Abstract
The production and fate of seaweed detritus is a major unknown in the global C-budget. Knowing the quantity of detritus produced, the form it takes (size) and its timing of delivery are key to understanding its role as a resource subsidy to secondary production and/or its potential contribution to C-sequestration. We quantified the production and release of detritus from 10 Laminaria hyperborea sites in northern Norway (69.6° N). Kelp biomass averaged 770 ± 100 g C m−2 while net production reached 499 ± 50 g C m−2 year−1, with most taking place in spring when new blades were formed. Production of biomass was balanced by a similar formation of detritus (478 ± 41 g C m−2 year−1), and both were unrelated to wave exposure when compared across sites. Distal blade erosion accounted for 23% of the total detritus production and was highest during autumn and winter, while dislodgment of whole individuals and/or whole blades corresponded to 24% of the detritus production. Detachment of old blades constituted the largest source of kelp detritus, accounting for > 50% of the total detrital production. Almost 80% of the detritus from L. hyperborea was thus in the form of whole plants or blades and > 60% of that was delivered as a large pulse within 1–2 months in spring. The discrete nature of the delivery suggests that the detritus cannot be retained and consumed locally and that some is exported to adjacent deep areas where it may subsidize secondary production or become buried into deep marine sediments as blue carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flow of dead organic matter (detritus) across ecosystem boundaries connects neighboring ecosystems and may fuel secondary productivity in recipient ecosystems where primary productivity is low. Detrital subsidies can alter patterns of species composition and food web structure in terrestrial and aquatic systems (e.g., Polis et al. 1997), but may be particularly important in aquatic environments due to greater connectivity than in terrestrial systems (Carr et al. 2003). The productivity of kelp-dominated ecosystems may exceed 2000 g C m−2 year−1 (Mann 1973; Abdullah and Fredriksen 2004) although rates in the range of 4–600 g C m−2 year−1 are more common (e.g., Krumhansl and Scheibling 2012; Pessarrodona et al. 2018; Wernberg et al. 2019). Direct grazing on live kelp is often low and ranges from 10 to 15% of the annual production (Krumhansl and Scheibling 2012), although grazing by sea urchins and herbivorous fishes can be high in disturbed systems (Poore et al. 2012; Wernberg et al. 2013; Steneck and Johnson 2013). Most kelp production is, therefore, channeled to the detrital pool within kelp systems or in adjacent systems such as beaches (Colombini and Chelazzi 2003; Ince et al. 2007), seagrass beds (Wernberg et al. 2006), distant reefs (Vanderklift and Wernberg 2008), deeper subtidal areas (Filbee-Dexter and Scheibling 2016) and submarine canyons (Vetter and Dayton 1999), where it may be consumed by detritivores, decompose or accumulate and, thus, contribute to C-sequestering (Cebrian 1999; Krause-Jensen and Duarte 2016).
Kelp detritus is generated through different processes such as continuous erosion and/or pruning of the blades and dislodgement of entire plants or whole blades, including phenologically determined losses of old blades in some species (Krumhansl and Scheibling 2012). Most studies to date have either quantified detritus formation through dislodgement or through blade erosion (Table 1 in Krumhansl and Scheibling 2012), while only three studies have conducted concurrent measurements of erosion and dislodgment rates that allow comparisons of the relative contribution of detritus formed by these different processes (Gerard 1976; de Bettignies et al. 2013b; Pessarrodona et al. 2018).
The relative importance of the mechanisms of detritus formation may be context dependent and vary as a function of species and environmental conditions. Dislodgement caused by strong water movement is often considered the main driver for production of kelp detritus, due to higher kelp mortality during periods of peak wave action (e.g., Ebeling et al. 1985; Seymour et al. 1989; Graham et al. 1997) and because large amounts of kelp detritus accumulate as beach cast or in adjacent deep habitats following storms (e.g., Griffiths et al. 1983; Filbee-Dexter and Scheibling 2012). Other studies have shown that the formation of detritus through distal erosion of blades can be significant and match annual blade production (e.g., Krumhansl and Scheibling 2011; de Bettignies et al. 2013b). Blade erosion may be positively correlated to water movement, but may also be stimulated by epiphytic load (e.g., bryozoans), grazing and seasonal patterns of reproduction that may weaken the blade tissue and make it more susceptible to scouring (Krumhansl et al. 2011; de Bettignies et al. 2012, 2013b; Mohring et al. 2012). Detritus generated by these different processes varies substantially in size (from small particles to whole thalli), which may affect dispersal range, consumption and decomposition rate.
Laminaria hyperborea (Gunnerus) Foslie is the dominant kelp species in terms of biomass along rocky shores in the NE Atlantic where it forms extensive forests that dominate coastal primary production (e.g., Smale et al. 2013; Pessarrodona et al. 2018; Wernberg et al. 2019). L. hyperborea produces one annual blade that begins to form in winter and grows to maximum size (~ 1 m2) during spring and early summer, after which it erodes during fall and winter. The remains of the blade are shed in spring as the new emerging blade is formed at the base of the old. A large proportion of the old blade biomass is thus discharged over a short period, which may result in a significant pulse of coarse detritus (Pessarrodona et al. 2018). The overall aim of this study was to quantify the spatial and seasonal variation in productivity and formation of detritus through erosion, dislodgement, and the spring cast of old blades for high latitude populations of L. hyperborea. We expected that physical forcing caused by waves would be an important driver for spatial and temporal variations in the formation of kelp detritus through erosion and dislodgment, while the spring cast of old blades would constitute a substantial pulse of coarse kelp detritus.
Methods
Study site
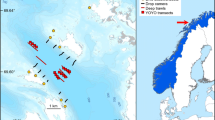
Our study took place around the mouth of Malangen fjord in northern Norway (69.6° N, 18.0° E). The area is heavily influenced by ocean swells, wind-generated waves and tides (± 1.5 m). The rocky subtidal is dominated by kelp Laminaria hyperborea to a depth of ca. 20–25 m. The study area covers 126 km2 of coastal ocean (Fig. 1), of which L. hyperborea covers ca. 22 km2 according to a predictive kelp forest model developed by the national Norwegian mapping of marine habitats (Bekkby et al. 2013). The model uses 12 years of monitoring data for the entire Norwegian coast along with wind, fetch, coastline and bathymetric data to predict the presence/absence of kelp. We selected ten study sites representing a range of wave exposure levels based on variations in effective fetch (Fig. 1), with the most exposed site on a shoal 2.4 km offshore (site 5), and the most protected site in a small bight 3.5 km in from the mouth of the fjord (site 10). The sites ranged from ‘moderately exposed’ to ‘very exposed’ according to the EUNIS classification system used to classify coastal habitats in Europe (Davies and Moss 2003). We quantified kelp density, biomass, production, and formation of detritus through different processes at each site during autumn 2016, winter 2016–2017, spring 2017 and summer 2017.
