. In 1934, a strong eruption formed a new crater (Petroeschevsky 1953), while another event in 1953 deposited ash to the northern part of the volcano. Five people were killed during this latter eruption (Hadikusumo 1961; Poorter et al. 1989).
Methodology and field measurements
Thermal infrared measurements
For the thermal survey, we used an OPTRIS PI400 miniature thermal infrared camera that weighs 320 g, including a lens of 62° × 49° FOV, f = 8 mm, and with a dynamic range equivalent to radiant temperatures from −20 to 900 °C. The detector has 382 × 288 pixels and the operating waveband is 7.5–13 μm. The maximum frame rate is 80 Hz. The camera was positioned at two locations, first on the northern part of the caldera rim (Fig. 2), affording a wide view of the degassing areas, and then in the southwestern part of the caldera, closer to the fumaroles (Fig. 2).
The surface temperature of a volcanic lake reflects the balance of heat sources and losses:
where ф VolcCond is the conductive heat input from the magma body, ф volc is the enthalpy of the volcanic-hydrothermal flux, ф SunRad is the short wave solar flux, and ф AtmRad is the longwave radiation from the atmosphere. Terms on the right hand side of the equation represent heat losses: they include the radiation from the lake surface ф LakeRad, the heat loss due to evaporation ф Evap, and conduction ф LakeCond associated with the energy transferred in heating meteoric influxes into the lake and heating of the air above the lake. The net radiative heat loss is given by the following:
where A lake is the lake surface (m2), ε is emissivity (0.97—Terada et al. 2012), σ is the Boltzmann constant (5.67 × 10−8 Wm−2 K−4), and T lake and T amb correspond to the lake surface and ambient air temperatures (in K).
where T VirtualLake and T virtualAmb are the virtual temperatures (K) of the lake surface and the overlying air, while e s and e a are the saturation water vapor pressures (mb) at the lake surface temperature and atmospheric temperature (mb). We take the wind speed (W) at the bottom of the caldera as ~1 m/s.
e x is the water vapor pressure at T x and P is the ambient air pressure (mb): (Pasternack and Varekamp 1997)
The conductive heat flux from the surface can be calculated as follows (Harris 2013; Brown et al. 1989):
where C B is the Bowen constant (0.61 m K−1).
DOAS measurements
DOAS measurements were performed from a fixed position in the main active zone using a scanning system (Fig. 2). To improve chances of catching each degassing source, measurements were performed with an angle of 30° from horizontal. Thirty spectra were collected during each scan with a step angle of 2.7°. Our spectrometer was an Ocean Optics USB2000+ with a spectral range of 290–440 nm and a spectral resolution of 0.5 FWHM. The SO2 column amounts (ppm m) were retrieved using DOAS calibration and analysis procedures (Platt and Stutz 2008). Reference spectra included in the non-linear fit were obtained by convolving high resolution SO2 (Bogumil et al. 2003) and O3 (Voigt et al. 2001) cross-sections with the instrument line shape. A Fraunhofer reference spectrum and ring spectrum, calculated in DOASIS, were also included in the fit. The total column amount of the plume cross section was then multiplied by the plume rise speed (estimated at 1 m/s using a thermal camera) to derive the SO2 emission rate.
Water and rock sampling
We collected water samples from the main crater lake and the sub-craters (Fig. 2). Water samples were analyzed with inductively coupled plasma-optical emissions spectroscopy (ICP-OES) and ion chromatography for cations (Al, Fe, Ca, Mg, K, Mn, B) and anions (SO4, Cl), respectively (Takano et al. 2000). NH3 was analyzed using calorimetric techniques (Eaton et al. 2005). Rock samples (collected on the inner slope of the caldera, to the north) were analyzed by X-ray fluorescence (XRF) to obtain whole-rock compositions (Johnson et al. 1999). Results are compared to available data to evaluate potential chemistry changes.
Multi-GAS
The Multi-GAS used in this work is a compact portable instrument from INGV (as used by Aiuppa et al. 2012, 2013, 2015). The system simultaneously acquired concentrations of H2O, CO2, SO2, H2S, and H2 at 0.1 Hz. H2O and CO2 were detected by non-dispersive infrared spectroscopy (LI-COR LI-840A; 0–60,000 ppm range), and SO2, H2S, and H2 via specific electrochemical sensors (respectively, models 3ST/F, EZ3H, and EZT3HYT “Easy Cal,” all from City Technology with calibration range of 0–200 ppm). Powered by a (6 Ah) 12-V LiPo battery, this lightweight multi-GAS sensor was carried along the most actively degassing areas of Sirung volcano (Fig. 2). Data were processed using Ratiocalc (Tamburello 2015) to calculate molar ratios between different volatile species.
