Abstract
The global phenomenon of mangrove encroachment into saltmarshes has been observed across five continents. It has been proposed that this encroachment is driven in part by rising atmospheric CO2 concentration and reduced salinity in saltmarshes resulting from rising sea levels enhancing the establishment success of mangrove seedlings. However, this theory is yet to be empirically tested at the community-level. In this study, we examined the effect of CO2 and salinity on seedling growth of two mangrove species, Aegiceras corniculatum and Avicennia marina, grown individually and in a model saltmarsh community in a glasshouse experiment. We found that the shoot (210%) and root (91%) biomass of the saltmarsh species was significantly greater under elevated CO2. As a result, both mangrove species experienced a stronger competitive effect from the saltmarsh species under elevated CO2. Nevertheless, A. marina seedlings produced on average 48% more biomass under elevated CO2 when grown in competition with the saltmarsh species. The seedlings tended to allocate this additional biomass to growing taller suggesting they were light limited. In contrast, A. corniculatum growth did not significantly differ between CO2 treatments. However, it had on average 36% greater growth under seawater salinity compared to hypersaline conditions. Avicennia marina seedlings were not affected by salinity. From these results, we suggest that although CO2 and salinity are not universal drivers determining saltmarsh–mangrove boundaries, it is likely that rising atmospheric CO2 concentration and reduced salinity associated with sea level rise will enhance the establishment success of mangrove seedlings in saltmarshes, which may facilitate mangrove encroachment in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saltmarsh and mangrove communities provide critically important habitat and feeding grounds for a range of invertebrates, shore-birds and fish as well as other important ecosystem services including coastal stabilisation, filtration and trapping of pollutants (Spencer et al. 2009; Kathiresan 2012). Economic modelling of the value of goods and services provided by saltmarsh and mangrove communities to coastal fisheries production, coastal protection, carbon sequestration and biodiversity conservation is estimated to be on average $USD 194,000 ha−1 year−1 (Costanza et al. 2014). Given that the global area of saltmarsh communities is estimated to be greater than two million hectares and mangrove communities is greater than 18 million hectares (Saintilan and Rogers 2015), we can calculate their total value at ~ $USD 4 trillion annually. Therefore, significant changes to the structure and distribution of saltmarsh and mangrove communities will not only impact the natural processes that they mediate, but also have a profound effect on human activities that utilise these communities (Kelleway et al. 2017).
Within the subtropics, an ecotone of 2°–3° of latitude occurs where mangrove and saltmarsh communities co-exist and compete for dominance, with saltmarshes usually found landwards of mangroves (Osland et al. 2013). Recently, the expansion of mangroves into saltmarsh communities has been observed worldwide (Saintilan et al. 2014), with examples from North America (Krauss et al. 2011; Comeaux et al. 2012), Central America (López-Medellín et al. 2011) and Australia (Saintilan and Williams 1999; Williamson et al. 2011). It has been suggested that this expansion of mangroves into saltmarsh communities is a result of changes in environmental factors associated with climate change such as increasing temperatures, rising atmospheric CO2 concentration and reduced salinity levels resulting from sea-level rise (Saintilan and Rogers 2013; Cavanaugh et al. 2014; Reef and Lovelock 2014; Alongi 2015). This suggestion is unsurprising considering the highest velocity of climate-driven change is occurring in the coastal zone (Loarie et al. 2009), where mangrove and saltmarsh ecosystems serve as sentinels of temperature, CO2 and sea-level impacts (McKee et al. 2012).
In terrestrial systems, it has been proposed that rising atmospheric CO2 concentration over the last 200 years may be a potential driver of woody plant encroachment in grasslands (Bond and Midgley 2000; Archer et al. 2017). The mechanism behind this theory is that the growth of woody plants that utilise the C3 photosynthetic pathway is promoted under elevated CO2, while the co-occurring C4 grass species obtain little or no benefit from additional CO2 (Ainsworth and Long 2005; Leakey et al. 2009). Despite the obvious parallels between terrestrial woody plant and mangrove encroachment, there has been limited research on the role of CO2 in determining saltmarsh–mangrove boundaries (Saintilan and Rogers 2015). The studies that have tested the response of mangrove species to elevated CO2 have shown that responses tend to be species-specific (Alongi 2015). For example, a study testing the growth responses of four Caribbean mangrove species reported that three of the species were not affected by elevated CO2 while the remaining species (Laguncularia racemosa) had a decline in biomass (Snedaker and Araujo 1998). Although CO2 is likely to be a key driver of woody plant encroachment in terrestrial grasslands, it is not yet clear if a similar mechanism will drive mangrove encroachment in saltmarshes. This lack of clarity is due to several additional factors in saltmarsh–mangrove systems: the unpredictable responses of mangrove species to elevated CO2, the relative abundance of C3 and C4 species in saltmarsh communities (Drake et al. 1989; Rozema et al. 1991; Arp et al. 1993), and the effect of salinity on mangrove recruitment (Alongi 2015).