Temperature, light and wave exposure
Water temperature and light intensity in the kelp forests (just above the canopy) were monitored hourly at each sampling site during the entire study period using HOBO data loggers (Pendant Temp–Light, Onset Computer Corporation) anchored to subsurface floats. Wave exposure level was calculated for each site from August 2016 to August 2017 using a modified version of the method presented by Fonseca and Bell (1998). Hourly wind data (mean velocity and direction) were obtained from Hekkingen Lighthouse weather station (the Norwegian Metrological Institute) located in the middle of the study area. Weighted effective fetch (WEF) for each sampling site was estimated by placing the center of a circle on all sites and subsequently dividing each of these into 8 sectors each with an angle of 45°, beginning at the N sector (337.5°–22.5°). The fetch (F in km) was measured along 5 radia (each with 11.25° spacing) within each sector and the weighted effective fetch for each sector (WEFi) was then estimated by first multiplying each fetch with the cosine of the angle (γ) of departure from the major heading (of the sector) and finally averaging the 5 values:
Relative wave exposure index (REI) was computed hour by hour for each site by multiplying hourly wind speeds with the relevant effective fetch:
where i is the ith compass heading [i.e., 1–8 (N, NE, E, etc.) in 45° increments] and Vi is the wind speed from direction i. Hourly estimates of REI were finally used to estimate mean and maximum REI for each site during autumn (18th Aug–25th Oct), winter (26th Oct–29th March), spring (2nd April–29th May) and summer (30th May–10th Aug), respectively. The maximum REI was estimated as the average of the 10% highest REI values in a season.
Kelp density and biomass
The density and biomass of kelp were quantified in August and October 2016 and in March, May and August 2017. SCUBA divers collected all canopy plants (i.e., plants with stipes longer than ca. 0.7 m, Pedersen et al. 2012) within 4–6 quadrats (area = 0.25 m2) at each site. The quadrats were placed haphazardly in the kelp forest at 5–7 m depth and with a minimum distance of 5 m apart. Density was quantified by counting the number of canopy plants in each quadrat. The fresh weight (FW) biomass of each individual stipe and blade (both old and new blades in March and May) was weighed to the nearest gram and total FW biomass per quadrat was estimated as the sum of all individual weights of canopy plants. Holdfasts were not collected, but they comprise ca. 13% (± 4) of the FW biomass of the whole thallus (Pedersen et al. 2012; Bekkby et al. 2014).
Blade growth and erosion
Modified versions of the hole punch methods were used to measure frond elongation (Parke 1948) and distal erosion of the blade (Tala and Edding 2005). Twenty kelp individuals were tagged for growth and erosion measurements at each site and field campaign and harvested during the succeeding campaign. The kelps were tagged with two holes in the lower, basal part of the blade for growth measurements (5 and 10 cm above the junction between the stipe and the blade, i.e., the meristem) and three holes in the distal part of the blade (10, 20 and 30 cm from the distal edge of the blade) for erosion measurements. Tagged individuals were marked with yellow cable ties around the top of the stipe to ease identification and harvest during the following field campaign. Blade elongation was quantified by measuring the distance from the lowest hole to the meristem (bd1) and the distance between the two basal holes (bd2). Blade elongation (BE) was calculated by subtraction of the sum of these two measures by 10 cm
The distance from the distal edge of the blade to each of the three terminal holes (td1, td2 and td3, respectively) was also measured and blade erosion (ER) was calculated by subtracting 10, 20 and 30 cm, respectively, from the measured distances from the edge to each of the three terminal holes and averaging the results:
Each blade was finally cut into 5 cm segments that were weighed (blotted FW). The heaviest segment from the basal half of the blade was used to calculate daily blade production per individual (BP, g FW individual−1 day−1) using Eq. 5:
where BE is the blade elongation (in cm), FWB is the length-specific biomass (g FW cm−1) of the heaviest segment from the basal half of the blade, and t is the number of days elapsed between tagging the plant and its harvest. The heaviest segment from the lower half of the lamina was used to calculate production because the density (g FW unit−1 area) continues to increase after the elongation rate has ceased. Blade production (g DW m−2) was finally estimated by multiplying daily blade production per individual (BP) with plant density and the number of days elapsed between sampling events. Stipe production was not measured in the present study but was estimated from measured stipe biomass from the above quadrat collections and P/B ratios for canopy plant stipe [P/B ratio = 0.234 ± 0.032 (mean ± SD)]; Pedersen et al. 2012).
Segments from the distal half of the blade were used to calculate the biomass of eroded blade material (BE) by multiplying the erosion length (ER) with the average length-specific biomass (FWD cm−1) of the distal half of the blade according to Eq. 6:
where t is the number of days elapsed between tagging the plants and its collection. Blade erosion losses (g DW m−2) were finally estimated by multiplying daily blade erosion per individual (BE) with plant density and the number of days elapsed between the sampling events.
Dislodgement and spring cast
Dislodgement of whole plants and blades was estimated as the proportion of tagged plants that was lost between sampling events and from the number of ‘fresh’ stipes without blades (i.e., with destroyed meristems) collected in the quadrats. The mass of kelp detritus formed by dislodged plants (DDIS) was estimated as the site-specific proportion of plants lost between sampling events (PL) multiplied by site-specific kelp density (D) and individual kelp biomass (BIND) to obtain daily losses in g FW m−2 between sampling events:
where t is the time elapsed between two succeeding sampling events. The biomass of old lamina lost during the spring cast (DCAST) was estimated from site-specific changes in the proportion of individuals carrying an old lamina (POB) between successive sampling events (i.e., winter to spring and spring to summer) multiplied by site-specific kelp density (D) and the individual biomass of old lamina (BLAM) to obtain daily losses in g FW m−2 between sampling events:
where t is the time elapsed between two succeeding sampling events. Units of FW were finally converted to units of carbon applying a DW:FW ratio of 0.163 ± 0.047 for blades and 0.135 ± 0.019 for stipes, respectively, and a C content of 33.0 ± 3.1% of DW for blades and 29.7 ± 2.6% of DW for stipes (own unpublished values for this species, n = 32).