Results
Water chemistry
Our water samples highlight an extreme acid brine on Sirung crater lake with a pH of 0.1–0.2, an elevated content of cations (Al, Fe, Ca, Mg, Na, K, and Mn), and very high concentrations of SO4 (32,000–38,000 mg/L) and Cl (13,440–16,980 mg/L) (Table 2; Fig. 3). The main crater lake is slightly more acidic than the sub-craters with higher sulfate and chlorine contents. Cation concentrations are also higher in the main crater lake, except for Mg and Mn. Mg/Cl and SO2/Cl ratios are relatively similar in the main crater (0.03 and 2.2, respectively) and the sub-craters (0.05 and 2.4, respectively). When compared with the few available reports in the literature (Table 2; Fig. 3), our data suggest that the extreme chemical concentrations observed at the sub-craters in 1984 (Poorter et al. 1989) have drastically declined in 30 years. Their composition in August 2015 was comparable to that of other known highly acidic crater lakes, e.g., Ijen, Ruapehu, Poas, or Ambae (Delmelle et al. 2000; Christensen 2000; Rowe et al. 1992; Bani et al. 2009).
Mixing plots of the main crater lake and sub-craters water chemistry at Sirung on different dates including 1984 (Poorter et al. 1989), March and October 2011 (Alfianti 2015) and 2015 (this work). The first two plots (above) display the cations evolution, the middle two plots show the anions while the bottom two plots give the ratios of Mg/Cl and SO4/Cl. The vertical dot lines denote the two eruptions that occurred within our dataset period
Whole-rock composition
Our collected lava samples have all a dacitic composition (Fig. 4); however, we note that Whitford et al. (1977) and Poorter et al. (1989) record a wider range of rock compositions, suggesting either rapid fractional crystallization or injections of new magma into Sirung reservoir. In any case, our samples collected on the surface of a large tephra deposit indicate that Sirung had hosted highly differentiated magma leading to some of the explosive eruption in the past (Table 1). We assume here, based on our lava sample, that the magmatic source of heat and gas beneath Sirung today is mainly dacitic (Table 3; Fig. 4).
Chemistry of Sirung lava samples placed into alkali versus SiO2 classification. Our samples collected on the northern inner slope of the caldera (in red) indicate a dacitic melt composition, referred to in this work. However, Whitford et al. (1977) (yellow circles) and Poorter et al. (1989) (green circles) suggest a much wider range melt composition beneath Sirung. Further work is required to address this question which is beyond the scope of this paper
Plume composition and emission budget
Our DOAS scanning results are summarized in Table 4. The cumulative SO2 flux from the different degassing sotes on Sirung amounts to 48 ± 11 t/day. This figure must be considered minimal since the scanning focused on the main active zone and neglected the emissions from the fumarolic zone 1 and from the main crater lake. The cross section profiles of SO2 column amounts display two peaks, which respectively correspond to emissions from the sub-craters B (and fumarolic zone 3) and to the cumulative gas contributions from fumarolic zones 2 and 4, mud pools and the sub-craters (Fig. 5). In order to distinguish the respective contributions of these degassing sources, we applied a Gaussian function (Fig. 5) to fit the mean column amount profile. Results indicate that sub-crater B alone contributes about 30% (14 t/day) of the SO2 emission budget from Sirung, the other sources providing the remaining 34 t/day (70%) of SO2 release.
SO2 column amount obtained in the plume of Sirung. Representative profiles a highlight two peaks that correspond, on the one hand, to the degassing from sub-crater B and its associated fumarole zone 3, and on the other hand, the combining emission from the fumarolic zones 2 and 4, mud pools, and sub-craters. Two Gaussian functions fit to the mean SO2 column amount b allow discriminate SO2 contributions from the two degassing groups
H2S/SO2 ratios obtained from Multi-GAS measurements vary widely, from 0.2 and 1.2, evidencing gas emissions with highly heterogeneous compositions for sulfur species on Sirung (Fig. 6; Table 5). The highest ratio of 1.2 characterizes the combined degassing from the sub-craters, mud pools, and fumarolic zone 2 (Fig. 6f). Note that due to access difficulties, it was not possible to attempt closer measurements of these sources. The lowest ratios correspond to fumarole zone 4 (H2S/SO2 = 0.2–0.3) (Fig. 6k). At the northwest border of the main crater lake (Fig. 6a), we measured a similar H2S/SO2 ratio of 0.4. Because this area is distant from any fumarolic emissions, we interpret this value as representative of gases bubbling across the main crater lake. Finally, gas from sub-crater B has a H2S/SO2 ratio of 0.5 (Fig. 6i).
Sirung gas composition changes along the Multi-GAS path (Fig. 2). Gases released through the main crater lake are enriched in H2S and CO2 (a, b). The fumarolic zone 1 releases notable amounts of H2S and CO2 compare to SO2 (c, d). H2O is also quantified at fumarolic zone 1 (e). The combined gas composition from sub-craters, fumarolic zone 2, and mud pools is the most enriched in H2S (f). CO2 and H2O are also quantified in this combined sources (g, h). Gas composition from sub-crater B has a moderate H2S and low CO2 contents (i, j), compared to other sources. The highest and strongest SO2 discharge occurs from fumarole zone 4 (k). H2O and CO2 are also measured at this zone (l, m)
The CO2 output from the main crater lake, sub-craters (+fumarolic zone 2 and mud pools), sub-crater B (+fumarolic zone 3), fumarolic zone 1, and zone 4 were also quantified with the Multi-GAS (Fig. 6; Table 5). Instead, H2O emission was successfully measured only on fumarolic zone 1, zone 4, and sub-craters. We obtain CO2/SO2 and H2O/SO2 ratios of 5.7 and 482 for fumarole zone 1, 1.7 and 143 for fumarole zone 4, then 1.3 and 170 for the sub-craters. For the main crater lake and sub-crater B, we obtain CO2/SO2 ratios of 8.0 and 1.2, respectively (Table 5). Based on these ratios and the corresponding SO2 flux (Table 4, Fig. 5), emission rate for H2S, CO2, and H2O amount to 31 t day−1, 46 t day−1, and 1500 t day−1, respectively.
