Abstract
Plant phenology differs largely among coexisting species within communities that share similar habitat conditions. However, the factors explaining such phenological diversity of plants have not been fully investigated. We hypothesize that species traits, including leaf mass per area (LMA), seed mass, stem tissue mass density (STD), maximum plant height (Hmax), and relative growth rate in height (RGRH), explain variation in plant phenology, and tested this hypothesis in an alpine meadow. Results showed that both LMA and STD were positively correlated with the onset (i.e., beginning) and offset (i.e., ending) times of the four life history events including two reproductive events (flowering and fruiting) and two vegetative events (leafing and senescing). In contrast, RGRH was negatively correlated with the four life phenological events. Moreover, Hmax was positively correlated with reproductive events but not with vegetative events. However, none of the eight phenological events was associated with seed size. In addition, the combination of LMA and STD accounted for 50% of the variation in plant phenologies. Phylogenetic generalized least squares analysis showed plant phylogeny weakened the relationships between species traits vs. phenologies. Phylogeny significantly regulated the variation in the ending but not the beginning of phenologies. Our results indicate that species traits are robust indicators for plant phenologies and can be used to explain the diversity of plant phenologies among co-occurring herbaceous species in grasslands. The findings highlight the important role of the combination of and trade-offs between functional traits in determing plant phenology diversity in the alpine meadow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant phenology, which is the timing of growth, development, and reproductive events, is crucial to species resource acquisition, reproductive success, and population regeneration (Nord and Lynch 2009; Armstrong et al. 2016). Numerous studies have recorded the diversity of vegetative (leafing and aboveground senescing) and reproductive (flowering and fruiting) phenologies of plant species along environmental gradients (Xia and Wan 2013; Liu et al. 2017) and within communities (Singh and Kushwaha 2006; Miller-Rushing and Forrest 2010; Sun and Frelich 2011; Polgar and Primack 2011; Wang et al. 2019). Although existing studies have demonstrated that abiotic factors such as photoperiod, temperature, water and nutrient availability, and the duration of snowpack are correlated with phenological differences between habitats (Xia and Wan 2013; Liu et al. 2017; Wang et al. 2019), it is doubtful that these abiotic factors fully account for the contrasting phenologies among coexisting species sharing the same local environmental conditions (Castro-Díez et al. 2005; Singh and Kushwaha 2006; Sun and Frelich 2011). Understanding the mechanisms underlying differences in plant phenology among species is of particular importance as it has been suggested to foster species co-existence and improve species diversity (Brody 1997; Mason et al. 2013; Fantinato et al. 2018).
Differences in species functional traits are hypothesized to influence interspecific variation in plant phenology (Sun and Frelich 2011; Mason et al. 2013). Two important hypotheses regarding species traits vs. plant phenologies relationships are proposed. One is the time-size tradeoff hypothesis, which predicts that seed size should be negatively correlated with the timing of flowering, and late-flowering species cannot produce seeds as large as early-flowering species because of the short time available for seed development (Bolmgren and Cowan 2008). This hypothesis has been supported by negative correlations between flowering timing and seed size in a north-temperate flora (Bolmgren and Cowan 2008) and an alpine Tibetan ecosystem in China (Du and Qi 2010; Jia et al. 2011). The other one is the height-time hypothesis, which assumes that low-stature species (with small maximum plant height) are more likely to emerge earlier and grow faster than high-stature species in temperate dense herbaceous communities because low-stature species are more likely to be shaded by late-flowering high-stature species and herbaceous species must maximize height to be successful when flowering (sensu Vile et al. 2006, Sun and Frelich 2011). Since relative growth rate (RGR) is the determinant for when plants can reach their maximum heights (Hmax; Westoby et al. 2002, Vile et al. 2006, Chave et al. 2009) and stem density (STD) and leaf mass per area (LMA) are factors affecting RGR, these species traits are suggested to be factors explaining interspecific variation in plant phenology (Sun and Frelich 2011). The hypothesis that functional traits can explain the variation of phenological events has been tested by the relationships between reproductive time and functional traits for perennial plant species in an old-field (Sun and Frelich 2011).
However, the two hypotheses have not been substantially tested. For example, the time-size tradeoff hypothesis has not been tested for species drawn from the same community. Importantly, both hypotheses were proposed to explain the relationship between species traits and reproductive phenologies but not vegetative phenologies of herbaceous species, although studies have frequently related stem anatomy and leaf traits to the leafing phenologies for woody species (Wang et al. 1992; Sun et al. 2006). Here, we propose that these two hypotheses can be extended to predict vegetative phenologies of herbaceous plants from species traits, considering the fact that herbaceous species rarely bear flowers and fruits before leafing and growing in height, and that the aboveground parts of these species usually senesce after flowering and fruiting. Consequently, it is reasonable to hypothesize that the species traits that account for the interspecific variation in reproductive phenologies (e.g., LMA, STD, Hmax and RGR, and seed size) should be correlated with vegetative phenologies (e.g., leafing and senescing).