Comparing detrital C-flux from L. kelp to that of other habitats
We compared finally the obtained values of detrital C donation by L. hyperborea to that of other terrestrial and coastal habitats using data obtained from the literature. Terrestrial habitats included temperate and tropical forests and shrubs, temperate and tropical grass lands, while coastal habitats included marine phytoplankton, non-kelp seaweeds, seagrasses, mangroves and marshes. The particulate detrital carbon donation included all types of litterfall and detritus (e.g., leaves, branches and twigs, reproductive structures), but in most cases not below-ground detritus production. Numbers and references are given in Supporting Information Table S1.
Statistical analyses
All values in the text are means ± 95% CI unless otherwise stated. Most data (i.e., REI, kelp density, individual biomass, biomass per unit area, blade growth, blade erosion, dislodgment of plants and loss of old blades) did not meet the assumptions for parametric analysis (especially homogeneity of variance) and were, therefore, compared across sites and seasons using non-parametric repeated measures ANOVA (i.e., Friedman’s test). Means were first compared across sites using season as a blocking factor and then compared across seasons using site as blocking factor. Multiple pairwise comparisons were conducted using the Tukey procedure for ranked data when the Friedman test provided significant results (Zar 1999). Correlations between net production, blade erosion, dislodgment and relative wave exposure level (REI) were tested using non-parametric Spearman Rank Correlation analysis. The detritus production in different ecosystem types was compared using one-way ANOVA. All tests were performed using α = 0.05.
Results
Temperature, light and relative wave exposure
Water temperature averaged 7.1 ± 2.3 °C (± SD) and ranged from 4.2 °C in spring (March–April) to 11.5 °C in late summer (August) (Fig. 2a). Daily light intensity reaching the canopy averaged 765 ± 855 lx (± SD) and ranged from 0 lx day−1 in December and January to 3877 lux day−1 late June (Fig. 2b). Wind speed (Fig. 2c) averaged 6.5 ± 4.0 m s−1 (± sd) and ranged from an average of 4 m s−1 in summer to 8 m s−1 in winter, while the maximum wind speed ranged from 18 m s−1 in autumn to 26 and 32 m s−1 in winter and spring, respectively. Mean and maximum wave exposure level (REI) varied between seasons and sites (Fig. 3a and b). Mean REI varied from 10- to 25-fold between sites depending on season (\(\chi_{r, 4,10}^{2}\) = 33.4; p < 0.001) and was significantly higher at sites 1–5 than at sites 6–10. Maximum REI followed largely the same pattern across sites (\(\chi_{r, 4,10}^{2}\) = 33.8; p < 0.001), but the variation was larger than for mean REI (30 to 53-fold variation depending on season). Mean REI was highest during winter and lowest in autumn (\(\chi_{r, 4,10}^{2}\) = 13.3; p = 0.004), while the maximum REI was highest in winter and lowest in summer (\(\chi_{r, 4,10}^{2}\) = 18.8; p < 0.001). Mean REI varied from 1.6- to 2.8-fold between seasons (depending on site), while maximum REI varied from 1.1- to 2.7-fold between seasons. Seasonal variation in REI was not consistent across all sites since some sites had larger seasonal variations in REI than others. This was likely due to seasonal variation in the dominant wind direction and showed that location and, thus, weighted effective fetch played an important role for REI.
Individual plant traits
Individual kelp biomass (i.e., stipe plus lamina) averaged 48.2 ± 12.9 g C (± SD) and ranged from 24.0 to 77.0 g C depending on site and season (Fig. 4a). Individual stipe biomass (mean ± SD = 19.2 ± 6.8 g C) was larger at sites 5, 6 and 7 than at the remaining sites (26.6 vs. 16.0 g C; \(\chi_{r, 10,4}^{2}\) = 28.0; p < 0.001), but did not vary seasonally (\(\chi_{r, 4,10}^{2}\) = 6.2; p = 0.188). Individual blade biomass (mean ± SD = 29.0 ± 8.6 g C; Fig. 4b) was larger in plants from sites 1 to 6 than from sites 7 to 10 (32.8 vs. 23.3 g C kelp−1; \(\chi_{r, 10,4}^{2}\) = 24.4; p = 0.004). Individual blade biomass was the only morphological variable that was correlated with REI (Spearman rank’s R = 0.745; p = 0.013). Blade biomass was lowest in late winter and largest in summer (22.9 vs. 33.8 g C; \(\chi_{r, 4,10}^{2}\) = 16.6; p = 0.002). New blades were initiated in early winter and increased in size during spring to reach maximum size in August. Old, fully grown blades lost 35.8 ± 18.6% of their biomass through erosion and pruning between late summer and the following spring where they were cast.
Laminaria hyperborea individual plant variables: a individual kelp biomass at the 10 study sites (averaged across seasons), b seasonal variation in individual kelp biomass (averaged across sites). Sites are ranked according to increasing wave exposure level (REI) along the x-axis. Mean values ± 95% CI
Kelp density, biomass and productivity
Kelp density averaged 16.6 ± 1.3 (± SD) individuals m−2 across sites and seasons (Fig. 5a, b). Density did not differ among sites (\(\chi_{r, 10,4}^{2}\) = 13.9; p = 0.126), but decreased slightly over the course of the study (\(\chi_{r, 4,10}^{2}\) = 12.8; p = 0.012; density in August 2016 being higher than in March, May and August 2017; all p < 0.015).
Laminaria hyperborea biomass and productivity: a kelp density at the 10 study sites (averaged across seasons), b seasonal variation in kelp density (averaged across sites), c kelp biomass per unit area at the 10 study sites (averaged a cross seasons), d seasonal variation in kelp biomass (averaged across sites), e blade production at each site in each of four seasons and, f seasonal variation in average blade production (averaged across sites). Sites are ranked according to increasing wave exposure level (REI) along the x-axis. Mean values ± 95% CI
Total kelp biomass per unit area averaged 770 ± 100 g C m−2 (± SD) across all sites and sampling dates (Fig. 5c, d). The total biomass was higher at sites 3, 5, 6, 7 and 8 than at the remaining sites (888 vs. 652 g C m−2; \(\chi_{r, 10,4}^{2}\) = 20.4; p = 0.015). The total stipe biomass per unit area averaged 313 ± 69 g C m−2 (± SD) across sites and sampling events, corresponding to ca. 41% of the total biomass. Stipe biomass was higher at sites 5–8 than at the other sites (415 vs. 245 g C m−2; \(\chi_{r, 10,4}^{2}\) = 28.3; p < 0.001), but did not vary seasonally (\(\chi_{r, 4,10}^{2}\) = 6.6; p = 0.161). Total blade biomass per unit area averaged 458 ± 64 (± SD) g C m−2 and was similar across sites, except for site 3, where it was higher than at all other sites (\(\chi_{r, 10,4}^{2}\) = 17.4; p = 0.043). Blade biomass varied seasonally (\(\chi_{r, 4,10}^{2}\) = 13.0; p = 0.011), being lowest in late winter (March) when the new blades were small and the old ones were heavily eroded, and highest in late summer.