Most mangrove species tend not to cope with the high salinity levels that can exist in saltmarsh communities, but rising sea levels may reduce salinity in these communities making conditions more favourable for mangroves (Rogers et al. 2006; Adam 2009). Furthermore, increased water-use efficiency under elevated CO2 may also increase the tolerance of mangroves to high salinity levels as has been shown to be the case for A. germinans (Reef et al. 2015). However, a previous study showed that the mangrove species Rhizophora apiculata and Rhizophora stylosa were only responsive to elevated CO2 under 25% seawater salinity but not 75% seawater salinity (Ball et al. 1997). Therefore, it is likely that mangrove responses to the interactive effects of CO2 and salinity will be complex, with some species thriving while others decline or show no change (Alongi 2015).
Currently, there is a critical gap in our knowledge of how climate change-associated environmental factors will interact to influence saltmarsh–mangrove boundaries. The first step in addressing this knowledge gap is to determine the effect these environmental factors have on the establishment success of mangrove seedlings, which is the crucial first step in mangrove encroachment. Therefore, the aim of this study was to determine the effect CO2 and salinity has on the establishment success of mangrove seedlings in saltmarsh communities. To test this aim, we established model saltmarsh communities under different CO2 (ambient = 400 ppm, elevated = 600 ppm) and salinity (seawater = 50 ds m−1, hypersaline = 100 ds m−1) treatments, then planted propagules of two mangrove species (Aegiceras corniculatum (L.) Blanco and Avicennia marina (Forssk.) Vierh.) into the established communities. The mangrove species were also grown individually. The saltmarsh and mangrove species used in the study are commonly co-occurring species along the entire coast of New South Wales (NSW), Australia, and all are C3 species. We predict that (1) the mangrove species will increase their growth under elevated CO2 and seawater salinity when grown individually; (2) the C3 saltmarsh species will also respond positively to elevated CO2 thus increasing their competitive effect on the mangrove species and preventing them from establishing, particularly in hypersaline conditions.
Materials and methods
Species selection
We selected three C3 species that commonly co-occur in saltmarsh communities along the entire coast of NSW, Australia (Daly 2013). The selected species were Ficinia nodosa (Rottb.) Goetgh., Muasya & D.A.Simpson (family: Cyperaceae), Juncus kraussii Hochst. (family: Juncaceae) and Selliera radicans Cav. (family: Goodeniaceae). It should be noted that Sarcocornia quinqueflora (Bunge ex Ung.Sternb.) A.J.Scott was initially included as a fourth saltmarsh species, but did not respond well to transplanting due to poor quality tube stock so was removed from the experiment after 6 weeks. Seeds for F. nodosa and J. kraussii and rhizomes of S. radicans were obtained from commercial suppliers (seeds from Nindethana Seed Service, Albany, WA, Australia; rhizomes from Bunya Native Nursery, Dural, NSW, Australia). The F. nodosa and J. kraussii seeds were germinated on freshwater-moistened paper towels within aluminium trays that were sealed with plastic wrap. For the mangrove species, A. corniculatum propagules were collected from Mickeys Point, NSW, Australia (33°97′79.4″ S, 151°02′30.4″ E) while A. marina propagules were collected from Empire Bay, NSW, Australia (33°29′32.0″ S, 151°21′22.0″ E).
Experimental design and treatments
Four individuals from each saltmarsh species were grown together in mesocosms (12 plants/mesocosm) using a fully factorial experimental design with two factors: CO2 (ambient and elevated) and salinity (seawater and hypersaline). The mesocosms consisted of 70 L tubs (60 cm long × 40 cm wide × 30 cm deep), with each tub containing 65 L of commercially obtained 80:20 sand soil mix (Australian Native Landscapes, North Ryde, NSW, Australia). This mix was selected for the experiment because the sand was obtained from sieved estuary sediment which is appropriate to our study system. The nutrient properties of the mix were 150 mg kg−1 total N and 100 mg kg−1 total P. Ficinia nodosa and J. kraussii seedlings were transplanted from the germination trays into the mesocosms at the stage of cotyledon emergence. For S. radicans, 5 cm cuttings of belowground rhizomes were transplanted into the mesocosms. All seedlings and rhizomes were planted within 10 h of each other in two rows of six with positions being randomly allocated. The seedlings and rhizomes were then allowed to establish in the mesocosms within a single glasshouse for 4 weeks after which the CO2 and salinity treatments were imposed beginning January 2018.