To test our hypothesis, we quantified the extent to which phenological differences among co-existing species correlate with functional trait differences among 16 herbaceous species in an alpine meadow using the phenology records and functional traits data collected in 2009 and 2010, and the supplementary traits data measured in 2019. For each species, we recorded four plant phenology events including two vegetative ones (leafing and senescing) and two reproductive ones (flowering and fruiting) and measured five functional traits (i.e., seed size, LMA, STD, Hmax, and RGR) that have been shown to be critically important for reproductive success (Bolmgren and Cowan 2008; Sun and Frelich 2011). To determine whether these 5 traits are powerful predictors of the phenological differences observed among the 16 species, a variety of correlation analyses were performed and compared in this study.
Materials and methods
Study site
Our study site was located in the Hongyuan Alpine Meadow Ecosystem Research Station of the Chinese Academy of Sciences, Sichuan Province of eastern Qinghai-Tibetan Plateau (32° 48′ N, 102° 33′ E). The altitude is 3500 m. The climate is cold, continental, and characterized by a short and cool Spring, Summer, and Autumn and a long Winter. In the period of 1961–2018, as recorded by Hongyuan County Climate Station (located 5 km from the study site), the annual mean temperature was 0.9 °C, with maximum and minimum monthly means of 10.9 and − 10.3 °C in July and January, respectively. The annual mean precipitation was 690 mm (80% of which occurs between May and August).
The meadow in the study site is dominated by sedges (e.g., Blysmus sinocompressus and Carex enervis subsp. chuanxibeiensis) and grasses (e.g., Deschampsia caespitosa). Forb species, including Anemone trullifolia var. linearis, Potentilla anserina, Haplosphaera himalayensis, Aster alpine, and Gentiana formosa, are also occasionally abundant. The total vegetation coverage in mid-summer is over 90%, the average maximum community height is about 30 cm in the meadow (Wu et al. 2011). The soil is characterized by a high organic content (250 g kg−1) and low total nitrogen (8 g kg−1) and phosphorus (5 mg kg−1).
Phenological observations
In 2007, a 0.5 ha plot was fenced. Vegetation in the meadow was so homogeneous that more than 90% of the indigenous angiosperm species could be found in any 25 × 25 cm2 patch (Liu et al. 2011). Ten randomly assigned 1 × 1 m2 area quadrats, each surrounded by a steel frame, were subsequently established in the plot. During 2009 and 2010 growing seasons (from middle April to late September), we recorded for each species every other day the number of plants that had leafed out, open flowers, fruits, and senesced plants, as well as plant height (the maximum height of photosynthetic tissue, see Cornelissen et al. 2003). Rather than counting individual flowers for graminoid, Compositae and Umbelliferae species (which was impractical), the number of flowering stems or inflorescences in each quadrat were counted. Plants having one fully expanded leaf were counted as having leafed out, those having one fully opened flower (or inflorescence) were counted as having flowered. Similarly, plants bearing one fully developed fruit were regarded as having fruited, and those with 10% leaves bearing yellow edges were counted as having senesced.
A total of 16 species were sufficiently abundant and robust for the purposes of this study. These included 11 forb, 3 sedge and 2 grass species (see Table S1) representing 16 genera and 8 families. These 16 species accounted for more than 80% of total aboveground biomass in the quadrats (data not shown). However, only 15 species were investigated in 2009 because the abundance of Leontopodium nanum was too low for analysis.
The onset and offset time of each life history events denote the date when 10% and 90% of plants leafed, flowered, fruited, and senesced. The dates of flowering and fruiting timing were determined with a quadratic function (Eq. 1) following Sun and Frelich (2011):
where Y was the number of flower or fruit existing in each observation, and x was the observation time. The dates when 10% and 90% flowers or fruits existed were calculated from Eq. (1) and referred as the onset and offset times of flowering and fruiting. The letters a, b, and c were constants.
The dates of leafing and senescing timing were determined by fitting an exponential equation (Eq. 2) using the percent of leafing (or senescing) individuals and the observation time (Julian day) for each species.
where Y was the percent of leafing (or senescing) individuals, and x was the observation time. The dates when 10% and 90% individuals leafed out and senesced were calculated from Eq. (1) and referred as the onset and offset times of leafing and senescing. Because some quadrats contained less than or more than ten plants per species, three species were represented in less than ten quadrats for calculating species averages (i.e., Chamaesium paradoxum, Potentilla ansrina and Caltha scaposa).
Functional trait measurements
Five functional traits were measured for each species in each quadrat, i.e., Hmax, relative growth rate of height (RGRH), LMA, STD (dry mass per fresh volume, mg mm−3), and seed size (seed mass per 1000 seeds, g). Plant height of five individuals for each species in each quadrat measured at each Julian day in 2009 (denoted by H) was used to calculate RGR and Hmax for each species by fitting observed values to a logistic function (Eq. 3) and solving for RGRH (see Table S3 for the results of model fitting):
where \(\alpha\) is a species-specific constant (see Kaufmann 1981; Hara et al. 1991), using the nonlinear least-square method (XLSTAT Win-2010) (see Sun and Frelich 2011).