Daily blade production per unit area averaged 1.16 ± 0.11 g C m−2 (Fig. 5e, f) and did not differ across sites (\(\chi_{r, 10,4}^{2}\) = 5.2; p = 0.813), but was much higher in spring than in other seasons (3.24 ± 0.51 vs. 0.06 ± 0.04 to 0.99 ± 0.12 g C m−2 day−1; \(\chi_{r, 3,10}^{2}\) = 26.0; p < 0.001). Blade production was not correlated to REI (R = − 0.248, p = 0.489). Annual blade production (August 2016 to August 2017) amounted to 426.2 ± 39.4 g C m−2, with more than 90% of that taking place within 3–4 months in spring. The annual production of stipe biomass amounted to 73.1 ± 16.2 g C m−2 yielding a total average productivity of 499.4 ± 49.9 g C m−2 year−1 across the ten study sites.
Detritus production
Erosion losses per unit area averaged 0.29 ± 0.05 g C m−2 day−1 (Fig. 6a, b) and did not differ across sites (\(\chi_{r, 10,4}^{2}\) = 3.5; p = 0.939) but differed between seasons, ranging from 0.05 ± 0.05 g C m−2 day−1 in spring to 0.61 ± 0.22 g C m−2 day−1 in late summer (\(\chi_{r, 3,10}^{2}\) = 25.6; p < 0.001). Erosion losses were not correlated to REI (R = 0.006, p = 0.987). Annual biomass losses through erosion amounted to 108.0 ± 7.2 g C m−2.
Laminaria hyperborea detritus production: a seasonal erosion rate at the 10 study sites, b seasonal variation in erosion rate (averaged across sites), c seasonal dislodgement at the 10 study sites, d seasonal variation in dislodgement rate (averaged across sites), e seasonal spring cast of old blades at the 10 study sites, and f seasonal variation in losses through spring cast of old blades (averaged across sites). Sites are ranked according to increasing wave exposure level (REI) along the x-axis. Mean values ± 95% CI
The number of kelp plants or whole blades lost through dislodgment averaged 18.6 ± 10.8% year−1 (data not shown) corresponding to an average biomass loss of 0.33 ± 0.19 g C m−2 day−1 (Fig. 6c, d). Losses through dislodgement did not differ among sites (\(\chi_{r, 10,4}^{2}\) = 7.0; p = 0.638), were not correlated to REI (R = − 0.430, p = 0.214) and did not vary seasonally (\(\chi_{r, 3,10}^{2}\) = 1.4; p = 0.711). Annual losses through dislodgment reached 114.5 ± 51.9 g C m−2 of which 46% was made up by stipe material while the remaining 54% was blade material.
More than 99% of the plants collected during late winter (March 2017) had an old blade attached to the distal end of their new blade, but this number fell to 37% in late May 2017. Most of the plants carrying an old blade in May lost them during our processing, so we assume that these would have been lost within days in the field. None of the plants sampled in August 2016 and 2017 carried an old blade. The spring cast of old blades corresponded to an average biomass loss of 255.5 ± 43.2 g C m−2 year−1 (Fig. 6e; no difference across sites: \(\chi_{r, 10,4}^{2}\) = 5.4; p = 0.803) with the majority being lost between late March and early May (Fig. 6f).
The total production of detritus from L. hyperborea averaged 478.0 ± 40.5 g C m−2 year−1 across the ten study sites. The formation of blade detritus through dislodgment and blade erosion was the least important form of detritus production, accounting for 24% and 23% of the total detritus production, respectively, while the spring cast of old blades represented 53% of the total detritus production (Fig. 7).
Discussion
Our study confirmed that high latitude kelp forests in Norway are very productive and deliver large amounts of particulate detritus that, depending on its form and timing of delivery, may support secondary production and/or contribute to Blue Carbon through permanent burial in marine sediments in deeper adjacent areas. The annual production of detritus from Laminaria hyperborea (478 g C m−2) was higher than that reported from southern England (202 g C m−2), but comparable to that found in northern Scotland (432 g C m−2; Pessarrodona et al. 2018). The study by Pessarrodona et al. (2018) is the only other one that reports rates of detritus production for L. hyperborea. However, grazing on live L. hyperborea is usually low (typically < 10% of the biomass production; Norderhaug and Christie 2011) and the formation of detritus can, therefore, be inferred from the annual production of biomass. The observed production in this study (499 g C m−2 year−1) is within the range of that reported for L. hyperborea along the west coast of Norway, Isle of Man (UK), Helgoland (Germany) and Normandy (France) (range 376–825 g C m−2 year−1; Lüning 1969; Jupp and Drew 1974; Sheppard et al. 1978; Sjötun et al. 1995; Pedersen et al. 2012), but higher than that reported from Iceland and Finmark in northernmost Norway (ca. 250 g C m−2 year−1; Gunnarsson 1991, Sjötun et al. 1993). The production of detritus from L. hyperborea seems thus to range from ca. 225 to ca. 750 g C m−2 year−1 (assuming grazing losses ~ 10% of NPP) across its distributional range, which is similar to the production of detritus in other kelp species (Table 1 in Krumhansl and Scheibling 2012). The production of detrital C from L. hyperborea included only particulate detritus (POC), but part of the C fixed in kelp photosynthesis is released as dissolved organic C (DOC), which may support pelagic microorganisms (e.g., Newell et al. 1982) or contribute to C-sequestration if transported below the mixed zone of the ocean (Krause-Jensen and Duarte 2016). Large uncertainties remain regarding the total production and fate of DOC from kelps, but the DOC released from kelps appears to range from 14 to 34% of the total production (POC plus DOC) depending on species and location (e.g., Newell et al. 1980; Abdullah and Fredriksen 2004; Reed et al. 2015), which would represent an important component of detrital production.