Heat loss from Sirung crater lake
Thermal images taken from the caldera rim (Fig. 7) indicate a homogeneous temperature of about 30 °C for the main crater lake (before sunrise), compared to and ambient temperature of 20 °C. An important heat flux is required to maintain such a temperature in the crater lake of 0.3 km2 area. The distance between the IR camera position 1 (Fig. 2) and the center of the lake was about 700 m, and the thermal camera measurement was not corrected for the atmospheric radiative transfer. In situ thermocouple measurement of the lake water indicated 35 °C, 5 °C higher than the thermal imaging data. Thermal data also indicate that the southwest part of the caldera concentrates most of the thermal emission of Sirung, in agreement with the observed degassing activity. Fumarolic zones are well identified in the thermal imaging (Fig. 7), more details being shown in Fig. 8 revealing the detail for different degassing zones. Fumarolic zone 4 exhibits the most energetic degassing regime with at-vent-temperature fluctuating from 90 to 270 °C. These values should be considered minimum since the thermal camera was about 100 m distant to the vents. Strong degassing was also observed at fumarolic zone 3 (sub-crater B) with temperature fluctuating between 120 and 180 °C. At the other degassing sites, temperatures were found to be more stable compared to fumarolic zones 4 and 3. Taking 20 °C as ambient temperature and 35 °C as the main crater lake temperature, we obtained a Ф lakeRad of 45 MW, a Ф Evap of 138 MW, and a ф LakeCond of 37 MW. Thus, the total heat loss from the main crater lake of Sirung through radiation, evaporation, and conduction is around 220 MW.
Comparative thermal and visual observation of the Sirung inner caldera. The main active zone (to the southwest) coincides with observed highest degassing activity. Sub-crater B appears to be the strongest site of gas discharge from the picture in coherent with the gas flux results. The temperature of the main crater lake is homogeneous suggesting no significant bubble release into the lake
Degassing dynamic at Sirung active vents, highlighted by thermal infrared recordings. Fumarolic zone 4 and zone 3 (sub-crater B) exhibit the most energetic degassing regime. The highest temperature of 270 °C was recorded at fumarolic zone 4. The main crater lake as well as the sub-craters and fumarole zone 1 display negligible temperature fluctuations
Discussion
Magmatic source
In 1984, Cl and cation concentrations were extremely high in the sub-craters, particularly in a boiling spring, compared to the main crater lake. These very high concentrations were attributed to the addition of Cl-rich magmatic fluids (Poorter et al. 1989). In contrast, the boiling spring was poor in SO4 as highlighted by the SO4/Cl ratio of 0.02 compared to 1.2 in the main crater lake. This S depletion was ascribed to subsurface sulfide deposition (Poorter et al. 1989). Moreover, much higher Mg/Cl ratio of 0.1 in the boiling spring, compared to 0.06 in the main crater lake (Table 2), indicated extensive rock leaching beneath the boiling spring. Fluid-rock interaction is also evidenced by the higher pH (0.2) of boiling spring compare to the main crater (pH = 0.08), given that rock dissolution consumes H+ (Delmelle and Bernard 2000; Bani et al. 2009).
Figure 3 reveals a significant increase of SO4/Cl ratio over time, basically in response to a drastic drop in Cl since 1984. The Mg/Cl ratio was stable between 1984 and March 2011 data. However, in October 2011, the Mg/Cl and SO4/Cl ratios increased in the main crater at relatively constant Cl, suggesting an enhanced supply of SO2-rich magmatic gas leading to increased fluid-rock interactions, possibly due to shallower magma input at depth (e.g., Giggenbach and Glover 1975; Bani et al. 2016). Mg, SO4 and Cl also increased in the sub-craters, but since Cl increased relatively faster, both Mg/Cl and SO4/Cl ratios tended to decrease by October 2011. This higher Cl input into the sub-crater water, although less pronounced than observed in 1984 indicates that the system receives a chlorine input via a concentrated hydrothermal brine. Chlorine can derive from the degassing of shallow (<100 MPa) magma, principally as gaseous HCl (Shinohara and Fujimoto 1994) and Cl partitioning into the fluid phase (Lukanin 2015). The increase of Cl, SO4 and cations in October 2011 and then August 2015 may thus reflect a shallower magma source beneath Sirung in that period.