Leaf mass per area was measured as the leaf dry mass divided by fresh leaf area. We collected mature and fully developed leaves from the least-shaded, middle part of the plant canopy of five individuals for each species in each quadrat in 2009, because basal leaves were typically shaded and largely different from more elevated leaves in both shape and size. This sampling strategy permitted uniform interspecific comparisons of LMA, although it was slightly different from the standard method that requires selecting leaves in full sunlight (Cornelissen et al. 2003). Freshly sampled collected leaves were scanned and digitized to calculate projection area, subsequently oven-dried for 48 h at 65 °C, and weighed to 0.1 mg.
Stem tissue density was measured for a minimum of 40 flowering individuals per species in 2019. One 5–10 cm-long segment was cut from stems at 1 cm above ground per individual. In the case of hollow or solid stems with elliptical cross sections, the major and minor (orthogonal) diameters of each cut surface were measured per stem segment and transverse areas were calculated as the elliptical area. Each segment was subsequently dried at 65 °C for 48 h and weighed. Stem density was calculated as the dry mass divided by fresh volume (calculated as ab/4 times segment length) regardless of whether stems were solid or hollow.
Seed size was measured for a minimum of four fruited individuals per species. Seeds were collected from ripen fruits, oven-dried for 48 h at 65 °C after recording the number of seeds, and weighed to 0.1 mg. Seed size was calculated as the seed mass divided by the number of seeds.
Data analysis
Phenological and functional trait data were averaged for each species and analysed using correlation, regression, and multivariate analysis protocols. Pearson correlations were used to evaluate interspecific relationships between functional trait pairs, between phenologies, and between functional traits and phenologies. This analysis identified statistically significant trait-phenology relationships and provided preliminary indications of the direction and strength (r) of these relationships in both years. The relationships between functional traits and phenologies were then evaluated using regression analyses to determine whether each of the traits significantly affected each of the phenologies. Last, stepwise multiple regressions were performed to evaluate the relationships between functional traits and each of phenologies. The input variables were selected based on the result of the simple regression. All analyses were performed using STATISTICA software (StatSoft Inc. 2001).
In addition, because cross-species correlations can be biased by phylogenetic relationships among species (Felsenstein 1985; Harvey and Pagel 1991), a phylogenetic generalized least squares (PGLS) analysis was used (Pagel 1999; Freckleton et al. 2002) to estimate the strength of the phylogenetic effect on the relationship between functional traits and plant phenology. The five functional traits were the independent variables in the analysis. A maximum likelihood framework was used to estimate the index of phylogenetic association, λ, which ranged from 0 (indicating phylogenetic independence) to 1 (indicating complete phylogenetic dependence). A phylogenetic tree was constructed using the program Phylomatic (Webb et al. 2008) and the "Flora of China" (ECCAS 1998), with constant branch lengths. The PGLS analysis was performed in R version 3.5 (R Development Core Team 2019) using the package ape (Paradis et al. 2004).
Results
Phenology
Strong interspecific variation in both vegetative and reproductive phenologies was observed in this study (Table S1). The earliest species (Anemone trullifolia var. linearis) leafed out in early April and flowered in middle May in both investigation years, which was more than 3 months earlier than the latest species (Gentiana formosa) that leafed out during middle July and flowered in middle September in both years. Likewise, several species began to senesce in middle July, when late species (like Gentiana formosa and Allium sikkimense) were leafing out.
Significant positive correlations were also observed among different phenological events (Fig. S1). The species that leafed out earlier tended to flower, fruit, and senesced earlier (and vice versa) as indicated by correlation analyses (Fig. S1). Moreover, onset and offset times were usually positively correlated for each life history event (Fig. S1). Nevertheless, late flowering species tended to have longer periods between leaf onset and flowering onset times (R2 = 0.79, P < 0.001).
Functional traits
Large differences in functional traits were observed among the 16 species (Table S2). For example, Hmax differed among species by over 20 fold, i.e., < 3 cm (e.g. Leontopodium nanum) and > 70 cm (e.g., Deschampsia caespitosa). Seed mass differed over 42 fold, i.e., from 0.05 g per 100 seeds (Leontopodium nanum) to 2.14 g per 100 seeds (Anemone trullifolia var. linearis). Likewise, stem tissue density (STD) ranged between 0.07 (Caltha scaposa) to 0.38 mg mm−3 (Gentiana formosa), whereas LMA ranged between 2.21 (Leontopodium nanum) and 15.95 mg cm−2 (Gentiana formosa). In contrast, RGRH differed little among the study species, i.e., from 0.014 (Swertia wolfangiana) to 0.092 (Ranunculus brotherusii) cm cm−1 day−1.
Significant correlations were found among some of the trait pairs. STD was significantly and positively correlated with Hmax (r = 0.604, P = 0.013) but negatively correlated with RGR (r = − 0.782, P < 0.001), whereas LMA was negatively correlated with RGRH (r = 0.556, P = 0.025, Fig. 2). However, no significant correlation was found between seed size and any other traits.