The processes through which kelp detritus is produced have implications for its transfer to other habitats and its turn over through consumption and decomposition. More than 75% of the detritus formed by L. hyperborea was delivered as coarse material formed through dislodgement of whole plants or the spring cast of old blades, while the rest was delivered as smaller particles and small blade fragments through erosion. This compares to the proportions reported by Pessarrodona et al. (2018) for this species. The large proportion of coarse detritus is comparable to that found in Macrocystis pyrifera where dislodgement accounts for almost 80% of the annual detritus production (Gerard 1976), but contrasts the pattern found in Ecklonia radiata where most (78%) detritus is formed through erosion (de Bettignies et al. 2013b). These inter-specific variations may be due to difference in morphology since the thallus of M. pyrifera extends 10 s of meters and forms floating canopies that are susceptible to wave forces (Seymour et al. 1989; Graham et al. 1997), whereas E. radiata is much shorter with scouring canopies that may stimulate erosion rate. The morphology of L. hyperborea is intermediate between these extremes; it has a longer stipe than E. radiata and no floating canopy like M. pyrifera so scouring and drag forces may be less important.
Water motion is often considered a major driver for the formation of kelp detritus. Blade erosion may be stimulated by water motion, although weakening of the blade tissue by formation of sori, grazing and encrustation by bryozoans can also play a role (Krumhansl et al. 2011; de Bettignies et al. 2012; Mohring et al. 2012). Erosion is correlated to water motion in some species (e.g., Laminaria digitata; Krumhansl and Scheibling 2011), but not in others (e.g., Saccharina latissima; Krumhansl and Scheibling 2011, E. radiata; de Bettignies et al. 2013b). Erosion rate in L. hyperborea was not correlated to REI when compared across sites although maximum REI varied from 30- to 53-fold, but varied instead seasonally with fast erosion coinciding with high REI in autumn and winter. Winter season is also the time where the blades get older and more fragile, which increases the erosion rate. The lack of correlation between erosion rate and REI when compared across sites suggests thus that seasonal aging of the blade is a more important driver of elevated erosion than water motion per se. Storms may cause dislodgement of whole kelps or their blades (Ebeling et al. 1985; Seymour et al. 1989; Filbee-Dexter and Scheibling 2012) as may weakening of the stipe caused by sea urchin grazing (de Bettignies et al. 2012), but dislodgement rate was neither correlated to REI nor to sea urchin density when compared across sites or seasons. Dislodgement rates in L. hyperborea were much lower than those in E. radiata (18% year−1 vs 44–55% year−1; de Bettignies et al. 2013b) and did not undergo any clear seasonal variation although storm events were more frequent and intense in autumn and winter (Fig. 2c). de Bettignies et al. (2015) found the same in a study on E. radiata and explained the low effect of water motion by small thallus size and, thus, reduced drag, in winter when wave exposure was highest (de Bettignies et al. 2013a). The blade of L. hyperborea is also slightly smaller in winter than in other seasons (Fig. 4b), but blade size was positively correlated to REI when compared across sites, so reduced drag in winter can hardly explain the low importance of water motion in the present study.
Most kelp detritus was delivered as coarse fragments, but these may be transformed to smaller size before reaching recipient communities outside the kelp forest. Once dislodged or cast, coarse detritus can break up mechanically due to scouring or grazers can shred it into smaller pieces or consume it and deliver the remains as fecal pellets. Such transformation is important for the fate of the detritus because size may affect its susceptibility to consumers, its dispersal capacity and its decomposition. Sea urchins feed intensively on coarse kelp detritus. The density of sea urchins (mainly the green sea urchin Strongylocentrotus droebachiensis) in the study area varied from 1 to 10 m−2 across sites and their consumption of kelp detritus inside and in the vicinity of our kelp forest sites corresponded to 60–65% of the total detritus production (Filbee-Dexter et al. 2019). Green sea urchins fed with fresh kelp detritus defecate 50–70% of the consumed detritus as small undigested, but fragmented material with approximately the same chemical composition as ‘intact’ kelp detritus (Mamelona and Pelletier 2005), which may support suspension and deposit feeders within and outside the kelp forest (Duggins et al. 1989; Fredriksen 2003; Leclerc et al. 2013; McMeans et al. 2013; Gaillard et al. 2017). However, the importance of kelp detritus as a food source has recently been questioned by a review showing that trophic studies based on stable C-isotope data alone may overestimate the trophic importance of kelp particles relative to that of phytoplankton (Miller and Page 2012).
Detritus that is not mineralized by consumers within and near the kelp forests will be prone to dispersal, decomposition or burial. Small kelp particles sink more slowly than larger fragments, whole blades or stipes, which allow for a wider dispersal (Wernberg and Filbee-Dexter 2018). Filbee-Dexter et al. (2019) used sinking rates for different sized kelp detritus and hydrodynamic modeling to simulate particle transport in the study area and found that the median dispersal range of whole kelp blades was 8.5 km (maximum range = 150 km) whereas it was 26 km (maximum > 300 km) for small kelp particles. Beach cast of kelp is often observed after storms (Griffiths et al. 1983; Seymour et al. 1989), but the coastline in the study area is steep and we did not observe substantial accumulations of kelp detritus on the shore. We hypothesize, therefore, that excess detritus is exported to the deeper parts in the area, which is supported by trawl collections and video observations in the study area (Filbee-Dexter et al. 2018). More than 50% of the kelp detritus was formed during the spring cast between April and May, coinciding with observations of large amounts of coarse kelp detritus within and around the kelp forests (Filbee-Dexter et al. 2018). Large amounts of coarse kelp detritus were subsequently (late May) observed below the kelp forests at depths from 20 to 80 m and in the deepest portions of the study area (~ 400 m) confirming that the detritus was exported several kilometers away from the source populations within days to weeks of its formation. The amount of visible kelp detritus was much lower and the fragments smaller in August, indicating that continuous fragmentation and transport to deeper sites in the study area occurred during summer.