The CO2 treatments were set to ambient (400 ± 20 ppm) and elevated (600 ± 20 ppm). These CO2 concentration ranges were maintained and monitored continuously by a CO2 dosing and monitoring system (Canary Company Pty Ltd, Lane Cove, NSW, Australia). The elevated CO2 treatment represents the predicted atmospheric CO2 concentration by 2060 under the RCP 4.5 emissions scenario (IPCC 2013). Each CO2 treatment (consisting of 12 mesocosms) was represented by one 55 m2 glasshouse with the glasshouses located next to each other (sharing a common wall). The temperature of the glasshouses was set for a minimum of 18 °C and a maximum of 25 °C and was continuously maintained by a fan coil unit using a water cooling and heating system. The fan coil unit was also responsible for circulating the air and evenly distributing the CO2 within each glasshouse. Relative humidity (RH) and photosynthetically active radiation (PAR) of the glasshouses was continuously monitored using a Multi-grow Controller System (Autogrow Systems, Auckland, New Zealand). The readings taken at 14:00 (time of maximum RH and PAR in the glasshouses) each day were then analysed using paired t tests to show that RH (GH1 = 87%, GH2 = 86%; p = 0.283) and PAR (GH1 = 818 mol m−2s−1, GH2 = 779 mol m−2s−1; p = 0.260) did not significantly differ between the glasshouses.
Salinity treatments were set to seawater (50 ± 5 ds m−1) and hypersaline (100 ± 5 ds m−1). We selected these treatments to represent the natural salinity range (20–95 ds m−1) reported for saltmarsh communities within the greater Sydney region, NSW, Australia (Sydney Environmental and Soil Laboratory 2018). These treatments were monitored fortnightly for each mesocosm by taking 100 g of soil from the mesocosm, mixing it well with distilled water (10:1 water soil), and leaving the solution overnight. The salinity of the solution was then measured using a HI98196 electrical conductivity meter (Hanna Instruments, Castle Hill, NSW, Australia). If salinity had dropped below the treatment thresholds (seawater = 40 ds m−1, hypersaline = 80 ds m−1), then the necessary amount of NaCl was dissolved in 1 L of water which was then added to the mesocosms (1 ds m−1 = 550 mg L−1 of NaCI). At each salinity treatment application, the amount of NaCl added to each mesocosm within a treatment was kept consistent. Replenishment of NaCl occurred five times during the entire experiment, three times pre-mangrove planting and twice post-mangrove planting.
Each treatment combination (CO2 × salinity) was replicated six times with treatments randomly assigned to mesocosms. This design resulted in a total of 24 mesocosms (two CO2 treatments × two salinity treatments × six replicates). The mesocosms were mist watered for 3 min per hour between 06:00 and 10:00 and 18:00 and 22:00. Watering was concentrated at these times to simulate tidal movements so by 10:00/22:00 the mesocosms were inundated with water, which would typically take 3 h to drain. Mist watering was used as the watering method to ensure inundation occurred gradually so NaCl was not flushed out of the mesocosms at a rapid rate.
The model saltmarsh communities were grown for 12 weeks after which three propagules from each of A. corniculatum and A. marina were planted into each mesocosm. Mangrove propagules were selected based on their fresh weight with A. corniculatum propagules having a weight of 4.3 ± 0.3 g and A. marina propagules having a weight of 0.8 ± 0.2 g. The mangrove propagules were planted in one row, between the two rows of saltmarsh plants, equidistant from each other in a random order. The propagules were then allowed to grow within the mesocosms for a period of 20 weeks. It should be noted that the cotyledons dropped off the mangrove seedlings ~ 4 weeks after planting. To minimise any glasshouse effect, the mesocosms along with the CO2 treatment were switched between the two glasshouses after 6 weeks (mid-way through the growth period before the mangrove propagules were planted) and 22 weeks (mid-way through the growth period after the mangrove propagules were planted). Throughout the duration of the experiment volumetric soil water content (VSWC) of the mesocosms was measured on a weekly basis at a depth of 15 cm using a Hydrosense II Portable Soil Moisture System (Campbell Scientific Australia Pty Ltd, Garbutt, QLD, Australia). As the soil was always at saturation point (~ 40%) due to being inundated daily we have not reported the VSWC data in the “Results” section below. In addition to the mangrove propagules planted in the model saltmarsh communities, three propagules of each species for each CO2 × salinity treatment combination were planted individually in 12 L pots containing 11.5 L of the same sand soil mix used for the mesocosms.