Relationships between functional traits and phenology
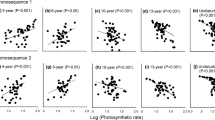
Majority of functional traits were highly related with phenology, although no significant correlation between phenological events and seed size was observed (Fig. S1). In contrast, LMA, RGRH and STD showed significant correlations with the onset and offset times for each of the four phenological events (Fig. S1). Hmax was related to all reproductive events (Fig. S1). LMA was positively correlated but RGRH was negatively correlated with the timings of each phenological event, i.e., early species tended to be characterized by low LMA and high RGRH compared to late species (Figs. 1, 2). Species with less dense stems typically flowered, fruited, and senesced earlier than species with denser stems (Fig. 3). High-stature species flowered and fruited later (Fig. 6, Table 1). LMA and STD contributed to ca. 50% of the cross-species variation observed for most of the observed phenologies (Table 1). Stepwise multiple linear regression analyses showed that LMA and STD significantly contributed to the variation observed in phenology (Table 1), while RGR significantly contributed to the variation observed in offset of senescing time (Table 1). Seed size and Hmax did not contribute significantly to phenological variation (Table 1).
Phylogenetic generalized least square analyses yielded different results to those obtained from interspecific regression analysis (Table 2). The maximum-likelihood values of λ were distinguishable from 0 but not from 1 in most of the functional trait vs. offset time of phenology relationships, indicating that phylogenetic relationships contribute in a meaningful way to the variation in offset time of phenology. In addition, although the λ values were not significantly different from 0 (at P = 0.05, Table 2) in any of the functional trait vs. onset time of phenology relationships, 18 of the 20 phenology-trait relationships examined using phylogenetic generalized least square analyses were not significant at the significant level of P < 0.05. These results indicate that phylogeny associations can weaken the relationship between onset time of phenology and functional traits.
Discussion
Main findings of the relationships between phenology and functional traits
We have shown that seed size is not significantly correlated with the phenological differences observed among our study species, inconsistent with the size-time tradeoff hypothesis, but our data partly support the height-time hypothesis. Importantly, we found that the leaf mass per area in tandem with stem tissue density serves in some cases as a powerful predictor for phenological differences, even though phylogenetic GLS analyses show that some phenological vs. functional trait relationships are influenced by the phylogenic relationships among species. Our findings highlight that plant phenology diversity could be better predicted by the combination of and trade-off among functional traits relative to single functional trait. The following sections provide a detailed discussion of each of these findings.
Seed size
The size-time hypothesis proposes a physiological mechanism underlying the vegetative growth–flowering phenology relationship (Bolmgren and Cowan 2008). According to this hypothesis, plants cannot reproduce much earlier than the time they have accumulated sufficient material resources nor can they delay reproduction significantly before the end of the growing season. Accordingly, early-flowering plants are posited to have relatively smaller resource availabilities but comparatively longer time to develop seeds, while tall, later-flowering species have shorter times for seed development (but might support higher reproductive capacities). Seed size (as determined by developmental time) has been successfully related to the variation in the onset time of flowering among perennial herb species (Bolmgren and Cowan 2008; Du and Qi 2010; Jia et al. 2011; Catorci et al. 2012). However, we did not observe a significant correlation between these two variables of interest.
The discrepancy between previous studies and the results reported here may result from the fact that not all early-flowering species have a long time to develop seeds. Some early-flowering species senesce early and lack a longer growing season compared to species flowering during the middle of the growing season (e.g., Caltha scaposa, Ranunculus brotherusii, Leontopodium nanum in this study). When these species are excluded from our analyses, a marginally negative correlation is observed between flowering onset time and seed size (r = − 0.56, P = 0.04 and r = − 0.52, P = 0.059 for 2009 and 2010, respectively). Moreover, the early-flowering species in our study are usually small in overall size (e.g., Leontopodium nanum), and may therefore be resource-limited. In contrast, larger plants (e.g., Blysmus sinocompressus) may have access to more resources that permits more rapid seed development, even if these species have less time to develop larger seeds. In addition, for any given resource level, the allocation tradeoff between seed size and number may differ even among species that flower at nearly the same time. For example, Anemone trullifolia var. linearis and Leontopodium nanum both flower early. Yet, they represent two extremes in seed size (the former has the biggest seeds, while L. nanum has the smallest seeds). This difference could be partly due to the fact that A. trullifolia var. linearis produces fewer seeds per fruit per flowering shoot than L. nanum (data not shown). Consequently, the time-size tradeoff hypothesis may not be applicable to all species in the same community, although it has been demonstrated for perennials at the regional scale (Bolmgren and Cowan 2008; Du and Qi 2010; Jia et al. 2011; Catorci et al. 2012).