Kelp detritus that is not consumed will ultimately decompose or become buried in deeper areas. Laboratory studies show that coarse detritus from L. hyperborea loses more than 40% of its initial C-biomass within 3–4 weeks and decomposes completely in less than 1 year under aerobic conditions, while decomposition under anoxic conditions (such as in deeper areas) stops after 5–6 months leaving 20–25% of the initial biomass to decompose at extremely low rates or not at all (Frisk 2017). Decomposition rate depends also on particle size. Fecal pellets from sea urchins fed with kelp detritus lose almost 80% of their initial C-mass in 2 weeks (Sauchyn and Scheibling 2009), which is much faster than that for larger kelp fragments. Decomposition of kelp detritus can thus be fast depending on the environmental conditions and the degree of fragmentation, while burial of significant quantities of kelp C requires rapid export to areas where the conditions disfavor mineralization through consumption or decomposition.
The potential export of detrital kelp C to the non-vegetated portions of the study area can be estimated from the production of kelp detritus per unit area and kelp coverage in the area. Kelp covered ca. 22 km2 of the 126 km2 covered by ocean in the study area (Fig. 1). The total production of kelp detritus in the study area amounts to 10517 T C year−1 or 101 g C m−2 year−1 if dispersed evenly over the 104 km2 of non-vegetated area and assuming no consumption and decomposition. The potential input of detrital kelp C is comparable to the vertical flux of POC (marine snow) from the pelagic zone, which ranges from 93 to 150 g C m−2 year−1 in the outer part of Malangen Fjord (Keck and Wassmann 1996). Kelp detritus may thus contribute significantly to the total input of C to the deeper portions of the study area, although the input is less than estimated above when consumption by sea urchins and rapid initial decomposition are taken into account.
The importance of Blue C has lately received increased attention (Mcleod et al. 2011; Duarte 2017; Raven 2017) and coastal habitats such as mangroves, marshlands and seagrasses are now recognized as significant C-sinks (Chmura et al. 2003; Donato et al. 2011; Fourqurean et al. 2012), while the role of kelps and other macroalgae is still being debated (Howard et al. 2017; Krause-Jensen et al. 2018; Smale et al. 2018). Quantifying the formation of kelp detritus is a first, but important step when evaluating the potential role of kelps as donors to C-sequestration. The production of detrital C by L. hyperborea reported here (~ 500 g C m−2 year−1) is well within the range of that in other kelps, seagrasses, and mangroves, but significantly lower than in marshes and significantly higher than in marine phytoplankton, non-kelp seaweeds and terrestrial habitats such as forests and grasslands (Fig. 8, one-way ANOVA: F7,488 = 13.8, p < 0.001; Suppl. information Table 1). The substantial production of detrital C from kelp forests suggests that kelp systems could play an important role as Blue C-donors to marine sediments. However, most of the studies on detritus production in grasslands, forests, mangroves and marshes report only above-ground litterfall and do not include below-ground production of detritus, which means that the numbers from these systems may be underestimated.
Annual per area production of detrital C in different habitats. The flow of C via detritus includes various kinds of litterfall and detritus production. One-way ANOVA revealed significant differences between habitats (F7,488 = 13.8, p < 0.001) and Dunnett’s test was subsequently used to compare the detrital production of each habitat to that of kelps. Asterisk indicates significant differences when compared to kelps (*p < 0.05, **p < 0.01, ns non-significant). Details and references are provided in Supporting Information Table S1. Values are means ± 95% CI
Blue C is defined as the sequestration of C from marine organisms that takes place when burial rates in sediments exceed long-term rates of erosion and decomposition. The importance for Blue C depends, therefore, not only on the amount of detrital C being produced, but also on re-mineralization of C through consumption by detritivores and/or through decomposition, which will determine how much of the C can be buried. Kelps usually grow on hard substrates and do not have below-ground tissues like seagrasses, mangrove trees and marsh plants. Thus, export of kelp detritus to marine sediments where the conditions disfavor consumption and/or decomposition plays an important role in the final fate of kelp C. Future studies should focus on the different fates of kelp detritus and explore how much is consumed, how much is exported to potential Blue C sediments and how fast and under which environmental conditions detrital C is re-mineralized through decomposition.
References
Abdullah MI, Fredriksen S (2004) Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. J Mar Biol Assoc UK 84:887–894
Bekkby T, Rinde E, Gundersen H, Norderhaug KM, Gitmark JK, Christie H (2014) Length, strength and water flow: relative importance of wave and current exposure on morphology in kelp Laminaria hyperborea. Mar Ecol Prog Ser 506:61–70
Bekkby T, Moy FE, Olsen H, Rinde E, Bodvin T, Bøe R, Steen H, Grefsrud ES, Espeland SH, Pedersen A, Jørgensen NM (2013) The Norwegian programme for mapping of marine habitats—providing knowledge and maps for ICZMP. In: Moksness E, Dahl E, Støttrup J (eds) Global challenges in integrated coastal zone management, vol II. John Wiley & Sons Ltd., Oxford
Carr MH, Neigel JE, Estes JA, Andelman S, Warner RR, Largier JL (2003) Comparing marine and terrestrial ecosystems: implications for the design of coastal marine reserves. Ecol Appl 13:90–107
Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154:449–468. https://doi.org/10.1086/303244
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Global Biogeochem Cy 17:1–11. https://doi.org/10.1029/2002GB001917
Colombini I, Chelazzi L (2003) Influence of marine allochthonous input on sandy beach communities. Oceanogr Mar Biol 41:115–159. https://doi.org/10.1201/9780203180570.ch3
Davies CE, Moss D (2003) EUNIS habitat classification. European Topic Centre on Nature Protection and Bio-diversity, Paris. http://eunis.eea.europa.eu/habitats.jsp. Accessed 1st Oct 2019