At the conclusion of the experiment (32 weeks) every plant (both mesocosm and individually grown plants) was individually harvested and washed free of soil except for the root biomass of the saltmarsh species, which was too intertwined to separate into individual plants. For the mangrove seedlings grown in the mesocosms, shoot height and root length were measured for each individual plant. The biomass components were then oven-dried at 70 °C for 72 h and weighed using an analytical electronic balance (Mettler Toledo, Port Melbourne, VIC, Australia).
Data analysis
To test the effect of the CO2 and salinity treatments on the shoot biomass of the model saltmarsh communities we used a three-way mixed model ANOVA. CO2 and salinity were treated as fixed factors while species was treated as a random factor. The data used for this analysis was the total shoot biomass of each species at the mesocosm level. It required log10 transformation to fulfil the normality assumption of ANOVA. The same analysis was conducted for root biomass with the exception of species being removed from the model, as we were unable to separate roots on an individual plant basis. This root data did not required transformation.
We then tested the effects of the CO2 and salinity treatments on the total biomass, shoot height and root length of each mangrove species using two-way ANOVAs. CO2 and salinity were treated as fixed factors. This analysis was conducted for mangrove seedlings grown in competition at the mesocosm-level and repeated for the mangrove seedlings grown individually. Total biomass and shoot height required log10 transformations to fulfil the normality assumption of ANOVA.
All statistical analyses were performed using R version 3.2.4. (R Development Core Team, R: A Language and Environment for Statistical Computing, Boston, MA, United States, http://www.rstudio.com), with the significance level set at 0.05.
Results
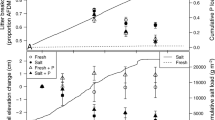
There was no significant interactive effect between any of the factors on the biomass (total, shoot and root) of the saltmarsh communities. The shoot (F1,60 = 45.40, p = 0.021) and root (F1,20 = 21.34, p < 0.001) biomass of the model saltmarsh communities were significantly greater under elevated compared to ambient CO2 (Fig. 1). In contrast, there was no significant effect of salinity (shoot: F1,60 = 0.86, p = 0.453; root: F1,20 = 0.15, p = 0.701; Fig. 1) or species (shoot only: F2,60 = 40.56, p = 0.058) on either the shoot or root biomass of the model saltmarsh communities.
Mean shoot dry biomass (± SE) of each saltmarsh species and their combined shoot and root dry weight biomass under all treatment combinations (ACO2-ambient CO2, ECO2-elevated CO2, SW-seawater salinity, HS-hypersaline). Mean dry weight biomass values are at the mesocosm-level. Letters indicate significant differences between CO2 × salinity treatments in shoot and root biomass for all saltmarsh species at p < 0.05
There was no significant interactive effect between CO2 and salinity on the biomass (total, shoot and root), shoot height and root length of the mangrove species grown individually and in mesocosms. The total biomass of A. corniculatum seedlings grown in mesocosms was significantly reduced under elevated CO2 (F1,20 = 8.43, p = 0.009; Fig. 2) while shoot height (F1,20 = 0.19, p = 0.668; Fig. 3) and root length (F1,20 = 2.45, p = 0.133; Fig. 3) did not differ between the CO2 treatments. In contrast, the total biomass of the A. corniculatum seedlings grown individually did not significantly differ between CO2 treatments (F1,8 = 0.35, p = 0.569; Fig. 2). The total biomass of A. corniculatum seedlings grown in mesocosms (F1,20 = 7.30, p = 0.014; Fig. 2) and individually (F1,8 = 10.91, p = 0.011; Fig. 2) were significantly greater under seawater salinity. Their shoot height was also greater under seawater salinity (F1,20 = 20.00, p < 0.001; Fig. 3) while their root length did not differ between the CO2 treatments but only marginally (F1,20 = 4.29, p = 0.052; Fig. 3).
Mean total dry biomass (± SE) of each mangrove species when grown in mesocosms (competition) and individually (no competition) under all treatment combinations (ACO2-ambient CO2, ECO2-elevated CO2, SW-seawater salinity, HS-hypersaline). Letters indicate significant differences between CO2 × salinity treatments within each competition treatment for each mangrove species at p < 0.05
Mean shoot height and root length (± SE) of each mangrove species when grown in mesocosms (competition) under all treatment combinations (ACO2-ambient CO2, ECO2-elevated CO2, SW-seawater salinity, HS-hypersaline). Letters indicate significant differences between CO2 × salinity treatments in shoot height and root length for each mangrove species at p < 0.05
The total biomass of A. marina seedlings grown in mesocosms (F1,20 = 15.71, p = 0.001) and individually (F1,8 = 9.26, p = 0.0.016; Fig. 2) was significantly greater under elevated CO2. Their shoot height was also greater under elevated CO2 (F1,20 = 7.72, p = 0.012; Fig. 3) while root length did not differ between the CO2 treatments (F1,20 = 0.42, p = 0.524; Fig. 3). In contrast to A. corniculatum, salinity did not have a significant effect on the growth of A. marina irrespective of whether it was grown in mesocosms or individually.