Maximum plant height (H max)
Many studies show that grassland herbaceous species gain a short-term overtopping advantage while flowering such that a positive correlation exists between Hmax and the onset time of flowering. For example, Vile et al. (2006) report that the flowering onset time of 34 Mediterranean old-field herbaceous species correlates well with Hmax. Bolmgren and Cowan (2008) and Jia et al. (2011), respectively report similar findings for north-temperate perennial herbs and 48 herbaceous species in Qinghai-Tibetan. The positive correlations between Hmax and reproductive phenologies support the results of previous studies (Vile et al. 2006; Bolmgren and Cowan 2008; Jia et al. 2011; Sun and Frelich 2011). Similarly, we observed significant or marginally significant and positive relationships between Hmax and reproductive phenologies. Nevertheless, the explanation power of Hmax on the phenologies (0.0–29.7%) is lower than LMA, stem tissue density, and relative growth rate. The low explanation power is due to the exceptional species (Allium sikkimense and Gentiana formosa) that are unusual in that they leaf out and grow slowly under the canopy of early-growing plants and subsequently flower after many other species senesce. Removing these two species from the dataset, results in a more powerful significant linear relationship between onset of reproductive time and Hmax (R2 > 0.610, and P < 0.01 for both 2009 and 2010). Once again, we see that life history details (and thus habitat species composition) can profoundly alter the extent to which phenological and functional traits correlate.
Relative growth rate in height (RGRH)
In accordance with Sun and Frelich (2011) who revealed a higher RGRH in earlier flowering species, our data also show that early-flowering species had higher RGRH compared to species flowering later in the season. We believe this feature is the result of selection for an “overtopping advantage” early in the season when light and soil resources are more abundant and space is not limiting. It is nevertheless interesting to note that the relationship between RGRH (and associated LMA and SD) and phenologies predicted by Sun and Frelich (2011) holds true for the late-flowering, short species in our study site (R2 = 0.547, P = 0.036 in 2009, and R2 = 0.722, P = 0.008 in 2010). These late-flowering species emerge and grow under taller plants in the middle of the growing season when climatic conditions are generally favorable for growth but when shading limits growth. As a result, these species have low Hmax and a protracted time between leafing and flowering (as indicated by the positive correlation between the onset times of leafing and flowering among the species), which correlates with (and may actually cause) their low RGRH.
Perhaps more important, our data indicate that RGRH is an excellent predictor for the correlations observed between vegetative phenologies and functional traits for perennial herbaceous species (Fig. 4, Table 1). This is presumably because of the developmental linkage between vegetative and reproductive phenologies. Unlike woody species, none of our species produce flowers before leafing out. Indeed, floral phenology is typically size-dependent in many herbaceous species (e.g., Rathcke and Lacey 1985; Jia et al. 2011; Catorci et al. 2012). In addition, many forbs are insect-pollinated species in grassland communities (e.g., Ranunculaceae species in our study site) and they flower only after reaching community canopy layer. To this end, the early-flowering species need leaf out earlier and grow faster than later flowering species, as evidenced by our data. Moreover, early-flowering species often have a shorter interval between leafing out and the onset of flowering compared to late flowering species (Table S1). The higher RGRH in the early-flowering species can be explained as the high tolerance to coldness in these species (Mo et al. 2018).
Stem tissue mass density (STD)
In this study, stem tissue density was a better predicator of the phenologies than RGRH and Hmax. This is consistent with a recent study of the semi-arid region of northeastern Brazil, where Lima and Rodal (2010) show that species that leaf out and abscise earlier are more likely to have low STD during the transition period between the rainy and the dry seasons as an adaptation for water storage. In a similar way, Wang et al. (1992) find that species incurring less loss of hydraulic conductivity by late winter tend to leaf out earlier in the spring than their other counterparts.
Stem density by definition is almost unavoidably negatively correlated with RGRH. Low STD allows for high RGRH (King et al. 2005; Poorter et al. 2006, 2008), which reflects the trade-off among the growth rate, plant height and shoot quality. Consequently, variations in STD mediate the relationship between RGRH and Hmax (Poorter et al. 2003, 2006, 2008; King et al. 2006a, b; Sun and Frelich 2011). In the current study, although the late flowering species Gentiana formosa is as short as many early-flowering species, it has a higher STD and a lower RGRH compared to early-flowering species. The ecological importance of STD should be recognized and more explored, provided that STD are much less studied for herbaceous plants (see Sun and Frelich 2011) than woody plants (Wang et al. 1992; Poorter et al. 2003, 2006; King et al. 2006a, b; Lima and Rodal 2010).
Leaf mass per area (LMA)
This functional trait was significantly correlated with each of the phenological attributes examined in our study. This finding, which is consistent with previous studies reporting a negative correlation between LMA and RGRH (e.g., Hunt and Cornelissen 1997; Poorter et al. 2003; Sun and Frelich 2011; Craine et al. 2012), makes sense from a biomechanical perspective, since rapid stem growth is often achieved with low density tissues, which cannot support leaves constructed out of very dense tissues. Indeed, prior work indicates that high LMA is typically associated with low nutrient concentrations and low photosynthetic capacity, while low LMA enables plants to maximize light interception (e.g.,Hunt and Cornelissen 1997; Sun et al. 2006; Fotis and Curtis 2017). In our study site, species that leaf out and flower early in the growing season tend to be small with comparatively high reproductive allocations. We speculate that this phenomenology requires low LMA to facilitate rapid growth. In addition, these species often senesce earlier than late flowering species, and therefore do not require physically tough leaves.