Sparse data for the main crater lake chemistry prevent a clear assessment of the temporal evolution of the magma-hydrothermal system in 2011–2015. However, a shallower magma source, as suspected above, may have been responsible for the eruption that succeeded in May 2012 (Table 1), then for the changes in surface and color of Sirung crater lake (Fig. 9), from light blue (in 2005) to gray (in 2012), suggesting re-mobilization of sediment and hydrothermal-magmatic inputs, accompanying the ratio changes. The lake surface area was reduced in 2012, compared to 2005 and 2015 likely due to evaporation induced by magmatic heating, although no juvenile magma was erupted.
Evolution of Sirung crater lake in terms of surface and color. Smaller surfaces occurred in 2009 and 2012 while in 2015 was the largest. In 2012, the lake water displayed a gray color due to the eruption that occurred in the same year. Note the occurrence of a new fumarole zone in 2012 (tagged as Fumarolic zone 4 in this work). These pictures clearly indicate that the sub-craters zone is periodically dry
In July 2015, another eruption occurred on Sirung. Measurements made just after this event showed a decrease of Mg/Cl and SO4/Cl ratios in the crater lake (Fig. 3). The higher Cl content, relative to SO4 and Mg, suggests reduced rock leaching and a lower magmatic gas supply after the July 2015 event (Table 2, Table 5). Furthermore, the larger area of the lake in 2015 indicates a reduced heating and consequent evaporation (Fig. 9). The occurrence of an eruption with limited fluid rock interaction and low gas discharge has been observed on other volcanoes, including Rokatenda (Primulayan et al., Primulyana et al. 2017) and was described as being triggered by the second crystal nucleation event that occur at shallow depth in degassed silicic magma (Melnik and Sparks (2005), a plausible scenario for this July 2015 eruption at Sirung. In any case, the total heat loss of 220 MW from the lake in August 2015 remains high and comparable to that of other active crater lakes at Kawah Ijen (Indonesia), Zao (Japan), or Copahue (Chile) (Pasternack and Varekamp 1997) and suggests sustained thermal heat energy input from the magmatic-hydrothermal system. A follow-up of such thermal measurements may contribute to the better monitoring of Sirung volcano, as demonstrated at Santa Ana volcano (Hernandez et al. 2007).
Heterogeneity of gas composition, oxidation state change, and rock alteration
The H2S/SO2 ratio of magmatic fluids is well known to depend on magma oxidation state, temperature, and pressure (e.g., Whitney 1984; Symonds et al. 1994; Rye 2005). The pressure effect on sulfur speciation in magmatic fluids is such that high pressure favors H2S and low pressure favors SO2 as the dominant gas species (Gaillard et al. 2011). Our Sirung results (H2S/SO2 of 0.2–1.2) support the hypothesis of SO2 release from a shallow degassing magma source. The high H2S/SO2 ratio at the sub-craters is thus unlikely to reflect magma degassing, but instead the consequence of disproportionation reactions of SO2, such as 4SO2 + 4H2O → 3H2SO4 + H2S (Holland 1965). The resulting H2S may react with Fe-bearing minerals to produce sulfides or may be oxidized in a steam-heated environment, or be simply vented to the surface (Rye 2005). Ultimately, these reactions may lead to an increase of H2S/SO2 ratio in the fluids (Ohmoto and Rye 1979; Rye 1993; Giggenbach 1997).
A slow upraise of magmatic fluids produce extensive fluid-rock interactions. However, if magmatic fluid pressure is suddenly released, before equilibrium with lower temperature rocks is achieved, then SO2-rich fluids can be vented at the surface (Rye 2005; de Moor et al. 2016a; b). This may be the case for fumarolic zone 4, where SO2 concentrations are the highest (H2S/SO2 of 0.3) and volcanic CO2 and H2O were also detected. There is no report on the formation of this fumarolic zone that was first observed in 2012 (Fig. 9) when Sirung volcano erupted (Table 1). We propose that this intense eruptive activity altered the geometry of the hydrothermal system and favored the opening of new gas channels that allowed deeply sourced SO2-rich fluids to that fumarolic zone 4, as highlighted by the energetic and hot degassing regime (Fig. 8).
Low H2S/SO2 ratios (of 0.4) in the gas emanating from the main lake surface (Fig. 6) are comparable to those measured in plumes from acidic lakes such a Poas (de Moor et al. 2016b) or Copahue (Tamburello et al. 2015). We conclude that fast interactions between magmatic gases and Sirung lake water hinder both SO2 scrubbing and the effects of disproportionation reactions (de Moor et al. 2016a; b). In contrast, high H2S/SO2 ratios of 1.2 at the sub-craters imply large extents of magmatic gas re-equilibration to hydrothermal conditions. Sub-crater B was likely formed during an eruption in 1934 (Petroeschevsky 1953; Petroeschevsky and Klompe 1950; Table 1). It is actually the least eroded structure of Sirung and probably the most recently formed. The intermediate H2S/SO2 ratios at this crater (0.5) may thus reflect a less developed hydrothermal system beneath this younger crater, and weaker disproportionation of SO2.