Discussion
The expansion of mangroves into saltmarsh communities has been observed worldwide (Saintilan et al. 2014), with a range of different environmental factors associated with climate change considered as drivers of this encroachment (Alongi 2015). More specifically, rising atmospheric CO2 concentration and reduced salinity levels because of rising sea levels have been identified as potential key drivers of mangrove encroachment (Alongi 2015). The crucial first step in mangrove encroachment is the successful establishment of mangrove seedlings, when they are most vulnerable to biotic and abiotic stresses, in saltmarsh communities (McKee and Rooth 2008). Therefore, the aim of this study was to determine the effect CO2 and salinity has on the establishment success of mangroves seedlings in saltmarsh communities.
Both shoot and root biomass of our C3 saltmarsh species increased, by an average of 210% and 91%, respectively, when grown under elevated CO2. All three saltmarsh species had at least a 180% increase in shoot biomass under elevated CO2 with this increase being most pronounced in J. kraussi (520% on average). Previous studies have shown similar strong growth responses to elevated CO2 in a number of different C3 saltmarsh species (Drake et al. 1989; Rozema et al. 1991; Arp et al. 1993). This suggests that the competitive effect of saltmarsh species on mangrove seedlings will likely increase under future CO2 conditions, reducing any direct positive effect of elevated CO2 on mangrove growth in mixed saltmarsh–mangrove assemblages. The strong response of C3 saltmarsh species to elevated CO2 may also help saltmarsh communities respond to other pressures associated with climate change (Saintilan and Rogers 2015). For example, a 2-year study of a saltmarsh community at Chesapeake Bay in the United States found that increased belowground biomass production in the C3 species Schoenoplectus americanus under elevated CO2 accelerated soil elevation gain by 3.9 mm year−1 (Cherry et al. 2009; Langley et al. 2009), providing a counterbalance against rising sea levels (Langley et al. 2009; see Lovelock et al. 2015 for exception). It is worth noting that, similarly to terrestrial systems, the responsiveness of saltmarsh communities to elevated CO2 has been shown to be dependent on ample nutrient supply (Langley et al. 2013). As our model saltmarsh communities experienced a strong response to elevated CO2, we can suggest that they were not nutrient limited.
From what we have learnt of woody plant encroachment in terrestrial grassland systems (Manea and Leishman 2015), it would be expected that an increase in saltmarsh community biomass under elevated CO2 would suppress the growth of co-occurring mangrove seedlings. This was the case for the mangrove species A. corniculatum, which produced on average 24% less total biomass under elevated compared to ambient CO2 when grown in competition with the saltmarsh species, despite it producing marginally more total biomass (10% on average) under elevated CO2 when grown individually. In contrast, the mangrove species A. marina produced on average 48% more total biomass when grown in the model saltmarsh communities under elevated CO2. However, this growth increase was also a reduction from the average 78% increase in total biomass it had when grown individually. Despite both mangrove species experiencing a similar strong competitive effect from the saltmarsh species under elevated CO2, the relatively stronger effect of CO2 on A. marina seedlings compared to A. corniculatum seedlings suggests they are more likely to ‘escape’ competition from saltmarsh species and become established in saltmarsh communities in the future. This suggestion is supported by a historical reconstruction of A. marina growth response in the Indo-Pacific region over the last two centuries which showed that the greater biomass gains in this species over this time period were a result of rising atmospheric CO2 concentration (Reef et al. 2015).
In terrestrial grassland systems, there are two main mechanisms by which woody plant seedlings can utilise their additional biomass gains under elevated CO2 to ‘escape’ competition from co-occurring grasses. They can grow taller which allows them to overtop the grass canopy and ‘escape’ any shading effect from the grasses (Bond and Midgley 2000) and/or they can send their roots deeper, giving them access to water and nutrient resources that the grasses cannot reach (Polley et al. 1997). For A. marina, it is likely that the ‘escape from shade’ scenario is true as it grew on average 30% taller under elevated CO2 but did not send its roots deeper. This increase in height enabled it to overtop the extremely dense lower S. radicans layer (~ 10 cm in height) in the mesocosms thus giving it access to more light. This result contrasts with the findings of a previous study that found belowground competition was more important in the establishment and growth of A. germinans seedlings when grown in competition with the C4 saltmarsh species, Spartina alterniflora (Howard et al. 2018). A possible explanation for these differing results is that because our model saltmarsh communities were not nutrient limited, it is likely that light was the most limiting growth resource which made it more advantageous for A. marina seedlings to use the additional CO2 to grow taller. This explanation is supported by the findings of previous studies that found A. germinans growth is only stimulated under elevated CO2 when nutrients is not limiting (McKee and Rooth 2008; Reef et al. 2016). Furthermore, these studies reported that the majority of this increased growth occurred in aboveground biomass (Reef et al. 2016) and resulted in increased shoot height (McKee and Rooth 2008), which is consistent with our findings for A. marina.