de Bettignies T, Thomsen MS, Wernberg T (2012) Wounded kelps: Patterns and susceptibility to breakage. Aquat Biol 17:223–233. https://doi.org/10.3354/ab00471
de Bettignies T, Wernberg T, Lavery PS (2013a) Size, not morphology, determines hydrodynamic performance of a kelp during peak flow. Mar Biol 160:843–851. https://doi.org/10.1007/s00227-012-2138-8
de Bettignies T, Wernberg T, Lavery PS, Vanderklift MA, Mohring MB (2013b) Contrasting mechanisms of dislodgement and erosion contribute to production of kelp detritus. Limnol Oceanogr 58:1680–1688. https://doi.org/10.4319/lo.2013.58.5.1680
de Bettignies T, Wernberg T, Lavery PS, Vanderklift MA, Gunson JR, Symonds G, Collier N (2015) Phenological decoupling of mortality from wave forcing in kelp beds. Ecology 96:850–861
Donato DC, Kaufmann JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4:293–297
Duarte CM (2017) Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 14:301–310. https://doi.org/10.5194/bg-14-301-2017
Duggins D, Simenstad C, Estes J (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:170–173
Ebeling AW, Laur DR, Rowley RJ (1985) Severe storm disturbances and reversal of community structure in a southern California kelp forest. Mar Biol 84:287–294
Filbee-Dexter K, Scheibling RE (2012) Hurricane-mediated defoliation of kelp beds and pulsed delivery of kelp detritus to offshore sedimentary habitats. Mar Ecol Prog Ser 455:51–64. https://doi.org/10.3354/meps09667
Filbee-Dexter K, Scheibling RE (2016) Spatial patterns and predictors of drift algal subsidy in deep subtidal environments. Estuaries Coasts 39:1724–1734. https://doi.org/10.1007/s12237-016-0101-5
Filbee-Dexter K, Wernberg T, Norderhaug KM, Ramirez-Llodra E, Pedersen MF (2018) Movement of pulsed resource subsidies from kelp forests to deep fjords. Oecologia 187:291–304. https://doi.org/10.1007/s00442-018-4121-7
Filbee-Dexter K, Pedersen MF, Fredriksen S, Norderhaug KM, Rinde E, Kristiansen T, Albretsen J, Wernberg T (2019) Carbon export is facilitated by marine shredders transforming kelp detritus. Oecologia. https://doi.org/10.1007/s00442-019-04571
Fonseca MS, Bell SS (1998) Influence of physical setting on seagrass landscapes near Beaufort, North Carolina, USA. Mar Ecol Prog Ser 171:109–121
Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen McGlathery KJ, Serrano O (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509. https://doi.org/10.1038/NGEO1477
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (13C and 15N) analysis. Mar Ecol Prog Ser 260:71–81
Frisk NL (2017) The effect of temperature and oxygen availability on decay rate and changes in food quality of Laminaria hyperborea detritus. Master thesis, Department of Science and Environment, Roskilde University, Denmark
Gaillard B, Meziane T, Tremblay R, Archambault P, Blicher ME, Chauvaud L, Rysgaard S, Olivier F (2017) Food resources of the bivalve Astarte elliptica in a sub-Arctic fjord: a multi-biomarker approach. Mar Ecol Prog Ser 567:139–156. https://doi.org/10.3354/meps12036
Gerard VA (1976) Some aspects of material dynamics and energy flow in a kelp forest in Monterey Bay, California. PhD dissertation, University of California, Santa Cruz, USA
Graham MH, Harrold C, Lisin S, Light K, Watanabe JM, Foster MS (1997) Population dynamics of giant kelp Macrocystis pyrifera along a wave exposure gradient. Mar Ecol Prog Ser 148:269–279
Griffiths CL, Stenton-Dozey J, Koop K (1983) Kelp wrack and the flow of energy through a sandy beach ecosystem. In: McLachlan A, Erasmus T (eds) Sandy beaches as ecosystems. W. Junk Publishers, The Hague, pp 547–556
Gunnarsson K (1991) Populations de Laminaria hyperborea et Laminaria digitata (Phéophycées) dans la baie de Breidifjördur, Islande. Rit Fiskideildar. J Mar Res Inst Reykjavik 12:1–148
Howard J, Sutton-Grier A, Herr D, Kleypas J, Landis E, Mcleod E, Pigeon E, Simpson S (2017) Clarifying the role of coastal and marine systems in climate mitigation. Front Ecol Environ 15:42–50. https://doi.org/10.1002/fee.1451
Ince R, Hyndes GA, Lavery PS, Vanderklift MA (2007) Marine macrophytes directly enhance abundances of sandy beach fauna through provision of food and habitat. Estuar Coast Shelf Sci 74:77–86
Jupp BP, Drew EA (1974) Studies on the growth of Laminaria hyperborea (Gunn) Fosl. I. Biomass and productivity. J Exp Mar Biol Ecol 15:185–196
Keck A, Wassmann P (1996) Temporal and spatial patterns of sedimentation in the subarctic fjord Malangen, northern Norway. Sarsia 80:259–276
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–742. https://doi.org/10.1038/ngeo2790
Krause-Jensen D, Lavery P, Serrano O, Marbà N, Masque P, Duarte CM (2018) Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biol Lett 14:20180236. https://doi.org/10.1098/rsbl.2018.0236
Krumhansl KA, Scheibling RE (2011) Detrital production in Nova Scotian kelp beds: patterns and processes. Mar Ecol Prog Ser 421:67–82. https://doi.org/10.3354/meps08905
Krumhansl KA, Scheibling RE (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302. https://doi.org/10.3354/meps09940
Krumhansl KA, Lee MJ, Scheibling RE (2011) Grazing damage and encrustation by an invasive bryozoan reduce the ability of kelps to withstand breakage by waves. J Exp Mar Biol Ecol 407:12–18
Leclerc J-C, Riera P, Leroux C, Lévêque L, Davoult D (2013) Temporal variation in organic matter supply in kelp forests: linking structure to trophic function. Mar Ecol Prog Ser 494:87–105
Lüning K (1969) Standing crop and leaf area index of the sublittoral Laminaria species near Helgoland. Mar Biol 3:282–286
Mamelona J, Pelletier É (2005) Green urchin as a significant source of fecal particulate organic matter within nearshore benthic ecosystems. J Exp Mar Biol Ecol 314:163–174. https://doi.org/10.1016/J.JEMBE.2004.08.026
Mann KH (1973) Seaweeds: their productivity and strategy for growth. Science 182:975–981. https://doi.org/10.1126/science.182.4116.975
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560. https://doi.org/10.1890/110004
McMeans BC, Rooney N, Arts MT, Fisk AT (2013) Food web structure of a coastal Arctic marine ecosystem and implications for stability. Mar Ecol Prog Ser 482:17–28. https://doi.org/10.3354/meps10278
Miller RJ, Page HM (2012) Kelp as a trophic resource for marine suspension feeders: a review based on isotope-based evidence. Mar Biol 159:1391–1402