The main rock-derived elements (RDE) in volcanic lake fluids are Na, Ca, K, Mg, Fe, and Al. From that list, Mg and Na are the easily leached elements, thus acid fluids that travel rapidly through a fresh volcanic rock matrix may produce high (Na+Mg)/(RDE) ratios whereas slow flowing fluids that have undergone more extensive reaction with fresh rock would have lower (Na+Mg)/(RDE) ratios (Varekamp et al. 2000). When applying this model to Sirung (Fig. 10a), one can observe that the (Na+Mg)/(RDE) ratios indicate a low to moderate degree of fluid-rock interaction, in comparison to high ratios observed at Voui and Ruapehu during their eruptive activities (Bani et al. 2009; Christensen 2000), and to low ratios at Poas and Ijen, in relation to slow flowing fluids and long-term fluid-rock interactions. Such a low to moderate (Na+Mg)/(RDE) ratios at Sirung uphold the possibility for new magmatic-hydrothermal fluids input into the lakes. Further, the high Ca content in the lake, as shown in the ternary diagram of Na+K-Ca-Mg (Fig. 10b), suggests re-mobilization of Ca from Ca-mineral phases such as gypsum, similar to Ruapehu (Christensen 2000). Finally, new arrival of fluids to the surface is usually enriched in Cl, leading to large gain of Mg in the lakes of Sirung (Fig. 10c).
Geochemistry of Sirung lakes. a The (Mg+Na)/Sum RFE ratios appear to be low to moderate compare to other crater lakes. Voui and Ruapehu display high ratio while Poas and Ijen highligh a rather low ratio. Poas shows a large variation in concentration while Ijen composition is very stable. b Na+K-Ca-Mg ternary diagram indicate high Ca content in Sirung and Ruapehu, likely due to re-mobilization of Ca-phase minerals such as gypsum. Voui and Capahue display distinct high Mg in relation rapid fluid-rock interaction during eruptive events. c A large varition of Cl and Mg is observed at Sirung, compare to low Cl and high Mg at Voui, Ijen and even Poas. Data sources: Rowe et al. 1992 (Poas), Delmelle et al. 2000 (Ijen), Christenson, Christensen 2000 (Ruapehu) and Bani et al. 2009 (Voui)
Volatile budget
Assuming that the gas fluxes obtained in this work are representative for the long-term degassing regime of Sirung volcano, we infer the annual discharge of ~17 Gg of SO2, ~11 Gg of H2S, ~17 Gg of CO2, and ~547 Gg of H2O (Table 5). This very first budget estimate is substantial: in terms of SO2 flux, Sirung volcano is comparable to Semeru in Java (21–71 Gg SO2; Smekens et al. 2015), Vulcano in Italy (16 Gg), and Augustine in Alaska (17.5 Gg) (Andres and Kasgnoc 1998). If integrating the figure into the new global volcanic emissions inventory of Carn et al. (2017), Sirung degassing constitutes 0.8% of the annual Indonesian volcanic SO2 emission budget (~2000 Gg/year), which subsequently represents ~0.07% of the global volcanic sulfur dioxide contribution (23,000 Gg/year) into the atmosphere. Our estimate for the volatile emissions from Sirung adds a new piece to better document the volatile emissions from the Indonesian arc (Nho et al. 1996; Andres and Kasgnoc 1998; Hilton et al. 2002; Pfeffer 2007; Bani et al. 2013; Aiuppa et al. 2015; Carn et al. 2017).
Poorter et al. (1989) estimated that, on Sirung, a complete degassing of 424,000 m3 of magma per year is required to accommodate the Cl discharged observed in 1984. However, this estimation did not take into account the gas budget to the atmosphere. Assuming that Sirung magmas contain 3.5% H2O (Poorter et al. 1989), and that the molar H2O/SO2 ratio of ~143 obtained for fumarolic zone 4 is representative of the magmatic output, then about 15 Mt of magma would be necessary to sustain the annual volcanic gas output into the atmosphere. Assuming a density of ~1600 kg/m3 for the volatile-saturated dacitic magma (Hoblitt and Harmon 1993), we infer a complete degassing of 9 × 106 m3 of magma per year. Comparing this figure with that estimated by Poorter et al. (1989), we show that a major proportion of the magmatic gases reaches the atmosphere and that a relatively small proportion dissolves into the lake.
Conclusion
Sirung is an active volcano with at least ten eruptions over the last hundred years. Located a thousand kilometers east of Java, it is one of the most isolated in the Indonesian archipelago—3 days are necessary to reach the volcano, from the capital city, Jakarta. Reflecting its remote location, very little is known about the volcano. We have found that its hyperacidic main crater lake releases around 220 MW of thermal energy sustained by the thermal heat carried to the surface by hot gases. A huge fraction of these latter gases released by the underground dacitic magma ultimately reaches the atmosphere, constituting an annual output of ~17 Gg of SO2, ~11 Gg of H2S, ~17 Gg of CO2, and ~550 Gg of H2O. Ignored in the past, this degassing budget should be integrated in the Indonesian and global volcanic emission budget, knowing that in terms of SO2 release, Sirung emission constitutes 0.8% of the Indonesian volcanic SO2 emission budget and about 0.07% of the global volcanic SO2 output through passive degassing.