Salinity tends to be higher in saltmarsh communities compared to mangrove communities due to a higher evaporation rate in the higher intertidal zone (Adam 2009). Consequently, saltmarsh species are generally adapted to cope with higher levels of salinity than mangrove species (Adam 2009). Consistent with the findings of Clarke and Hannon (1970), we found that A. corniculatum seedlings produced more total biomass under seawater salinity irrespective of competition (on average 36% in mesocosms and 51% individually). Surprisingly, the growth of A. marina seedlings was not influenced by salinity despite previous studies reporting the contrary (Clarke and Hannon 1970). These results suggest that the encroachment of certain mangrove species into saltmarsh communities will be favoured by rising sea levels in the future. However, as discussed above, the soil elevation gains in saltmarsh communities that may occur in the future may negate this to some extent (Saintilan and Rogers 2015).
From our study, we can suggest that rising atmospheric CO2 concentration and reduced salinity as a result of sea-level rise are likely to enhance the establishment success of mangrove seedlings in saltmarsh communities thus facilitating mangrove encroachment. However, it is important that we acknowledge the caveats of this study that need to be considered when applying our results to natural environments. Firstly, as our study was conducted in an enclosed glasshouse the impact from herbivores was non-existent. Herbivory has been shown to significantly reduce mangrove seedling survival in the field, which may negate any positive effect of CO2 on their growth in saltmarsh communities (McKee and Rooth 2008). Secondly, it would have been ideal to have included C4 or CAM species in our model saltmarsh communities (sourcing problems for Sporobolus virginicus and poor quality S. quinqueflora tube stock prevented this from happening), considering mangrove encroachment is most prevalent in C4 and CAM saltmarsh communities. Because of this our results may be viewed as conservative when applied to C4 and CAM saltmarsh communities due to these species having less of a competitive effect on mangrove seedlings than C3 species under elevated CO2. Thirdly, the salinity treatments we used were above the natural salinity range (< 50 ds m−1) which some of our species typically thrive in (e.g. J. kraussii; see Clarke and Hannon 1970). Despite this, no individuals of these species experienced mortality with J. kraussi actually preferring higher salinity levels under elevated CO2. This suggests the salinity treatments we imposed were reasonable for the survival and growth of our study species. Finally, by maintaining the salinity treatment levels during the experiment, we may have eliminated the CO2 driven benefit of reduced salinity in the mesocosms resulting from the improved water-use efficiency of the plants. This is a potentially important feedback mechanism in the field because mangroves have strong growth responses when salinity is reduced (Gilman et al. 2008; Ward et al. 2016). This means that the growth responses of the mangroves under elevated CO2 in our experiment may be conservative compared to the field. Irrespective of these caveats, this study provides a valuable insight into how climate change-associated environmental factors may influence saltmarsh–mangrove boundaries in the future.