The effect of phylogenetic associations
Our results show that despite phylogenetic effect on phenology was only observed in the case of some offset time of phenologies, most of the relationships between functional traits and phenologies are weakened after removing phylogeny effects. This result approve that phylogenetic effects exist in the regulation of phenological variation in the meadow. For example, the four species representing the Ranunculaceae have relatively low SLA and end their flowering time earlier than the two species representing the Gentianaceae, which flower late and are characterized by high stem tissue densities and low RGRH. Similarly noticeable phylogeny effects on flowering phenology have been reported for angiosperm flora of China (Du et al. 2015), south-eastern Brazil (Staggemeier et al. 2010), animal pollinated floras of North and South Carolina and temperate Japan (Kochmer and Handel 1986), and for the endemic flora of the southern and south-western Cape Province, South Africa (Johnson 1993). Likewise, Davis et al. (2012) show that phenological responses to climate change are often similar among closely related taxa, even between those in geographically disjunct communities. These results have demonstrated the regulation of evolutionary on flowering time (Gaudinier and Blackman, 2020). In addition, the low λ value indicates no phylogenetic effect on the variation in onset of phenological traits. We believe that this reflects the non-conservative evolutionary nature of onset pehnological traits, which contrasts with the conservative nature of offset phenological traits (e.g., Ollerton and Lack 1992). For example, while beginning time of leafing, flowering and senescing phenologies are known to vary with abiotic and biotic factors as is evident from studies of global climate change (Cleland et al. 2007), ending time of phenologies should have evolved adaptively and generally to favorable periods during the growing season, particularly in climatically stressful habitats such as alpine meadows. Furthermore, the weakened relationships between the onset time of phenology and functional traits in association with the low λ value may be ascribed to the phylogenetic regulation on functional traits, which has been widely reported. For example, phylogenetic signal of maximum plant height was observed in a mountain ecosystem in northeastern China (Xu et al. 2017). Phylogenetic signal of leaf traits was observed in Acer species in Japan (Nakadai et al. 2014). Our results indicate that phylogenetic associations must be considered in the study on the interspecific variation in functional traits.
Conclusions
Our data reveal significant correlations between species traits and plant phenologies for co-existing herbaceous species in an alpine meadow. Plant species with higher stem tissue density has lower relative growth rate, higher leaf mass per area, and higher maximum plant height in the alpine meadow. Species with early leaf-out also had early occurrence of all other studied phenological events. Leaf mass per area and relative growth rate in height provide a robust predictor of vegetative phenologies, while stem tissue mass density and leaf mass per area are good predictors for reproductive phenologies. Phylogeny has some effects on the offset time of phenology, but not on the onset time of phenology. Phylogeny weakened the correlations between functional traits and offset time of phenological events. Our findings highlight that plant phenology diversity could be better predicted by the combination of and trade-off among functional traits relative to single functional trait. Future studies are required to determine whether these results can be extrapolated to other habitats dominated by grassland herbaceous species.
References
Armstrong JB, Takimoto G, Schindler DE, Hayes MM, Kauffman MJ (2016) Resource waves: phenological diversity enhances foraging opportunities for mobile consumers. Ecology 97:1099–1112. https://doi.org/10.1890/15-0554.1
Bolmgren K, Cowan DP (2008) Time-size tradeoffs: a phylogenetic comparative study of flowering time, plant height and seed mass in a north-temperate flora. Oikos 117:424–429. https://doi.org/10.1111/j.2007.0030-1299.16142.x
Brody AK (1997) Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology 78:1624–1631. https://doi.org/10.1890/0012-9658(1997)078[1624:EOPHAS]2.0.CO;2
Castro-Díez P, Milla R, Sanz V (2005) Phenological comparison between two co-occurring Mediterranean woody species differing in growth form. Flora 200:88–95. https://doi.org/10.1016/j.flora.2004.07.002
Catorci A, Cesaretti S, Gatti R, Tardella FM (2012) Trait-related flowering patterns in submediterranean mountain meadows. Plant Ecol 213:1315–1328. https://doi.org/10.1007/s11258-012-0090-9
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366. https://doi.org/10.1111/j.1461-0248.2009.01285.x
Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shifting plant phenology in response to global change. Trends Ecol Evol 22:357–365. https://doi.org/10.1016/j.tree.2007.04.003