Sirung volcanic manifestations include 3 main sites: (i) the main crater lake, (ii) an area combining at least two small craters (called sub-craters in this work) that host mud pools, and (iii) a well-defined crater (refer to as sub-crater B in this work), formed in 1934. At least four main fumarolic zones exist on Sirung, the latest of which (called fumarole zone 4 in this work) was possibly formed during a strong eruption in 2012. Most of these active sites are concentrated in the southwestern part of Sirung caldera, suggesting a shift of the magmatic discharge to the southwest. Magmatic-hydrothermal fluids discharged at Sirung display distinct gas compositions and different lake water chemistry (in the sub-craters and the main crater lake). These differences are related to variable extents of magma-derived SO2 scrubbing by shallow hydrothermal fluids. In the conduits beneath the main crater lake and fumarolic zone 4, where the gas flux is the strongest, magmatic fluids are rapidly transferred to the surface and suffer minor re-equilibration with lower temperature rocks/fluids, leading to emissions of more SO2-rich fluids. In contrast, SO2 is largely converted (by disproportionation) into H2S at the sub-craters, where it can further react with Fe-bearing minerals in the conduit to produce sulfide deposits.
References
Aiuppa A, Giudice G, Liuzzo M, Tamburello G, Allard P, Calabrese S, Chaplygin I, McGonigle AJS, Taran Y (2012) First volatile inventory for Gorely volcano, Kamchatka. Geophys Res Lett 39:L06307. doi:10.1029/2012GL051177
Aiuppa A, Tamburello G, Di Napoli R, Cardellini C, Chiodini G, Giudice G, Grassa F, Pedone M (2013) First observations of the fumarolic gas output froma restless caldera: implications for the current period of unrest (2005–2013) at Campi Flegrei. Geochem Geophys Geosyst 14:4153–4169
Aiuppa A, Bani P, Moussallam Y, Di Napoli R, Allard P, Gunawan H, Hendrasto M, Tamburello G (2015) First determination of magma-derived gas emissions from Bromo volcano, eastern Java (Indonesia). J Volcanol Geotherm Res 304:206–213
Alfianti H (2015) Laporan, Pemantauan Kegiatan Gunungapi Sirung, Nusa Tenggara Timur. Pusat Vulkanologi dan Mitigasi Bencana Geologi, 30 pp
Andres RJ, Kasgnoc AD (1998) A time-average inventory of subaerial volcanic sulfur emissions. J Geophys Res 103:25251–25261
Bani P, Oppenheimer C, Varekamp JC, Quinou T, Lardy M, Carn S (2009) Remarkable geochemical changes and degassing at Voui crater lake, Ambae volcano, Vanuatu. J Volcanol Geotherm Re 188:347–357
Bani P, Surono, Hendrasto M, Gunawan H, Primulyana S (2013) Sulfur dioxide emissions from Papandayan and Bromo, two Indonesian volcanoes. Nat Hazards Earth Syst Sci 13:2399–2407. doi:10.5194/nhess-13-2399-2013
Bani P, Boudon G, Balcone-Boissard H, Delmelle P, Quiniou T, Lefèvre J, Garaebiti BE, Shinohara H, Lardy M (2016) The 2009-2010 eruption of Gaua volcano (Vanuatu archipelago): eruptive dynamics and unsuspected strong halogens source. J Volcanol Geotherm Res 322:63–75. doi:10.1016/j.jvolgeores.2015.06.023
Bogumil K, Orphal J, Homann T, Voigt S, Spietz P, Fleischmann OC, Vogel A, Harmann M, Kromminga H, Bovensmann H, Frerick J, Burrows JP (2003) Measurements of molecular absorption spectra with SCIAMACHY preflight model: instrument characterization and reference data for atmospheric remote sensing in the 230–2380 nm region. J Photochem Photobiol A Chem 157:167–184
Bowin C, Purdy GM, Johnston C, Shor GG, Lawver L, Hartono HMS, Jezek P (1980) Arc continent collision in Banda Sea region. Am Assoc Pet Geol Bull 64:868–915
Brown G, Rymer H, Dowden J, Kapadia P, Stevenson D, Barquero J, Morales LD (1989) Energy budget analysis for Poas crater lake: implications for predicting volcanic activity. Nature 339:370–373
Carn SA, Fioletov VE, McLinden CA, Li C, Krotkov NA (2017) A decade of global volcanic SO2 emissions measured from space. Sci Rep 7:44095. doi:10.1038/srep44095
Chamalaun FH, Lockwood K, White A (1976) The Bouguer gravity field of eastern Timor. Tectonophysics 30:241–259
Christensen BW (2000) Geochemistry of fluids associated with the 1995–1996 eruption of Mt. Ruapehu, New Zealand: signatures and processes in the magmatic-hydrothermal system
Data Dasar Gunung api Indonesia (2011) Kementerian Energi dan Sumber daya Mineral, Badan Geologi. edisi kedua, 208–219
Delmelle P, Bernard A (1994) Geochemistry, mineralogy and chemical modeling of the acid crater lake of Kawah Ijen volcano, Indonesia. Geochem Cosmochim Acta 58:2445–2460
Delmelle P, Bernard A, Kusakabe M, Fischer TP, Takano B (2000a) Geochemistry of the magmatic-hydrothermal system of Kawah Ijen volcano, East Java, Indonesia. J Volcanol Geotherm Res 97:31–53
Delmelle P, Bernard A (2000b) Volcanic Lakes. In: Sirgurdsson H, Houghto BF, McNutt SR, Rymer H, Stix J (Eds.), Encyclopedia of volcanoes. Academic Press, San Diego, pp. 877–895
de Moor JM, Aiuppa A, Avard G, Wehrmann H, Dunbar N, Muller C, Tamburello G, Giudice G, Liuzzo R, Moretti R, Conde V, Galle B (2016a) Turrialba volcano (Costa Rica): degassing and eruptive processes inferred from high-frequency gas monitoring. J Geophys Res Solid Earth 121:5761–5775. doi:10.1002/2016JB013150
de Moor JM, Aiuppa A, Pacheco J, Avard G, Kern C, Liuzzo M, Martinez M, Giudice G, Fisher TP (2016b) Short-period volcanic gas precursors to phreatic eruptions: insights from Poas volcano, Costa Rica. Earth Planet Sci Lett 442:218–227
Eaton AD, Clesceri LS, Rice EW, Greenberg AE, Franson MAH (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, D.C.