References
Adam P (2009) Australian saltmarshes in global context. In: Saintilan N (ed) Australian saltmarsh ecology. CSIRO Publishing, Australia, pp 1–21
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Alongi D (2015) The impact of climate change on mangrove forests. Curr Clim Change Rep 1:30–39. https://doi.org/10.1007/s40641-015-0002-x
Archer S, Andersen E, Predick K, Schwinning S, Steidl R, Woods S (2017) Woody plant encroachment: causes and consequences. In: Briske DD (ed) Rangeland systems: processes, management and challenges. Springer, New York, pp 25–84
Arp WJ, Drake BG, Pockman WT, Curtis PS, Whigham DF (1993) Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetatio 104(105):133–143. https://doi.org/10.1007/BF00048149
Ball MC, Cochrane MJ, Rawson HM (1997) Growth and water use of the mangroves Rhizophora apiculata and R. stylosa in response to salinity and humidity under ambient and elevated concentrations of atmospheric CO2. Plant Cell Environ 20:1158–1166. https://doi.org/10.1046/j.1365-3040.1997.d01-144.x
Bond WJ, Midgley GF (2000) A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob Change Biol 6:865–869. https://doi.org/10.1046/j.1365-2486.2000.00365.x
Cavanaugh K et al (2014) Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc Natl Acad Sci 111:723–727. https://doi.org/10.1073/pnas.1315800111
Cherry J, McKee K, Grace J (2009) Elevated CO2 enhances biological contributions to elevation change in coastal wetlands by offsetting stressors associated with sea-level rise. J Ecol 97:67–77. https://doi.org/10.1111/j.1365-2745.2008.01449.x
Clarke LD, Hannon NJ (1970) The mangrove swamp and salt marsh communities of the Sydney district: III. Plant growth in relation to salinity and waterlogging. J Ecol 58:351–369. https://doi.org/10.2307/2258330
Comeaux R, Allison M, Bianchi T (2012) Mangrove expansion in the Gulf of Mexico with climate change: implications for wetland health and resistance to rising sea levels. Estuar Coast Shelf Sci 96:81–95. https://doi.org/10.1016/j.ecss.2011.10.003
Costanza R et al (2014) Changes in the global value of ecosystem services. Glob Environ Change 26:152–158. https://doi.org/10.1016/j.gloenvcha.2014.04.002
Daly T (2013) Coastal saltmarsh. Primefact first edition. NSW Department of Primary Industries. Orange, NSW, Australia
Drake BG, Leadley PW, Arp WJ, Nassiry D, Curtis PS (1989) An open top chamber for field studies of elevated atmospheric CO2 concentration on saltmarsh vegetation. Funct Ecol 3:363–371. https://doi.org/10.2307/2389377
Gilman EL, Ellison J, Duke NC, Field C (2008) Threats to mangroves from climate change and adaptation options: a review. Aquat Bot 89:237–250. https://doi.org/10.1016/j.aquabot.2007.12.009
Howard RJ, Stagg CL, Utomo HS (2018) Early growth interactions between a mangrove and an herbaceous salt marsh species are not affected by elevated CO2 or drought. Estuar Coast Shelf Sci 207:74–81. https://doi.org/10.1016/j.ecss.2018.03.026
IPCC (2013) Climate change 2013: a physical science basis. In: Joussaume S, Penner J, Tangang F (eds) Working Group I contribution to the IPCC fifth assessment report. Cambridge University Press, Cambridge
Kathiresan K (2012) Importance of mangrove ecosystem. Int J Marine Sci 2:70–89. https://doi.org/10.5376/ijms.2012.02.0010
Kelleway JJ et al (2017) Review of the ecosystem service implications of mangrove encroachment into salt marshes. Glob Change Biol 23:3967–3983. https://doi.org/10.1111/gcb.13727
Krauss KW, From AS, Doyle TW, Doyle TJ, Barry MJ (2011) Sea-level rise and landscape change influence mangrove encroachment onto marsh in the ten thousand Islands region of Florida, USA. J Coast Conserv 15:629–638. https://doi.org/10.1007/s11852-011-0153-4
Langley JA, McKee KL, Cahoon DR, Cherry JA, Megonigal JP (2009) Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proc Natl Acad Sci 106:6182–6186. https://doi.org/10.1073/pnas.0807695106
Langley A, Mozdzer T, Shepard K, Hagerty S, Megonigal P (2013) Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Glob Change Biol 19:1495–1503. https://doi.org/10.1111/gcb.12147
Leakey A, Ainsworth E, Bernacchi C, Rogers A, Long S, Ort D (2009) Elevated CO2 effects on plant carbon, nitrogen and water relations: six important lessons from FACE. J Exp Bot 60:2859–2876. https://doi.org/10.1093/jxb/erp096
Loarie S, Duffy P, Hamilton H, Asner G, Field C, Ackerly D (2009) The velocity of climate change. Nature 462:1052–1055. https://doi.org/10.1038/nature08649
López-Medellín X, Ezcurra E, González-Abraham C, Hak J, Santiago L, Sickman J (2011) Oceanographic anomalies and sea-level rise drive mangroves inland in the Pacific coast of Mexico. J Veg Sci 22:143–151. https://doi.org/10.1111/j.1654-1103.2010.01232.x
Lovelock CE et al (2015) The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526:559–563. https://doi.org/10.1038/nature15538