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. https://doi.org/10.1071/BT02124
Craine JM, Wolkovich EM, Towne EG, Kembel SW (2012) Flowering phenology as a functional trait in a tallgrass prairie. New Phytol. 193:673–682. https://doi.org/10.1111/j.1469-8137.2011.03953.x
Davis CC, Willis CG, Primack RB, Miller-Rushing AJ (2012) The importance of phylogeny to the study of phenological response to global climate change. Philos Trans R Soc B 365:3201–3213. https://doi.org/10.1098/rstb.2010.0130
Du G, Qi W (2010) Trade-offs between flowering time, plant height, and seed size within and across 11 communities of a QingHai-Tibetan flora. Plant Ecol 209:321–333. https://doi.org/10.1007/s11258-010-9763-4
Du YJ, Mao LF, Queenborough SA, Freckleton RP, Chen B, Ma KP (2015) Phylogenetic constraints and trait correlates of flowering phenology in the angiosperm flora of China. Glob Ecol Biogeogr 24:928–938. https://doi.org/10.1111/geb.12303
ECCAS (1998) Editor Committee of the Chinese Academy of Sciences for Flora of China. Flora of China Science Press, Beijing
Fantinato E, Del Vecchio S, Giovanetti M, Acosta ATR, Buffa G (2018) New insights into plants co-existence in species-rich communities: the pollination interaction perspective. J Veg Sci 29:6–14. https://doi.org/10.1111/jvs.12592
Felsenstein J (1985) Confidence limits phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Fotis AT, Curtis PS (2017) Effects of structural complexity on within-canopy light environments and leaf traits in a northern mixed deciduous forest. Tree Physiol 37:1426–1435. https://doi.org/10.1093/treephys/tpw124
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726. https://doi.org/10.1086/343873
Gaudinier A, Blackman BK (2020) Evolutionary processes from the perspective of flowering time diversity. New Phytol 225:1883–1898. https://doi.org/10.1111/nph.16205
Hara T, Kimura M, Kikuzawa K (1991) Growth patterns of tree height and stem diameter in populations of Abiesveitchii, A. mariesii and Betulaermanii. J Ecol 79:1085–1098. https://doi.org/10.2307/2261100
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Hunt R, Cornelissen JHC (1997) Components of relative growth rate and their interrelations in 59 temperate plant species. New Phytol 135:395–417. https://doi.org/10.1046/j.1469-8137.1997.00671.x
Jia P, Bayaerta T, Li XQ, Du GZ (2011) Relationships between flowering phenology and functional traits in eastern Tibet alpine meadow. Arct Ant Alp Res 43:585–592. https://doi.org/10.1657/1938-4246-43.4.585
Johnson SD (1993) Climatic and phylogenetic determinants of flowering seasonality in the Cape flora. J Ecol 81:567–572. https://doi.org/10.2307/2261535
Kaufmann KW (1981) Fitting and using growth curves. Oecologia 49:293–299. https://doi.org/10.1007/BF00347588
King DA, Davies SJ, Nur Supardi MN, Tan S (2005) Tree growth is related to light interception and wood density in two mixed dipterocarp forests of Malaysia. Funct Ecol 19:445–453. https://doi.org/10.1111/j.1365-2435.2005.00982.x
King DA, Davies SJ, Tan S, Noor NSM (2006a) The role of wood density and stem support costs in the growth and mortality of tropical trees. J Ecol 94:670–680. https://doi.org/10.1111/j.1365-2745.2006.01112.x
King DA, Davies SJ, Noor NSM (2006b) Growth and mortality are related to adult tree size in a Malaysian mixed dipterocarp forest. For Ecol Manag 223:152–158. https://doi.org/10.1016/j.foreco.2005.10.066
Kochmer JP, Handel SN (1986) Constraints and competition in the evolution of flowering phenology. Ecol Monogr 56:303–325. https://doi.org/10.2307/1942549
Lima ALA, Rodal MJN (2010) Phenology and wood density of plants growing in the semi-arid region of northeastern Brazil. J Arid Environ 74:1363–1373. https://doi.org/10.1016/j.jaridenv.2010.05.009
Liu Y, Reich PB, Li G, Sun S (2011) Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology 92:1201–1207. https://doi.org/10.1890/10-2060.1
Liu Y, Miao R, Chen A, Miao Y, Liu Y, Wu X (2017) Effects of nitrogen addition and mowing on reproductive phenology of three early-flowering forb species in a Tibetan alpine meadow. Ecol Eng 99:119–125. https://doi.org/10.1016/j.ecoleng.2016.11.033
Mason NWH, Pipenbaher N, Škornik S, Kaligarič M (2013) Does complementarity in leaf phenology and inclination promote co-existence in a species-rich meadow? Evidence from functional groups. J Veg Sci 24:94–100. https://doi.org/10.1111/j.1654-1103.2012.01451.x
Miller-Rushing AJ, Forrest J (2010) Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc B 365:3101–3260. https://doi.org/10.1098/rstb.2010.0145
Mo L, Luo P, Mou CX, Yang H, Wang J, Wang ZY, Li YJ, Luo C, Li T, Zuo DD (2018) Winter plant phenology in the alpine meadow on the eastern Qinghai-Tibetan Plateau. Ann Bot Lond 1226:1033–1045. https://doi.org/10.1093/aob/mcy112
Nakadai R, Murakami M, Hirao T (2014) Effects of phylogeny, leaf traits, and the altitudinal distribution of host plants on herbivore assemblages on congeneric Acer species. Oecologia 175:1237–1245. https://doi.org/10.1007/s00442-014-2964-0