Gaillard F, Scaillet B, Arndt NT (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478:229–232. doi:10.1038/nature10460
Giggenbach WF, Glover RB (1975) The use of chemical indicators in the surveillance of volcanic activity affecting the crater lake on Mt. Ruapehu, New Zealand. Bull Volcanol 39:1–12
Giggenbach WF (1997) The origin and evolution of fluids in magmatic-hydrothermal systems. In: Barnes HL (ed) Geochemistry of hydrothermal ore deposits, 3rd edn. Wiley, New York, pp 737–796
Global Volcanism Program (2012) Report on Sirung (Indonesia). In: Sennert SK (ed.) Weekly Volcanic Activity Report, 9 May-15 May 2012. Smithsonian Institution and US Geological Survey
Global Volcanism Program (2013) Sirung (264270) in Volcanoes of the World, v. 4.5.0. Venzke E (ed.). Smithsonian Institution. Downloaded 30 Aug 2016 (http://volcano.si.edu/volcano.cfm?vn=264270). http://dx.doi.org/10.5479/si.GVP.VOTW4-2013
Global Volcanism Program (2015) Report on Sirung (Indonesia). In: Sennert SK (ed.) Weekly Volcanic activity report, 8 July-14 July 2015. Smithsonian Institution and US Geological Survey
Hadikusumo D (1961) Bulletin of Volcanological Survey of Indonesia for the period 1950–1957, 100 pp
Hamilton W (1979) Tectonics of the Indonesian region. U.S. Geol. Surv.. Prof. Pap. 1078, 345 pp
Harris AJL (2013) Thermal remote sensing of active volcanoes: a user’s manual. Cambridge University Press, Cambridge 728 pp
Hernandez PA, Perez NM, Varekamp JC, Henriquez B, Hernandez A, Barrancos J, Padron E, Calvo D, Melian G (2007) Crater lake temperature changes of the 2005 eruption of Santa Ana volcano, El Salvador, Central America. Pure Appl Geophys 164:2507–2522. doi:10.1007/s00024-007-0275-7
Hilton DR, Fischer TP, Marty B (2002) Noble gases and volatile recycling at subduction zones. Rev Mineral Geochem 47
Hoblitt RP, Harmon RS (1993) Bimodal density distribution of cryptodome dacite from the 1980 eruption of Mount St. Helens, Washington. Bull Volcanol 55:421–437. doi:10.1007/BF00302002
Holland HD (1965) Some applications of thermochemical data to problems of ore deposits: II. Mineral assemblages and the compositions of ore-forming fluids. Econ Geol 60:1101–1166
Johnson DM, Hooper PR, Conrey RM (1999) XRF analysus of rocks and minerals for major and trace elements on a single low dilution Li-tetraborate fused bead. JCPDS-International Centre for Diffraction Data
Lukanin OA (2015) Chlorine partitioning between melt and aqueous chloride fluid during granite magma. Degassing I. Decompression induced melt degassing. Geochem Int 53:786–810
Melnik O, Sparks RSJ (2005) Controls on conduit magma flow dynamics during lava dome building eruptions. J Geophys Res 110:B02209. doi:10.1029/2004JB003183
Nho EY, Le Cloarec M-F, Ardouin B, Tjetjep WS (1996) Source strength assessment of volcanic trace elements emitted from the Indonesian arc. J Volcanol Geotherm Res 74:121–129
Ohmoto H, Rye RO (1979) Isotopes of sulfur and carbon. In: Barnes HL (ed) Geochemistry of hydrothermal ore deposits, 2nd edn. Wiley, New York, pp 509–567
Pasternack GB, Varekamp JC (1997) Volcanic lake systematics I. Physical constraints. Bull Volcanol 58:528–538
Petroeschevsky WA, Klompe THF (1950) Het vulkanologisch onderzoek in Indonesia. Chron Nat 106:187–204
Petroeschevsky WA (1953) The volcanic activity in Indonesia during the period of 1942–1948. Commun. Volcanol. Surv. Indonesia, I: 17–30
Pfeffer MA (2007) The relative influences of volcanic and anthropogenic emissions on air pollution in indonesia as studied with a regional atmospheric chemistry and climate model. Reports on earth system science. Max Planck Institute for Meteorology, Hamburg
Platt U, Stutz J (2008) Differential optical absorption spectroscopy, principal and applications, Springer, 597 pp
Poorter RPE, Varekamp JC, Van Bergen MJ, Kreulen R, Sriwana T, Vroon PZ, Wirakusumah AD (1989) The Sirung volcanic boiling spring: an extreme chloride-rich, acid brine on Pantar (Lesser Sunda Island, Indonesia). Chem Geol 76:215–228