Mohring MB, Wernberg T, Kendrick GA, Rule MJ (2012) Reproductive synchrony in a habitat-forming kelp and its relationship with environmental conditions. Mar Biol 160:119–126. https://doi.org/10.1007/s00227-012-2068-5
Newell RC, Field JG, Griffiths CL (1982) Energy balance and significance of microorganisms in a kelp bed community. Mar Ecol Prog Ser 8:103–113
Newell RC, Lucas MI, Velirnirov B, Seiderer LJ (1980) Quantitative significance of dissolved organic losses following fragmentation of Kelp (Ecklonia maxima and Laminaria pallida). Mar Ecol Prog Ser 2:45–59
Norderhaug KM, Christie H (2011) Secondary production in a Laminaria hyperborea kelp forest and variation according to wave exposure. Est Coast Shelf Sci 95:135–144
Parke M (1948) Studies of British Laminariaceae. I. Growth in Laminaria saccharina (L.) Lamour. J Mar Biol Ass UK 27:651–709
Pedersen MF, Nejrup LB, Fredriksen S, Christie H, Norderhaug KM (2012) Effects of wave exposure on population structure, demography, biomass and productivity of the kelp Laminaria hyperborea. Mar Ecol Prog Ser 451:45–60
Pessarrodona A, Moore PJ, Sayer MDJ, Smale DA (2018) Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob Change Biol 24:4386–4398. https://doi.org/10.1111/gcb.14303
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Ann Rev Ecol Syst 28:289–316. https://doi.org/10.1146/annurev.ecolsys.28.1.289
Poore AG, Campbell AH, Coleman RA, Edgar GJ, Jormalainen V, Reynolds PL, Sotka EE, Stachowicz JJ, Taylor RB, Vanderklift MA, Duffy JE (2012) Global patterns in the impact of marine herbivores on benthic primary producers. Ecol Lett 15:912–922. https://doi.org/10.1111/j.1461-0248.2012.01804.x
Raven JA (2017) The possible role of algae in restricting the increase in atmospheric CO2 and global temperature. Eur J Phycol 52:506–522. https://doi.org/10.1080/09670262.2017.1362593
Reed DC, Carlson CA, Halewood ER, Nelson JC, Harrer SL, Rassweiler A, Miller RJ (2015) Patterns and controls of reef-scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera. Limnol Oceanogr 60:1996–2008
Sauchyn L, Scheibling R (2009) Degradation of sea urchin feces in a rocky subtidal ecosystem: implications for nutrient cycling and energy flow. Aquat Biol 6:99–108. https://doi.org/10.3354/ab00171
Seymour RJ, Tegner MJ, Dayton PK, Parnell PN (1989) Storm wave induced mortality of giant kelp, Macrocystis pyrifera, in Southern California. Estuar Coast Shelf Sci 28:277–292. https://doi.org/10.1016/0272-7714(89)90018-8
Sheppard CRC, Jupp BP, Sheppard A, Bellamy DJ (1978) Studies on the Growth of Laminaria hyperborea (Gunn.) Fosl. and Laminaria ochroleuca De La Pylaie on the French Channel Coast. Bot Mar 21:109–116. https://doi.org/10.1515/botm.1978.21.2.109
Sjötun K, Fredriksen S, Lein TE, Rueness J, Sivertsen K (1993) Population studies of Laminaria hyperborea from its northern range of distribution in Norway. Hydrobiologia 260(261):215–221
Sjötun K, Fredriksen S, Rueness J, Lein TE (1995) Ecological studies of the kelp Laminaria hyperborea (Gunnerus) Foslie in Norway. In: Skjoldal HR, Hopkins C, Erikstad KE, Leinaas HP (eds) Ecology of fjords and coastal waters. Elsevier Science BV, Amsterdam, pp 525–536
Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3:4016–4038
Smale DA, Moore PJ, Queirós AM, Higgs ND, Burrows MT (2018) Appreciating interconnectivity between habitats is key to blue carbon management. Front Ecol Environ 16:71–73. https://doi.org/10.1002/fee.1765
Steneck RS, Johnson CR (2013) Kelp Forests: Dynamic patterns, processes, and feedbacks. In: Bertness M, Silliman B, Stachowitz J (eds) Marine community ecology. Sinauer, Sunderland, pp 315–336
Tala F, Edding M (2005) Growth and loss of distal tissue in blades of Lessonia nigrescens and Lessonia trabeculata (Laminariales). Aquat Bot 82:39–54
Vanderklift MA, Wernberg T (2008) Detached kelps from distant sources are a food subsidy for sea urchins. Oecologia 157:327–335. https://doi.org/10.1007/s00442-008-1061-7
Vetter E, Dayton P (1999) Organic enrichment by macrophyte detritus, and abundance patterns of megafaunal populations in submarine canyons. Mar Ecol Prog Ser 186:137–148
Wernberg T, Filbee-Dexter K (2018) Grazers extend blue carbon transfer by slowing sinking speeds of kelp detritus. Sci Rep 8:17180. https://doi.org/10.1038/s41598-018-34721-z
Wernberg T, Vanderklift MA, How J, Lavery PS (2006) Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia 147:692–701. https://doi.org/10.1007/s00442-005-0318-7
Wernberg T, Smale DA, Tuya F, Thomsen MS, Langlois TJ, de Bettignies T, Bennett S, Rousseaux CS (2013) An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat Clim Change 3:78–82. https://doi.org/10.1038/nclimate1627
Wernberg T, Krumhansl K, Filbee-Dexter K, Pedersen MF (2019) Status and trends for the worlds kelp forests. In: Sheppard C (ed) World Seas: an environmental evaluation, vol III. Ecological issues and environmental impacts. Elsevier, Amsterdam, pp 57–78
Zar J (1999) Biostatistical analysis. Prentice-Hall Inc, Upper Saddle River, p 929
Acknowledgements
This study was funded by the Norwegian Research Council through the KELPEX project (NRC Grant No. 255085) and TW’s participation was further supported by the Australian Research Council (DP160100114). We would like to thank Sabine Popp, Eva Ramirez-Llodra, Amanda Poste, and Hjalte Hjarlgaard Hansen for valuable help with some of the field works at Sommarøy and two anonymous reviewers for valuable comments to an earlier version of this manuscript.
Author information
Authors and Affiliations
Contributions
MFP, KN, SF, and TW conceived the study with help from KFD. All authors developed the experimental design and conducted the fieldwork. MFP analyzed the data and led the writing of the manuscript, with assistance from KFD, TW, KN, NLF, and SF. CWF contributed to data presentation. All authors discussed the results.
Corresponding author
Additional information
Communicated by James Fourqurean.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pedersen, M.F., Filbee-Dexter, K., Norderhaug, K.M. et al. Detrital carbon production and export in high latitude kelp forests. Oecologia 192, 227–239 (2020). https://doi.org/10.1007/s00442-019-04573-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04573-z