Nord EA, Lynch JP (2009) Plant phenology: a critical controller of soil resource acquisition. J Exp Bot 60:1927–1937. https://doi.org/10.1093/jxb/erp018
Ollerton J, Lack AJ (1992) Flowering phenology: an example of relaxation of natural selection? Trends Ecol Evol 7:274–276. https://doi.org/10.1016/0169-5347(92)90175-B
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884. https://doi.org/10.1038/44766
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogentics and evolution in R language. Bioinformatics 20:289–290. https://doi.org/10.1093/bioinformatics/btg412
Polgar CA, Primack RB (2011) Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytol 191:926–941. https://doi.org/10.1111/j.1469-8137.2011.03803.x
Poorter L, Bongers F, Sterck FJ, Woll H (2003) Architecture of 53 rain forest tree species differing in adult stature and shade tolerance. Ecology 84:602–608. https://doi.org/10.1890/0012-9658(2003)084[0602:AORFTS]2.0.CO;2
Poorter L, Bongers L, Bongers F (2006) Architecture of 54 moist-forest tree species: traits, trade-offs, and functional groups. Ecology 87:1289–1301. https://doi.org/10.1890/0012-9658(2006)87[1289:AOMTST]2.0.CO;2
Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, Harms KE, Licona JC, Martínez-Ramos M, Mazer SJ, Muller-Landau HC, Peña-Claros M, Webb CO, Wright IJ (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920. https://doi.org/10.1890/07-0207.1
R Development Core Team (2019) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Evol Syst 16:179–214. https://doi.org/10.1146/annurev.es.16.110185.001143
Singh KP, Kushwaha CP (2006) Diversity of flowering and fruiting phenology of trees in a tropical deciduous forest in India. Ann Bot 97:265–276. https://doi.org/10.1093/aob/mcj028
Staggemeier VG et al (2010) The shared influence of phylogeny and ecology on the reproductive patterns of Myrteae (Myrtaceae). J Ecol 98:1409–1421. https://doi.org/10.1111/j.1365-2745.2010.01717.x
Statsoft Inc. (2001) Statistica 6.0 Tulsa, Oklahoma, USA
Sun S, Jin D, Li R (2006) Timing of leaf emergence in relation to leaf traits of woody species in eastern Chinese Quercus fabri forests. Act Oecol 30:212–222. https://doi.org/10.1016/j.actao.2006.04.001
Sun S, Frelich LE (2011) Flowering phenology and height growth pattern are associated with maximum plant height, relative growth rate and stem tissue mass density in herbaceous grassland species. J Ecol 99:991–1000. https://doi.org/10.1111/j.1365-2745.2011.01830.x
Vile D, Shipley B, Garnier E (2006) A structural equation model to integrate changes in functional strategies during old-field succession. Ecology 87:504–517. https://doi.org/10.1890/05-0822
Wang J, Ives NE, Echowicz MJL (1992) The relation of foliar phenology to xylem embolism in trees. Funct Ecol 6:469–475. https://doi.org/10.2307/2389285
Wang GC, Huang Y, Wei YR, Zhang W, Li TT, Zhang Q (2019) Inner Mongolian grassland plant phenological changes and thei r climatic drivers. Sci Tot Environ 683:1–8. https://doi.org/10.1016/j.scitotenv.2019.05.125
Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24:2098–2100. https://doi.org/10.1093/bioinformatics/btn358
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecology strategies: some leading dimensions of variation between species. Annu Rev Eco Evol Syst 33:125–159. https://doi.org/10.1146/annurev.ecolsys.33.010802.150452
Wu X, Duffy JE, Reich PB, Sun SC (2011) A brown-world cascade in the dung decomposer food web of an alpine meadow: effects of predator interactions and warming. Ecol Monogr 81:313–328. https://doi.org/10.1890/10-0808.1
Xia J, Wan S (2013) Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Ann Bot 111:1207–1217. https://doi.org/10.1093/aob/mct079
Xu J, Chen Y, Zhang L, Chai Y, Wang M, Guo Y, Li T, Yue M (2017) Using phylogeny and functional traits for assessing community assembly along environmental gradients: a deterministic process driven by elevation. Ecol Evol 7:5056–5069. https://doi.org/10.1002/ece3.3068
Acknowledgements
We thank Jian Feng, Yibin Yuan, and Ang Zhang for the help with data collection; Xinqiang Xi and Xiaoli Hu for the help on phylogenetic regression analyses.
Funding
This study was funded by National Natural Science Foundation of China (Grant number 31530007, 31670477, and 31770522).
Author information
Authors and Affiliations
Contributions
YL, GL, and SS collected the data and wrote the first draft of the manuscript. YL and XW analyzed the data. YL, GL, KJN, ZY, and SS contributed substantially to technical support and English revisions. SS design the experiment.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Data accessibility
All basic data deposited in the Appendix as support information Table S1 and Table S2.
Additional information
Communicated by Jonathan A. Myers.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, G., Wu, X. et al. Linkage between species traits and plant phenology in an alpine meadow. Oecologia 195, 409–419 (2021). https://doi.org/10.1007/s00442-020-04846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04846-y