Primulyana S, Bani P, Harris A (2017) The effusive-explosive transitions at Rokatenda 2012–2013: unloading by extrusion of degassed magma with lateral gas flow. Bull Volcanol 79:22. doi:10.1007/s00445-017-1104-1
Rowe GL Jr, Ohsawa S, Takano B, Brantley SL, Fernandez JF, Barquero J (1992) Using crater lake chemistry to predict volcanic activity at Poas volcano, Costa Rica. Bull Volcanol 54:494–503
Rye RO (1993) The evolution of magmatic fluids in the epithermal environment: the stable isotope perspective. Econ Geol 88:733–753
Rye RO (2005) A review of the stable-isotope geochemistry of sulfate minerals in selected igneous environments and related hydrothermal systems. Chem Geol 215:5–36
Shinohara H, Fujimoto K (1994) Experimental study in the system albite-andalousite-quartz-NaCl-H2O at 600 degrees C and 400 to 2000 bars. Geochim Cosmochim Acta 58:4857–4866
Silver EA, Reed D, McCaffrey R (1983) Back arc thrusting in the eastern sunda arc, Indonsia: a consequence of arc-continent collision. J Geophys Res 88(B9):7429–7448
Smekens JF, Clarke AB, Burton MR, Harijoko A, Wibowo HE (2015) SO2 emissions at Semeru volcano, Indonesia: characterization and quantification of persistent and periodic explosive activity. J Volcanol Geotherm Res 300:121–128
Symonds RB, Rose WI, Bluth GJS, Gerlach TM (1994) Volcanic-gas studies: methods, results, and applications. In: Carroll, M.R., Holloway, J.R. (Eds.), Volatiles in magmas. Rev Mineral 30(691):1–66
Tamburello G (2015) Ratiocalc: software for processing data from multicomponent volcanic gas analyzers. Comput Geosci 82:63–67
Tamburello G, Agusto M, Caselli A, Tassi F, Vaselli O, Calabrese S, Rouwet D, Capaccioni B, Di Napoli R, Cardellini C, Chiodini G, Bitetto M, Brusca L, Bellomo S, Aiuppa A (2015) Intense magmatic degassing through the lake of Copahue volcano, 2013–2014. J Geophys Res Solid Earth 120:6071–6084. doi:10.1002/2015JB012160
Terada A, Hashimoto T, Kagiyama T (2012) A water flow modem of the active crater lake at Aso volcano, Japan: fluctuations of magmatic gas and groundwater fluxes from the underlying hydrothermal system. Bull Volcanol 74:641–655
Takano B, Fazlullin SM, Delmelle P (2000) Analytical laboratory comparison of major and minor constituents in an active crater lake. J Volcanol Geotherm Res 97:497–508
Varekamp JC, Pasternack GB, Rowe GL Jr (2000) Volcanic lake systematics II. Chemical constraints. J Volcanol Geotherm Res 97:161–179
Voigt S, Orphal J, Bogumil K, Burrows JP (2001) The temperature dependence (203–293 K) of the absorption cross-sections of O3 in the 230–850 nm region measured by Fourier-transform spectroscopy. J Photochem Photobiol A 143:1–9
Whitford DG, Comston W, Nicholls IA, Abbott MJ (1977) Geochemistry of the late Cenozoic lavas from the eastern Indonesia—role of subducted sediments in petrogenesis. Geology 5:571–575
Whitney JA (1984) Volatiles in magmatic systems. In: Robertson, J.M. (Ed.), Fluid–mineral equilibria in hydrothermal systems, Rev. Econ. Geol., vol. 1, pp. 155–175
Acknowledgements
This work was achieved thanks to the collaboration between Center of Volcanology and Geological Hazards Mitigation (CVGHM) and Institut de Recherche pour le Développement (IRD) under the JEAI-Commission program. We gratefully acknowledge the technical assistance from Sirung Observatory. We thank the two anonymous referees for their beneficial review and T. Fischer for editorial handling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: T.P. Fischer
Rights and permissions
About this article
Cite this article
Bani, P., Alfianti, H., Aiuppa, A. et al. First study of the heat and gas budget for Sirung volcano, Indonesia. Bull Volcanol 79, 60 (2017). https://doi.org/10.1007/s00445-017-1142-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00445-017-1142-8