Manea A, Leishman MR (2015) Competitive interactions between established grasses and woody plant seedlings under elevated CO2 levels are mediated by soil water availability. Oecologia 177:499–506. https://doi.org/10.1007/s00442-014-3143-z
McKee KL, Rooth JE (2008) Where temperate meets tropical: multi-factorial effects of elevated CO2, nitrogen enrichment, and competition on a mangrove-salt marsh community. Glob Change Biol 14:971–984. https://doi.org/10.1111/j.1365-2486.2008.01547.x
McKee KL, Rogers K, Saintilan N (2012) Response of salt marsh and mangrove wetlands to changes in atmospheric CO2, climate, and sea level. Global change and the function and distribution of wetlands. Springer, New York, pp 63–96
Osland M, Enwright N, Day R, Doyle T (2013) Winter climate change and coastal wetland foundation species: salt marshes vs. mangrove forests in the southeastern United States. Glob Change Biol 19:1482–1494. https://doi.org/10.1111/gcb.12126
Polley HW, Mayeux HS, Johnson HB, Tischler CR (1997) Viewpoint: atmospheric CO2, soil water, and shrub/grass ratios on rangelands. J Range Manag 50:278–284. https://doi.org/10.2307/4003730
Reef R, Lovelock CE (2014) Historical analysis of mangrove leaf traits throughout the 19th and 20th centuries reveals differential responses to increases in atmospheric CO2. Glob Ecol Biogeogr 23:1209–1214. https://doi.org/10.1111/geb.12211
Reef R, Winter K, Morales J, Adame MF, Reef DL, Lovelock CE (2015) The effect of atmospheric carbon dioxide concentrations on the performance of the mangrove Avicennia germinans over a range of salinities. Physiol Plant 154:358–368. https://doi.org/10.1111/ppl.12289
Reef R et al (2016) The effects of CO2 and nutrient fertilisation on the growth and temperature response of the mangrove Avicennia germinans. Photosynth Res 129:159–170. https://doi.org/10.1007/s11120-016-0278-2
Rogers K, Wilton K, Saintilan N (2006) Vegetation change and surface elevation dynamics in estuarine wetlands of southeast Australia. Estuar Coast Shelf Sci 66:559–569. https://doi.org/10.1016/j.ecss.2005.11.004
Rozema J et al (1991) Effect of elevated atmospheric CO2 on growth, photosynthesis and water relations of salt marsh grass species. Aquat Bot 39:45–55. https://doi.org/10.1016/0304-3770(91)90021-V
Saintilan N, Rogers K (2013) The significance and vulnerability of Australian saltmarshes: implications for management in a changing climate. Mar Freshw Res 61:66–79. https://doi.org/10.1071/MF12212
Saintilan N, Rogers K (2015) Woody plant encroachment of grasslands: a comparison of terrestrial and wetland settings. New Phytol 205:1062–1070. https://doi.org/10.1111/nph.13147
Saintilan N, Williams R (1999) Mangrove transgression into saltmarsh environments in New South Wales, Australia. Glob Ecol Biogeogr 8:117–124. https://doi.org/10.1046/j.1365-2699.1999.00133.x
Saintilan N, Wilson NC, Rogers K, Rajkaran A, Krauss KW (2014) Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob Change Biol 20:147–157. https://doi.org/10.1111/gcb.12341
Snedaker S, Araujo R (1998) Stomatal conductance and gas exchange in four species of Caribbean mangroves exposed to ambient and increased CO2. Marine Freshw Res 1:325–327. https://doi.org/10.1071/MF98001
Spencer J, Monamy V, Breitfuss M (2009) Saltmarsh as habitat for birds and other vertebrates. In: Saintilan N (ed) Australian saltmarsh ecology, vol 7. CSIRO Publishing, Clayton, pp 143–159
Sydney Environmental and Soil Laboratory (2018) Soils of the saltmarsh. Sydney Environmental and Soil Laboratory, Australia
Ward RD, Friess DA, Day RH, MacKenzie RA (2016) Impacts of climate change on mangrove ecosystems: a region by region overview. Ecosyst Health Sustain 2:e01211. https://doi.org/10.1002/ehs2.1211
Williamson G, Boggs G, Bowman D (2011) Late 20th century mangrove encroachment in the coastal Australian monsoon tropics parallels the regional increase in woody biomass. Reg Environ Change 11:19–27. https://doi.org/10.1007/s10113-010-0109-5
Acknowledgements
We gratefully acknowledge the Plant Invasion and Restoration Ecology Laboratory (PIREL) of Macquarie University for their input throughout the experiment and Muhammad Masood for assistance in the glasshouses. A scientific license (SL101685) issued by the New South Wales Office of Environment and Heritage granted permission for the collection of mangrove propagules from the field.
Author information
Authors and Affiliations
Contributions
AM and MRL conceived and designed the experiments. AM and IG performed the experiments. AM analysed the data. AM, IG and MRL wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yu-Long Feng.
Rights and permissions
About this article
Cite this article
Manea, A., Geedicke, I. & Leishman, M.R. Elevated carbon dioxide and reduced salinity enhance mangrove seedling establishment in an artificial saltmarsh community. Oecologia 192, 273–280 (2020). https://doi.org/10.1007/s00442-019-04563-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04563-1